Maternal effect on body measurement and meat production traits in purebred Duroc pigs
Abstract
We investigated maternal effect on nine body measurement traits (body height, body length, front width (FW), chest width (CW), hind width (HW), chest depth, chest girth (CHG), front cannon circumference (FCC) and rear cannon circumference (RCC)) measured at the end of performance testing and five meat production traits (ages at the start and end of performance testing (D30 and D105), average daily gain (ADG), backfat thickness and loin muscle area) in purebred Duroc pigs. Genetic parameters for each trait were estimated by using six single-trait models with and without common litter environmental effect, maternal genetic effect and direct-maternal genetic correlation. The value of Akaike's information criterion was lowest with the model including direct additive genetic and common litter environmental effects for 10 traits. The estimated proportion of common litter environmental variance to phenotypic variance was approximately ≥0.1 for D30, D105, ADG, FW, CW, HW, CHG, FCC and RCC. Using a model without common litter environmental effect would overestimate the direct heritability of most traits. Standard errors of estimated genetic parameters tended to be larger in models including maternal genetic effect. The results indicate that a compromise could be made for accurate genetic parameter estimation for body measurement traits, as well as meat production traits, in pigs by considering common litter environmental effect.
1 INTRODUCTION
In pig breeding, selection has been aimed mainly at improving growth efficiency and the number of piglets weaned. Investigation of the genetic relationships of meat production with lifetime productivity traits and linear-type traits for exterior conformation and leg conformation (Aasmundstad, Olsen, Sehested, & Vangen, 2014; Kadowaki, Suzuki, Ogawa, & Itoh, 1998; Le, Madsen, Lundeheim, Nilsson, & Norberg, 2016; López-Serrano, Reinsch, Looft, & Kalm, 2000; Nikkilä et al., 2013; Sobczyńska, Blicharski, & Tyra, 2013; Van Steenbergen, 1989) shows the importance of selection while considering the soundness of body shape (Song, Zhang, Zhang, & Ding, 2019; Yazaki et al., 2020). Body measurement traits measured as continuous variables, which are harder to obtain, are more objective than linear-type traits scored on a scale (Ohnishi & Satoh, 2018). Several studies have reported results of genetic parameter estimation for different body measurement traits measured as continuous variables at different ages or body weights in different pig breeds (Do et al., 2014; Fukawa, Sugiyama, Kusuhara, Kudoh, & Kameyama, 2001; Ishida, Kuroki, Harada, & Fukuhara, 2001; Kadowaki et al., 1998; Ohnishi & Satoh, 2018; Song et al., 2019; Yazaki et al., 2020).
Substantial effects of a shared environment on phenotypic measurements in younger pigs have been reported, such as common litter environmental effects and maternal genetic effects (Flori et al., 2011; Hoque, Suzuki, Kadowaki, Shibata, & Oikawa, 2007; Suzuki et al., 2005; Tomiyama, Kanetani, Tatsukawa, Mori, & Oikawa, 2010; Yang et al., 2018). Statistical models that ignore maternal effects might bias estimates of direct heritability (Dube, Mulugeta, & Dzama, 2014; Satoh, Hicks, Ishii, & Furukawa, 2002). Therefore, it is important to choose an appropriate model for accurate genetic parameter estimation. Many studies of meat production traits reported genetic parameters estimated through the use of statistical models that include various kinds of effects concerned with shared environment before phenotypes are measured (Do, Strathe, Jensen, Mark, & Kadarmideen, 2013; Hoque, Kadowaki, Shibata, & Suzuki, 2008; Johnson, Chewning, & Nugent, 2002). Studies of body measurement traits have used statistical models that ignore (Ishida et al., 2001; Kadowaki et al., 1998; Yazaki et al., 2020) or include common litter environmental effects (Ohnishi & Satoh, 2018), contemporary group effects (Fukawa et al., 2001) or effects for herd (Do et al., 2014; Song et al., 2019). However, those models did not include maternal genetic effects, so little is known about those effects on body measurement traits in pigs. A selection strategy that simultaneously exploits direct and maternal genetic effects might improve traits more efficiently (Roehe & Kennedy, 1993). Therefore, it is important to assess whether it is better to consider maternal effects on body measurement traits as shared environmental effects at a younger age in pigs.
Here, we estimated genetic parameters for body measurement and meat production traits in purebred Duroc pigs using six statistical models differing in ignoring or considering maternal effects.
2 MATERIALS AND METHODS
All procedures involving animals were performed in accordance with the National Livestock Breeding Center's guidelines for care and use of laboratory animals. We analysed the same data set used in Yazaki et al. (2020), using an animal model that ignores common litter environmental effect and maternal genetic effect, excluding intramuscular fat content, pastern posture, body mass index and body density. We excluded intramuscular fat content because few phenotypic records were available. Pastern posture is a linear-type trait scored from 1 to 5, so it would be difficult to interpret the results. And we concluded that there is no additional merit in improving body mass index or body density.
2.1 Phenotypic information
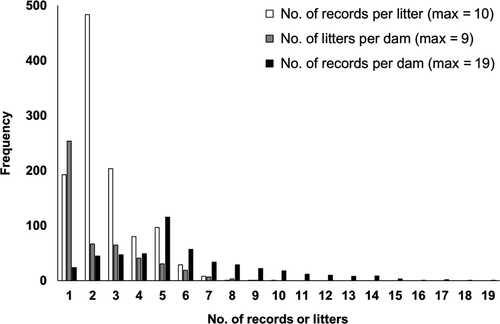
Records were available for performance testing of 3,046 purebred Duroc pigs born at the Japan National Livestock Breeding Center's Miyazaki Station between May 2009 and April 2017. Piglets were weaned at 21 days of age. All piglets weaned from the same litter were grouped into a single weaning pen. At 60 days of age, pigs were preselected as selection candidates from each litter mainly by their growth performance. Four individuals of each sex were reared in a single growing pen (2.4 m2 floor space per individual) and group-fed in a concrete-floored building. Pigs were provided ad libitum access to commercial feed (75% total digestible nutrients, 12.15% digestible crude protein) and had free access to water. Traits studied were ages at start and end of performance testing (from 30 to 105 kg live body weight: D30 and D105), average daily gain (ADG) during the performance testing, backfat thickness (BF) and loin muscle area (LMA) measured on the left side at 2 cm from the position of half the body length (BL) at D105 by ultrasound (B-mode; Super eye meat 900; Fujihira Industry Co., Ltd.) as meat production traits; and body measurement traits of body height (BH), BL, fore width (FW), chest width (CW), hind width (HW), chest depth (CHD), chest girth (CHG), and fore and rear cannon circumference (FCC, RCC) at D105. FCC and RCC were measured at the narrowest parts of the left legs. Records of 2,835 pigs with D105 within ±3 SD of its average were used (Yazaki et al., 2020) (Table 1). These pigs were progenies of 488 dams that produced 1,098 litters, of which 333 dams had their own records (Figure 1).
| Trait, unit | Abbreviation | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Body height, cm | BH | 61.5 | 2.0 | 50.0 | 69.6 |
| Body length, cm | BL | 106.7 | 3.6 | 95.5 | 121.0 |
| Front width, cm | FW | 34.0 | 1.5 | 29.2 | 42.5 |
| Chest width, cm | CW | 29.0 | 1.7 | 19.0 | 39.6 |
| Hind width, cm | HW | 31.8 | 1.3 | 27.6 | 38.8 |
| Chest depth, cm | CHD | 35.7 | 1.3 | 24.9 | 48.0 |
| Chest girth, cm | CHG | 110.1 | 3.1 | 85.0 | 120.0 |
| Front cannon circumference, cm | FCC | 18.5 | 0.8 | 16.0 | 23.5 |
| Rear cannon circumference, cm | RCC | 19.0 | 0.7 | 16.3 | 22.5 |
| Age at start of test, day | D30 | 69.6 | 6.7 | 50.0 | 104.0 |
| Age at end of test, day | D105 | 141.5 | 9.7 | 113.0 | 172.0 |
| Average daily gain, g/day | ADG | 1,061.9 | 107.5 | 760.0 | 1,639.1 |
| Backfat thickness, cm | BF | 2.8 | 0.6 | 1.2 | 5.0 |
| Loin muscle area, cm2 | LMA | 34.4 | 3.3 | 23.8 | 50.6 |
- Abbreviations: Max, maximum value; Min, minimum value; SD, standard deviation.
2.2 Statistical models
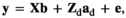 (Model 1)
(Model 1)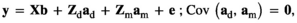 (Model 2)
(Model 2)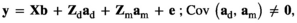 (Model 3)
(Model 3) (Model 4)
(Model 4)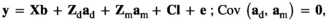 (Model 5)
(Model 5)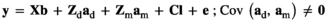 (Model 6)
(Model 6)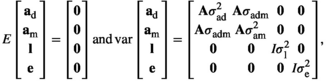
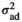 is the direct additive genetic variance;
is the direct additive genetic variance; 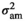 is the maternal additive genetic variance;
is the maternal additive genetic variance;  is the direct-maternal additive genetic covariance;
is the direct-maternal additive genetic covariance; 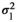 is the common litter environmental variance;
is the common litter environmental variance; 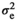 is the residual error variance; A is the additive relationship matrix constructed from pedigree information of 11,631 individuals; and I is the identity matrix. The value of
is the residual error variance; A is the additive relationship matrix constructed from pedigree information of 11,631 individuals; and I is the identity matrix. The value of  was fixed to 0 in Models 2 and 5. We used the single-trait REML procedure to estimate variance components with the average-information algorithm in ASREML software version 4.1 (Gilmour, Gogel, Cullis, Welham, & Thompson, 2015). Values of Akaike's information criterion (AIC) were compared among the six models:
was fixed to 0 in Models 2 and 5. We used the single-trait REML procedure to estimate variance components with the average-information algorithm in ASREML software version 4.1 (Gilmour, Gogel, Cullis, Welham, & Thompson, 2015). Values of Akaike's information criterion (AIC) were compared among the six models:
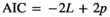
3 RESULTS AND DISCUSSION
3.1 Model comparison
Model 1 gave estimated direct heritabilities for body measurement traits ranging from 0.24 for HW to 0.45 for RCC, and those for meat production traits were from 0.33 for D30 to 0.64 for LMA (Table 2); these values were moderate to high. Previous studies also reported moderate direct heritabilities for body measurement traits in many cases, whether or not the common litter environmental effect was considered (Do et al., 2014; Fukawa et al., 2001; Ishida et al., 2001; Kadowaki et al., 1998; Ohnishi & Satoh, 2018; Song et al., 2019; Yazaki et al., 2020). Models 2 and 4 gave lower estimated values for several traits than Model 1. This implies that Model 1 somewhat overestimated the direct heritability (Clément et al., 2001; Ohnishi & Satoh, 2018; Satoh et al., 2002). Model 5 gave values similar to or slightly lower than those of Models 2 and 4. Models 3 and 6, which estimated the direct-maternal additive genetic covariance, gave greater values of the estimated direct heritabilities and their standard errors in most cases (Meyer, 1992; Solanes et al., 2004; Thompson, 1976).
| Trait | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BH | BL | FW | CW | HW | CHD | CHG | FCC | RCC | D30 | D105 | ADG | BF | LMA | |
| Model 1 | 0.30 | 0.38 | 0.30 | 0.27 | 0.24 | 0.26 | 0.39 | 0.42 | 0.45 | 0.33 | 0.40 | 0.40 | 0.61 | 0.64 |
| SE | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Model 2 | 0.29 | 0.33 | 0.30 | 0.24 | 0.24 | 0.26 | 0.29 | 0.39 | 0.41 | 0.14 | 0.28 | 0.34 | 0.58 | 0.63 |
| SE | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 |
| Model 3 | 0.33 | 0.47 | 0.35 | 0.31 | 0.28 | 0.31 | 0.37 | 0.43 | 0.40 | 0.25 | 0.37 | 0.40 | 0.63 | 0.58 |
| SE | 0.06 | 0.08 | 0.06 | 0.06 | 0.06 | 0.06 | 0.07 | 0.07 | 0.06 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 |
| Model 4 | 0.28 | 0.35 | 0.27 | 0.23 | 0.21 | 0.24 | 0.35 | 0.38 | 0.40 | 0.17 | 0.33 | 0.33 | 0.60 | 0.64 |
| SE | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Model 5 | 0.28 | 0.33 | 0.27 | 0.23 | 0.21 | 0.24 | 0.32 | 0.38 | 0.40 | 0.10 | 0.27 | 0.33 | 0.59 | 0.64 |
| SE | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 |
| Model 6 | 0.31 | 0.47 | 0.35 | 0.29 | 0.33 | 0.31 | 0.35 | 0.39 | 0.47 | 0.13 | 0.32 | 0.37 | 0.65 | 0.64 |
| SE | 0.06 | 0.08 | 0.07 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 | 0.08 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 |
Note
- Model 1, animal model without maternal effects. Model 2, animal model with maternal genetic effects and zero direct-maternal genetic correlation. Model 3, animal model with maternal genetic effects and non-zero direct-maternal genetic correlation. Model 4, animal model with common litter environmental effects. Model 5, animal model with common litter environmental effects, maternal genetic effects, and zero direct-maternal genetic correlation. Model 6, animal model with common litter environmental effects, maternal genetic effects, and zero direct-maternal genetic correlation. See Table 1 for abbreviations of trait names.
As the values of estimated genetic parameters differed among the models, we sought the model with the lowest AIC. AIC was lowest when using Model 1 for LMA, Model 5 for D30 and D105, Model 6 for BL and Model 4 for the rest (Table 3). AIC was lower when using Model 4 for D30, D105 and BL than when using Model 1. By log-likelihood value, which favours more complicated models (Ohnishi & Satoh, 2018), Model 4 was selected for FW, HW, CHD, FCC and RCC, Model 3 for LMA and Model 6 for the rest. The computation failed to converge when using Model 6 for FW, HW, CHG, FCC, RCC and LMA. Finally, AIC and log-likelihood chose the same model only for BL, FW, HW, CHD, FCC and RCC in the present study.
| Trait | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BH | BL | FW | CW | HW | CHD | CHG | FCC | RCC | D30 | D105 | ADG | BF | LMA | |
| Model 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Model 2 | 1.8 | −3.5 | 2.0 | −3.1 | 2.0 | 2.0 | −15.1 | −2.5 | −1.1 | −117.8 | −41.2 | −9.2 | −2.8 | 1.8 |
| Model 3 | 2.7 | −8.6 | 3.0 | −4.4 | 2.5 | 5.6 | −15.6 | −1.3 | 0.8 | −130.0 | −43.5 | −8.6 | −1.7 | 2.8a |
| Model 4 | −2.0 | −6.4 | −27.0a | −15.8 | −15.8a | −4.4a | −47.9 | −51.6a | −44.0a | −253.6 | −57.0 | −76.1 | −7.6 | 1.5 |
| Model 5 | 0.0 | −5.8 | −25.0 | −14.1 | −13.8 | −2.4 | −46.7 | −49.6 | −42.0 | −265.3 | −66.8 | −74.1 | −6.5 | 3.4 |
| Model 6 | 1.3a | −9.7a | −20.2 | −14.3a | −11.0 | 3.2 | −44.9a | −47.6 | −31.7 | −263.7a | −65.9a | −72.7a | −5.5a | 9.3 |
Note
- Each value was calculated as the AIC value obtained with the model minus that obtained with Model 1. Bold show the model with the lowest AIC value for a given trait.
- a The model with the lowest log-likelihood value for a given trait. See Table 1 for abbreviations of trait names. See Table 2 for the contents of models.
3.2 Genetic parameters for body measurement and meat production traits
When using the model with the lowest AIC value, estimated values of direct heritabilities ranged from 0.10 for D30 to 0.64 for LMA (Table 4). Ohnishi and Satoh (2018) reported similar values estimated by using an animal model including common litter environmental effect on the same traits as here, except for D30 and RCC, in purebred Duroc pigs. A model including common litter environmental effect on meat production traits in purebred Duroc pigs estimated direct heritabilities of 0.47 for ADG, 0.72 for BF and 0.45 for LMA (Suzuki et al., 2005). Using a model including common litter environmental effect, Hoque et al. (2007) estimated a direct heritability of 0.37 for D105. Uemoto et al. (2017) estimated moderate values of direct heritabilities for ADG, BF and LMA using three animal models—with an additive genetic relationship matrix, a genomic relationship matrix (Christensen, 2012; Su et al., 2016; VanRaden, 2008) and a haplotype-based relationship matrix (Eding & Meuwissen, 2001; Hayes & Goddard, 2008; Zhang et al., 2012)—but did not consider common litter environmental effect. Using an animal model ignoring it, Ito et al. (2018) reported direct heritabilities of 0.45 for ADG, 0.53 for BF and 0.35 for LMA. Hoque et al. (2008) estimated direct heritabilities for ADG, BF and LMA using four models including or ignoring maternal genetic effect, direct-maternal genetic correlation and maternal permanent environmental effect, although they did not compare models. Using an animal model including parity number of dams and number born alive as fixed effects and pen as random effects, Gjerlaug-Enger, Kongsro, Odegård, Aass, and Vangen (2012) estimated the direct heritability of ADG in purebred Landrace and Duroc populations. Do et al. (2013) used an animal model including random pen effect to estimate genetic parameters for ADG and BF in purebred Landrace, Large White, and Duroc boars. Do et al. (2014) and Song et al. (2019) used animal models including herd effects as fixed effects, although the definitions of herds were not found.
| Trait | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BH | BL | FW | CW | HW | CHD | CHG | FCC | RCC | D30 | D105 | ADG | BF | LMA | |
| Phenotypic variance | 3.77 | 12.53 | 1.96 | 2.21 | 1.51 | 1.50 | 7.91 | 0.50 | 0.43 | 35.1 | 73.8 | 9,852.6 | 0.24 | 9.87 |
| SE | 0.12 | 0.45 | 0.06 | 0.07 | 0.05 | 0.05 | 0.27 | 0.02 | 0.02 | 1.2 | 2.6 | 342.9 | 0.01 | 0.38 |
| Direct heritability | 0.28 | 0.47 | 0.27 | 0.23 | 0.21 | 0.24 | 0.35 | 0.38 | 0.40 | 0.10 | 0.27 | 0.33 | 0.60 | 0.64 |
| SE | 0.04 | 0.08 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 |
| Proportion of common litter variance | 0.04 | 0.03 | 0.10 | 0.09 | 0.09 | 0.05 | 0.13 | 0.14 | 0.13 | 0.28 | 0.11 | 0.17 | 0.04 | – |
| SE | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | – |
| Maternal heritability | – | 0.07 | – | – | – | – | – | – | – | 0.08 | 0.07 | – | – | – |
| SE | – | 0.03 | – | – | – | – | – | – | – | 0.03 | 0.03 | – | – | – |
| Direct-maternal genetic correlation | – | −0.59 | – | – | – | – | – | – | – | – | – | – | – | – |
| SE | – | 0.13 | – | – | – | – | – | – | – | – | – | – | – | – |
Note
- See Table 1 for abbreviations of trait names.
Estimated values of the proportion of phenotypic variance explained by common litter environmental effect were <0.1 for BH, BL, CHD and BF, ~0.1 for FW, CW, HW, CHG, FCC, RCC, D105 and ADG, and ~0.3 for D30. Ohnishi and Satoh (2018) estimated values of approximately 0.05–0.10, depending on the trait; the value was larger for D30 than for D105, possibly because of the difference in age at measurement. Estimated values of the proportion varied among the body measurement traits, maybe explained by the difference in growth patterns or proportions of body composition (bone, muscle and fat) among body parts (Landgraf et al., 2006; Moughan, Smith, & Stevens, 1990; Wagner, Schinckel, Chen, Forrest, & Coe, 1999). However, the contributions of animal management, including cross-fostering, movements and pen density, could also affect the results of common litter environmental effect. Here, the effect could be confounded by the effect of weaning pen due to the data structure (Solanes et al., 2004; Tomiyama et al., 2010). Therefore, it is difficult to pursue the common litter environmental effect in detail here.
Maternal heritability was estimated to be low—0.07 for BL, 0.08 for D30 and 0.07 for D105—and the estimated direct-maternal genetic correlation for BL was −0.59. It might be beneficial for efficient breeding that some traits are affected by maternal genetic effect (David et al., 2015; Roehe & Kennedy, 1993; Satoh et al., 2002), although we cannot suggest why a non-zero direct-maternal genetic correlation was estimated only for BL. Interpreting negative estimates of direct-maternal genetic correlation has been discussed in terms of biological mechanism, data structure and other factors (Bijma, 2006; David et al., 2015; Dodenhoff, Van Vleck, & Wilson, 1999; Dube et al., 2014; Heydarpour, Schaeffer, & Yazdi, 2008; Lee & Pollak, 1997; Maniatis & Pollott, 2003; Robinson, 1996; Willham, 1980). As an additional analysis, we ran 200 simulations of the phenotypic data of 2,835 pigs with a direct heritability of 0.3 and without maternal genetic or common litter environmental effect, based on the pedigree information of 11,631 pigs, with an infinitesimal model (Bulmer, 1980). Analysis of the simulated data using Model 3 without fixed effects gave estimated values of direct-maternal genetic correlation within the parameter space in 132 of the 200 cases, with a mean ± SD of −0.02 ± 0.40. Analysis of the same data using Model 6 without fixed effects gave estimated values of direct-maternal genetic correlation within the parameter space in 119 cases. These results could reflect the difficulty in correctly inferring the presence of non-zero direct-maternal genetic correlation, owing to low reliability of the estimates obtained using Models 3 and 6.
3.3 General discussion
More attention should be paid to body shape in pig breeding (Song et al., 2019; Yazaki et al., 2020). Therefore, it is important to estimate genetic parameters for traits relating to body shape as accurately as possible. Among previous studies that estimated genetic parameters for body measurement traits, only Ohnishi and Satoh (2018) compared two animal models with and without common litter environmental effect, and none examined the importance of maternal genetic effect. We estimated genetic parameters for body measurement traits using six models differing in considering or ignoring maternal effect in a shared environment at a younger age, together with meat production traits. We chose the most appropriate model for each trait on the basis of AIC value. Except for LMA, including common litter environmental effect (Model 4) gave lower AIC values than ignoring it (Model 1) (Table 3). Including maternal genetic effect further reduced AIC values for D30 and D105 when the direct-maternal genetic correlation was fixed at 0 (Model 5), and for BL when the correlation was estimated (Model 6). On the other hand, Model 6 led to non-convergence of REML estimation for many traits. The results obtained using the models with the lowest AIC values indicate that there seems to be a maternal effect, not only environmental but occasionally also genetic, on body measurement traits in pig (Table 4), although possible causes require careful consideration. We also showed that considering maternal effect might reduce bias in genetic parameter estimation, which would lead to more accurate genetic evaluation and prediction of expected genetic gain for body measurement traits. Therefore, these results could contribute to future breeding strategies considering the balance of body shape in pigs.
If the aim is to seek a true model for each body measurement trait, a larger data set with more detailed information about shared environment and more sophisticated modelling is needed. For example, more accurate inference about the impact of maternal genetic effects and direct-maternal genetic correlation could become possible when the number of phenotypes is larger. Also, analyses might become available using models that assume changes in maternal genetic effects through the age or parity of dams (Baldi, Albuquerque, & Alencar., 2010; Chaudhary et al., 2019; Molina, Menéndez-Buxadera, Valera, & Serradilla., 2007) and that include social genetic effects of litter- or pen-mates (Bergsma, Kanis, Knol, & Bijma, 2008; Canario, Lundeheim, & Bijma, 2017; Chen, Kachman, Johnson, Newman, & Van Vleck, 2008). On the other hand, our data set seems to be not large enough for fitting more complicated models, although we used at least as many phenotypic records as previous studies of purebred Duroc pigs (Fukawa et al., 2001; Ishida et al., 2001; Kadowaki et al., 1998; Ohnishi & Satoh, 2018; Yazaki et al., 2020). Furthermore, the effect of shared environment on the variability of phenotypic measurements could depend on the timing of measurement and on rearing situation. The results of D30 and D105 suggest that the effect of shared environment on phenotypic variation could be larger when a trait is measured at a younger age. On the basis on these results and in agreement with Ohnishi and Satoh (2018), we conclude that it is better for accurate genetic parameter estimation to consider the effect of shared environment at a younger age and that a compromise could be made by using statistical models including common litter environmental effects for body measurement traits as operational models, as well as for meat production traits, in pigs.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Development of Breeding Technology for Animal Life Production).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
S.O. and M.S. conceived the study. C.O. and K.I. prepared the raw data. S.O. carried out the analyses and drafted the manuscript. N.Y. helped carry out the analyses. M.S. contributed to writing and improving the manuscript. Y.U. participated in the design of the study. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Restrictions apply to the availability of these data, which were used under licence for this study.




