Ferulsinaic acid attenuation of advanced glycation end products extends the lifespan of Caenorhabditis elegans
Abstract
Objectives Ferulsinaic acid is the first member of a new rearranged class of sesquiterpene coumarins of the genus Ferula. The genus Ferula can be used for the treatment of skin infections, hysteria and for stomach disorders, such as a febrifuge and a carminative agent. The effect of ferulsinaic acid on the lifespan of the nematode Caenorhabditis elegans has been examined. Novel data explaining the effect of ferulsinaic acid on the lifespan of C. elegans and its antioxidant power were obtained.
Methods C. elegans was cultivated under standard laboratory conditions in absence and presence of different ferulsinaic acid. Also, animals were cultivated under heat and chemical stress conditions in absence and presence of ferulsinaic acid. Life span assay, determination of protein concentration, assay of malondialdehyde and ELISA for determination of AGEs were performed.
Key findings Under standard laboratory conditions and in presence of ferulsinaic acid (500 nm, 10 µm and 100 µm), mean life span of wild type animals was significantly lengthened in a dose-dependent manner from 18.64 ± 0.19 days (control) to 19 ± 0.19 (P = 0.695), 20.76 ± 0.25 (P = 0.043) and 22.3 ± 0.29 (P = 0.0291), respectively. Interestingly, in C. elegans resistance for heat stress at 35°C and oxidative stress induced by paraquat were significantly improved with ferulsinaic acid. Ferulsinaic acid was found to significantly attenuate both lipid peroxidation and the formation of advanced glycation end products in the wild-type animals under standard laboratory conditions.
Conclusions Ferulsinaic acid had therapeutic efficacy as an antioxidant with the possibility of its use as an antioxidant drug.
Introduction
Many plants synthesize an array of chemical compounds that are not involved in their primary metabolism. These ‘secondary compounds’ instead of serving a variety of ecological functions, they ultimately enhance the plant's survival during stress.[1] In addition, these compounds may be responsible for the beneficial effects of fruits and vegetables on an array of health related measures.[2]
Herbal medicine has existed for more than 5000 years. Today there are more than 3000 kinds of medicinal herbs. These herbal combinations are believed to act synergistically to harmonize beneficial effects and to neutralize or minimize the toxic or adverse effects of individual constituent herbs. Recently, numerous efforts have been made to investigate the mechanisms of action of Chinese herbal formulae using modern scientific methodology.[3–5] In-vitro and in-vivo studies on the individual herbs or constituents of classic formulae have been reported and many herbal medicines have immunomodulatory or antioxidant effects that may offer clinically relevant therapeutics for patients with disorders associated with ageing.[6,7]
The exclusively old-world genus Ferula, belonging to the family Apiaceae, has some 130 species distributed throughout the Mediterranean area and Central Asia. These plants are often used as spices and in the preparation of local drugs. The resins are reported to be used for stomach disorders, such as a febrifuge and carminative agent. Some species are used in traditional medicine for the treatment of skin infections and hysteria.[8] Previous work on members of this genus revealed that the main constituents are sesquiterpenes and sesquiterpene coumarins.[9]
Ferulsinaic acid is the first member of a new rearranged class of sesquiterpene coumarins from the genus Ferula with molecular formula C24H30O5. Its structure was proved in a previous work and is shown in Figure 1.[10] The effects of ferulsinaic acid on parameters related to ageing have been investigated. A study was designed to determine whether ferulsinaic acid could delay ageing and prolong the lifespan in a whole organism. An organism with a relatively short lifespan was required that could be assayed reproducibly and robustly, for which the genetic and environmental factors affecting lifespan have been well defined. The experimental organism that could best accommodate these requirements was the nematode, Caenorhabditis elegans, which has become a popular model for studying ageing and longevity.[11,12]
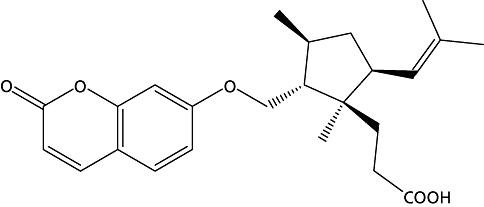
Structure of ferulsinaic acid.
C. elegans is a small (adult size approximately 1 mm length), transparent, free-living, bacteriovorus vermiform with a mean lifespan of approximately 20 days under laboratory conditions. Under these conditions, C. elegans are predominantly (>99%) self-fertilizing hermaphrodites whose population can be maintained effectively isogenic, since each individual hermaphrodite produces approximately 300 clonal progeny during the first week of adult life.[11]C. elegans hermaphrodite adults contain 959 somatic cells, 302 of which are neurons. The complete cell lineage is known, including those cells that undergo apoptosis during development.[13]
Ageing is the progressive accumulation of different changes over time that is associated with or is responsible for the increasing susceptibility to different diseases and death.[14] Although there are several theories that discuss the ageing process, the free radical theory of ageing seems to be a significant contributor in the process.[15] Free radicals are highly reactive by-products of metabolism seeking stability by gaining an extra electron from any source. This results in protein, lipid, and DNA damage. Once produced, free radicals are normally cleared by the body's naturally occurring antioxidants, but as organisms age, the declining efficiency of this mechanism allows for the accumulation of free radicals in the body.[15,16]
Studies have shown that specific genetic and environmental factors influence ageing and lifespan of C. elegans.[17,18] In addition, there are aspects of ageing which are similar between nematodes and mammals, including humans. For instance, sarcopenia, the loss of muscle mass, is a major feature of ageing in humans and C. elegans, and it contributes to ageing-related behavioural decline in both species.[18,19] Second, calorie restriction, the only known intervention that successfully extends lifespan in mammals, can prolong the lifespan of C. elegans.[20] Finally, oxidative stress appears to be a major factor limiting lifespan in C. elegans and in humans.[21,22] These findings show that studies of ageing on C. elegans provide useful stepping stones for identifying genes and compounds that can prolong lifespan in humans.
The aim of this work was to study the effect of ferulsinaic acid on the lifespan and stress resistance in C. elegans to evaluate its power as an antioxidant and its therapeutic potential.
Materials and Methods
Chemicals
The isolation of ferulsinaic acid has been detailed in a previous study.[10] 5′Fluorodeoxyuridine (FUDR), ammonium sulfate, thiobarbituric acid, 1,1′,5,5′tetramethoxy propane and butyl hydroxyl toluene were obtained from Sigma-Aldrich (Deisenhofen, Germany). 1,1′-Dimethyl-4,4′-bipyridinum dichloride x-hydrate (paraquat) was obtained from Riedel-de Haen (Germany).
Selected agar, yeast extract and peptone were obtained from Invitrogene (Karlsruhe, Germany). N-ε-Carboxymethyllysine (CML) ELISA kit and protease inhibitor tablets were from Roche (Mannheim, Germany). Tris buffer was purchased from BDH (Poole, UK). Phosphate buffered saline (PBS) was obtained from Biowhittaker (Walkerville MD, Germany). A protein concentration kit was obtained from Pierce (Rockford, USA). The remaining chemicals were purchased from ADWIC (Cairo, Egypt).
C. elegans strains, maintenance and culture conditions
Strains were maintained and manipulated under standard conditions.[23] The strain used in this study was Bristol N2 (wild type) and was kindly provided by Dr Michael Morcos, Department of Internal Medicine I (Endocrinology, Metabolism and Clinical Chemistry), Faculty of Medicine, Lüprecht-Karls University, Heidelberg, Germany.
All nematodes were cultivated on nematode growth medium (NGM) agar on 60 mm Petri plates and maintained at 20°C in a temperature-controlled incubator. Living Escherichia coli bacteria (OP50) provided the food source, which was added to the surface of NGM plates at 100 µl of a standardized overnight culture, using standard protocols.
To obtain age-synchronized nematodes, self-fertilizing hermaphrodites (three-days-old) were left to lay eggs and then removed to set the eggs in synchrony. After hatching and reaching adulthood, adult worms were transferred to new plates every day during the egg-laying period. To obtain large amounts of age-synchronous worms, young adult worms were transferred to NGM agar plates containing 40 µm FUDR to prevent eggs from hatching.[16,24,25]
Lifespan assay
The lifespan assay was conducted at 20°C as described previously in the presence of 40 µm FUDR to prevent eggs from hatching.[16,24–26]
The worms were observed and counted daily in the absence (control) or in the presence of different concentrations of ferulsinaic acid (500 nm, 10 µm and 100 µm) to determine whether they were alive. They were prodded gently with the tip of a thin platinum wire, and when they no longer responded, they were considered to be dead. Dead worms were removed from the plates. The number of worms for each analysis was approximately 100. For all lifespan experiments, assays were repeated at least three times.
Thermotolerance assays
Thermotolerance assays were performed with hermaphrodites on adult day 7, after the majority of egg-laying had ceased. Animals were transferred to 3-cm NGM agar plates supplemented as indicated and then incubated at 35°C for 16 h for N2 strain in the presence and in the absence of different concentrations of ferulsinaic acid.[27] Survivals were scored as the number of animals responsive to gentle touch as a fraction of the original number of animals on the plate. Thermotolerance assay was repeated three times.
Oxidative stress resistance assays
Paraquat assay was performed with 30 worms per trial at 20°C. To assay paraquat sensitivity, 7-day-old worms were placed overnight on agar plates containing 40 µm FUDR or 40 mm paraquat in the presence or absence of different concentrations of ferulsinaic acid (500 nm, 10 µm and 100 µm).[24,28] Viability was assayed over 12 h.
Isolation of protein extracts from C. elegans
A large number of worms was incubated with different concentrations of ferulsinaic acid (500 nm, 10 µm and 100 µm) or without ferulsinaic acid (control) until day 7 in the presence of 40 µm FUDR to prevent production of progeny. Subsequent to the incubation times, worms were harvested from plates using M9 buffer and washed free of bacteria by sucrose floatation and suspended in a 2-fold volume of homogenization buffer (0.05 m Tris-HCl, pH 7.9, 25% glycerol, 0.1 mm EDTA, 0.32 m (NH4)2SO4) containing a protease inhibitor tablet. The lysates were homogenized on ice using a Polytron homogenizer. The homogenates were centrifuged at 12 000 rev/min for 5 min at 4°C, and the supernatants assayed for protein concentration.
Lipid peroxidation
Lipid peroxidation was determined by measuring malondialdehyde (MDA), an index of lipid peroxidation according to the method used in previous work, using 1,1′,3,3′-tetramethoxy propane as the standard.[29–31] In brief, 8.1% SDS was added to the worm lysate (prepared as described above) and incubated for 10 min at room temperature, followed by boiling with 20% acetic acid and 0.6% thiobarbituric acid for 60 min in a water bath. On cooling, a mixture of butanol : pyridine (15 : 1 v/v) was added and centrifuged at 1200 rev/min for 5 min. Absorbance of the upper coloured layer was measured at 532 nm and the concentration of MDA was expressed in terms of nmol/g protein. Butylhydroxy toluene (0.01%) was added to each assay mixture to prevent undesirable autoxidation of the sample during the assay.[32]
ELISA of N-ε-(carboxymethyl) lysine-modified proteins
N-ε-Carboxymethyllysine (CML) concentrations were determined by a competitive enzyme-linked immunosorbent assay (Roche Diagnostics, Germany) using streptavidin-coated microtitre plates as described.[33] Biotinylated bovine serum albumin, which was glycated with glucose over three weeks, binds to a mouse monoclonal antibody specific for the CML epitope, which was labelled with horseradish peroxidase. 2,2-Azino-di-(3-ethylbenzthiazoline sulphonate) diammonium salt (ABTS) plus sodium perborate served as substrate for the indicator enzyme. ABTS is a trademark of a member of the Roche group. CML-modified proteins from the sample compete for the antibody binding site. As a standard, the monomeric epitope N-carboxymethyl-aminocaproate was used, yielding results as ‘ng CML’. The limit of detection was 5 ng CML/ml.
Statistical analysis
Statistical analysis was performed using SPSS software for calculation of Kaplan-Meier survival curves and comparison of lifespan. P-value calculations comparing different lifespans were made using the Kruskal-Wallis test. Mean and maximum lifespan values and Kaplan-Meier curves were the result of data pulled from three different experiments, performed independently at different time-points. Individual differences between the various treatments were then identified using Dunn's test (post hoc test). A value of P < 0.05 was considered as statistically significant.
Results
Ferulsinaic acid extended the lifespan of C. elegans
To study the effect of ferulsinaic acid on the lifespan of wild-type C. elegans, N2 worms were cultivated on agar plates under standard laboratory conditions at 20°C either in the absence (control) or in the presence of different concentrations of ferulsinaic acid (500 nm, 10 µm or 100 µm). Data in Figure 2 show that wild-type animals grown under standard laboratory conditions at 20°C had a mean lifespan of 18.64 ± 0.19 days and an average maximum lifespan of 24 days.
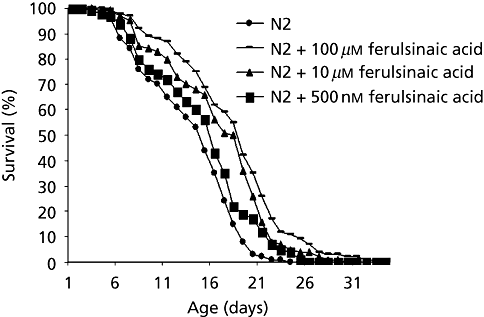
Survival curve of Caenorhabditis elegans (N2) on agar cultures of ferulsinaic acid compared with control group (without). Values represent the mean from three independent experiments each including 100 worms. Mean lifespan of: control = 18.64 ± 0.19 days; 100 µm ferulsinaic acid = 22 ± 0.29 days P = 0.0291; 10 µm ferulsinaic acid = 20.76 ± 0.25 days P = 0.043; 500 nm ferulsinaic acid = 19 ± 0.19 days P = 0.695.
On media containing 500 nm, 10 µm or 100 µm ferulsinaic acid, mean lifespan of wild-type animals was significantly lengthened in a dose-dependent manner from 18.64 ± 0.19 (control) to 19 ± 0.19 (P = 0.695), 20.76 ± 0.25 (P = 0.043), and 22.3 ± 0.29 (P = 0.0291) days, respectively. The percentage of mean lifespan extension was found to be 1.93, 11.37 and 18.03%, respectively.
Maximum lifespan was also increased in a dose-dependent manner from 24 days in the control group to 26, 30 and 34 days (percent of lifespan extension were 8.33, 25 and 41.6%), respectively, in the ferulsinaic acid-treated groups.
Effects of ferulsinaic acid on intrinsic stress resistance
Ferulsinaic acid treatment improved survival under conditions of oxidative stress. Resistance to oxidative stress was examined by exposing animals to paraquat, an intracellular free-radical-generating compound. Ferulsinaic acid treatment significantly increased the survival in the presence of paraquat in a dose-dependent manner as shown in Figure 3. Thermotolerance was increased significantly by ferulsinaic acid treatment dose dependently. In wild-type animals, after 16 h at 35°C, treatment with ferulsinaic acid (500 nm, 10 µm or 100 µm) was correlated with a 1.8-, 2.31- and 3.1-fold increase in survival, respectively, as shown in Figure 4.
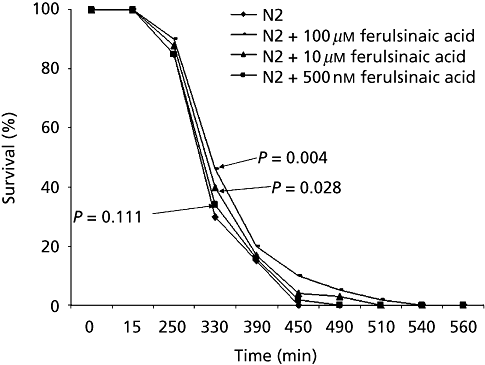
Average survival times of Caenorhabditis elegans under oxidative stress. Treatment with ferulsinaic acid significantly improved oxidative stress resistance induced by 40 µm paraquat on seven-day-old N2 worms. Control, without ferulsinaic acid. The values are the average of four independent experiments with an average of 30 individual animals used per experiment.
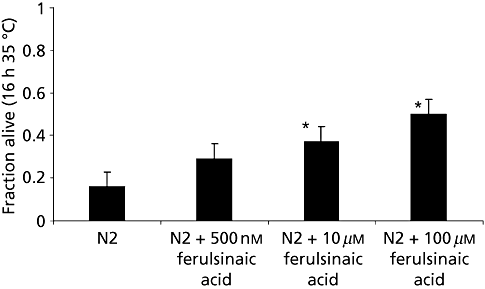
Average survival times of Caenorhabditis elegans under heat stress. Treatment with ferulsinaic acid significantly improved thermotolerance. Fractional survival at 35°C for seven-day-old N2 worms and for worms treated with ferulsinaic acid. The values are the average of four independent experiments with an average of 30 individual animals used per experiment. Error bars are the standard error of the mean. *P < 0.05 compared with N2/control strain.
Effects of ferulsinaic acid on lipid peroxidation
Lipid peroxidation has been and remains one of the most widely used indicators of oxidant/free radical formation in vitro and in vivo. Reactive-oxygen species (ROS) play a central role in the formation of active aldehydes e.g. glyoxal, methyl glyoxal and 3-deoxyglucosone. The role of ferulsinaic acid in attenuating lipid peroxidation was tested by measuring the concentration of MDA in the control and ferulsinaic acid-treated animals. Ferulsinaic acid was found to significantly decrease MDA in treated animals. The decrease in MDA was dose dependent also, as shown in Figure 5.
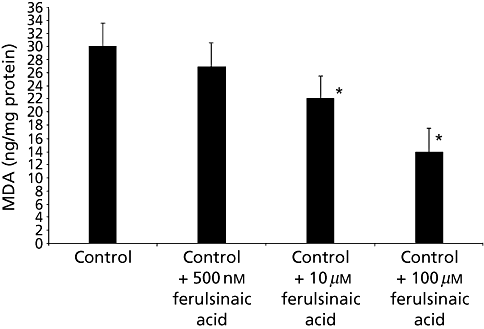
Malondialdehyde (MDA) content in seven-day-old Caenorhabditis elegans in the presence of ferulsinaic acid. Vertical bars represent SEM from five replicate experiments. *P < 0.05 compared with N2/control strain.
Effects of ferulsinaic acid on protein glycosylation
Formation of advanced glycation end products (AGEs) e.g. CML, is correlated with oxidative stress and formation of dicarbonyl compounds. AGE was found to play a major role in decreasing lifespan of C. elegans.[16] To test the degree of CML formation in worms cultivated under standard laboratory conditions at 20°C in the absence (control) and the presence of different concentrations of ferulsinaic acid, CML concentration was determined using ELISA. The data in Figure 6 showed that ferulsinaic acid significantly decreased CML formation in wild-type animals in a dose-dependent manner (at all concentrations P < 0.001).

N-ε-Carboxymethyllysine (CML) ELISA for seven-day-old Caenorhabditis elegans in the absence (control) or in the presence of ferulsinaic acid. Vertical bars represent SEM from three independent experiments. *P < 0.05 compared with N2/control strain.
Discussion
We have examined in vivo the antioxidant effect of ferulsinaic acid and its potential to extend the lifespan of C. elegans. Although ageing mechanisms have been well studied in model organisms such as yeast and nematodes, a feasible pharmaceutical intervention for humans has not yet been discovered. Yet many compounds have been demonstrated to delay ageing and age-related decline in model organisms.[27,34–36] In this study, we found that ingestion of different concentrations of ferulsinaic acid significantly extended the lifespan of C. elegans, increased stress resistance and reduced levels of MDA, which measures the degree of lipid peroxidation in the C. elegans wild-type under standard culture conditions. This reduction was found to be dose-dependent as shown in 2-5. High levels of active carbonyl compound in the cell may have been correlated to oxidative stress and generation of ROS. ROS as indicated in several publications have the ability to cause cellular damage.[16] The power of ferulsinaic acid to decrease the level of MDA confirmed its potential to reduce oxidative stress.
Ferulsinaic acid was found to significantly modulate the formation of AGEs, in particular CML in the wild-type animals under standard laboratory conditions at 20°C in a dose-dependent manner as shown in Figure 6. These observations indicated that glycosylation of proteins and ROS generation in the animals was significantly attenuated as a result of ferulsinaic acid supplementation. This suggested an antioxidant power of ferulsinaic acid or its ability to activate the antioxidant enzymes of the animals.
Lifespan of C. elegans is affected by many factors. Oxidative stress plays an important role in C. elegans longevity. Many studies have shown that reduction of oxidative stress resulted in lifespan extension of C. elegans.[16,24] To test that this was true in this study, the lifespan of the wild-type animals under standard laboratory conditions at 20°C was determined. Ferulsinaic acid was found to significantly extend the lifespan of C. elegans in a dose-dependent manner (Figure 2). These data were in line with those obtained in 5, 6. Decreasing ROS production and attenuation of AGE formation could be attributed to the antioxidant effect of ferulsinaic acid. The obtained data from Figure 6 were in line with previous studies.[16,21,37,38]
Interestingly, the lifespan extension was observed under heat stress at 35°C and induced oxidative stress conditions. Lifespan extension in both cases was found to be significant and dose-dependent as shown in 4, 3, respectively. Also, lifespan extension under both heat stress at 35°C and induced oxidative stress conditions by paraquat may have been attributed to the antioxidant effect of ferulsinaic acid, which decreased ROS production and attenuated AGE formation.
Oxidative stress has been demonstrated to be a major factor limiting lifespan in C. elegans and humans.[21,24] It has gained widespread support as a preeminent theory of ageing.[14,15] Oxidative stress initiates the production of ROS such as glyoxal, methyl glyoxal, peroxides and radicals. All these compounds can react with the amino group of lysine or the terminal amino group of proteins, and with the guanidine group of arginine and with cysteine. Interactions among the compounds formed are followed with formation of cyclic compounds and of aggregates. Moreover, AGEs can propagate free-radical reactions that may catalyse further damage of proteins, lipids or DNA.[39–41]
These findings were in line with the postulates of the theory of ageing. The obtained data indicated that ferulsinaic acid attenuated lipid peroxidation and protein glycosylation, which resulted in lifespan extension and increased stress resistance of C. elegans. These data indicated the antioxidant or radical scavenging activity of ferulsinaic acid.
Further studies are required to determine the exact genetic mechanism by which ferulsinaic acid extended the lifespan of C. elegans. These studies could use the long-lived mutants, such as age-1, clk-1, and spe-26 which have increased resistance to several stressors, including reactive oxidants. Also, toxicity experiments, for example LD50, are required to examine there is a possible restriction to the interest of ferulsinaic acid prolonging life in humans.
Conclusion
Lifespan extension of ferulsinaic acid in the nematode C. elegans might be attributed to its direct ROS scavenging activity and indirect free radical-scavenging activity through upregulating stress-resistance-associated genes such as SOD, hsp, and daf 2, clk, mev, and skn-1. These interesting findings highlight the therapeutic efficacy of ferulsinaic acid as an antioxidant and the possibility of its use as an antioxidant drug. Further studies using different mutants of C. elegans are required to examine the exact mechanism of ferulsinaic acid action and the optimal dose required.
Declarations
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements
The author wishes to acknowledge the scientific guidance of Dr Michael Morcos, Department of Internal Medicine I (Endocrinology, Metabolism and Clinical Chemistry), Faculty of Medicine, Lüprecht-Karls University, Germany, who provided C. elegans strains, the CML ELISA kit and some fine chemicals, and for his kind and continuous advice.




