Development and Clinical Evaluation of A PCR Assay Targeting the Metalloprotease Gene (mprA) of B. pseudomallei
Summary
A PCR assay targeting the metalloprotease gene (mprA) of Burkholderia pseudomallei was developed for the specific detection of this organism in pure cultures and clinical samples. All other closely related organisms including B. mallei the causative agent of glanders, and B. thailandensis tested negative. Burkholderia pseudomallei DNA was successfully amplified from paraffin-embedded lung tissue of a camel with a generalized B. pseudomallei infection. The developed PCR assay can be used as a simple tool for the specific and sensitive detection of B. pseudomallei.
Introduction
Melioidosis is an infectious disease of animals and humans caused by Burkholderia pseudomallei. Burkholderia pseudomallei is a motile, gram-negative, oxidase-positive rod genetically closely related to B. mallei the causative agent of glanders in solipeds (Yabuuchi et al., 1992). Its genetic information is encoded by two chromosomes, 4.03 and 3.17 Mbp in size (Holden et al., 2004). Melioidosis occurs in tropical areas between latitudes 20°N and 20°S, predominantly in South-east Asia and Northern Australia. Burkholderia pseudomallei is a saprophyte that can be regularly isolated from soil and muddy water in endemic areas. It has a high natural resistance to antibiotics, disinfectants and environmental living conditions, such as a soil pH value as low as pH 4, minimal humidity of 10–15% and temperatures ranging from 4 to 42°C (Nachiangmai et al., 1985; Tong et al., 1996; Cheng et al., 2003). It is commonly accepted that importation of the bacterium occurs with (subclinically) infected animals, which start to excrete bacteria with faeces or through abscesses due to stress caused by travelling, climate or poor confinement (Ketterer et al., 1975, 1986; Dodin, 1992; Currie et al., 2000). Thus, new (timely limited) endemic foci may establish even in the cooler European climate (Dodin, 1992). The spreading of B. pseudomallei clearly profits from globalization of animal trade, modern mass production involving crowding and the use of enormous amounts of potable water (Sprague and Neubauer, 2004). Consequently, cases in animals have not only been reported from Australia, Papua New Guinea, Malaysia, Thailand, Viet Nam, Singapore and Taiwan, but also from China, the Indian subcontinent, Turkey, Iran, Saudi Arabia, United Arab Emirates, Madagascar, Egypt, Chad, Niger, South Africa, Aruba, USA, Brazil, UK, France and Spain (Sprague and Neubauer, 2004). Occasional infection in a multitude of warm- and cold-blooded animals can occur by inhalation, ingestion, via skin wounds in contact with contaminated water or dust particles (Thomas et al., 1988). The clinical picture can present itself in various forms: acute fulminate septicaemia, local infection, subacute illness, chronic infection and subclinical disease. Acute melioidosis is often a fulminate infection with sepsis and high mortality, especially in young animals. The incubation period for naturally occurring infection in animals is not known. In solipeds and camelids melioidosis can easily be mistaken for glanders (Sprague and Neubauer, 2004).
Although B. pseudomallei is not a fastidious bacterium, isolation and identification of the agent are often unsuccessful. No optimized technique or media for enrichment and cultivation exists (Ashdown and Clarke, 1992; Brooks et al., 1997). Using biochemical identification by conventional and automated identification systems such as APITM stripes, VitekTM cards (both BioMérieux, Nurtingen, Germany) or the PhoenixTM (Becton Dickinson, Heidelberg, Germany), B. pseudomallei is often misdiagnosed as B. cepacia, Chromobacterium violaceum or not identified at all (Sprague and Neubauer, 2004; Glass and Popovic, 2005). Assays for a sensitive and specific detection of antigens are not commonly available. The development of specific PCR assays is hampered by the fact that B. pseudomallei and B. mallei are genetically closely related on the basis of their genetic information (Yabuuchi et al., 1992; Godoy et al., 2003; Holden et al., 2004; Nierman et al., 2004). Therefore, various PCR and real-time PCR systems have been described with a low detection limit but not capable of discriminating both pathovars e.g. flagellin C gene specific assays (Hagen et al., 2002; Sonthayanon et al., 2002; Sprague et al., 2002; Tomaso et al., 2004, 2005). Recently, strategies for the detection of B. pseudomallei from pure cultures have been proposed using real-time PCR and multiplex PCR technology (Thibault et al., 2004; Lee et al., 2005). However, the assays developed are highly sophisticated.
In this article, we describe the development and evaluation of a simple single target PCR assay with a low detection limit for the detection of B. pseudomallei DNA in pure cultures and lung tissue of a naturally infected dromedary.
Materials and Methods
Bacterial strains
A representative panel of 20 B. pseudomallei strains isolated from different environmental or clinical specimens and 53 strains of closely related species and genera were investigated (Table 1). Strains or DNA were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), the National Collection of Type Cultures of the Central Public Health Laboratory (NCTC, London, UK), B. Niederwöhrmeier (WIS, Munster, Germany), J. Ellis (Defence Science and Technology Laboratory, Porton Down, UK), N. Anuntagool (Mahidol University, Bangkok, Thailand), K. Nöckler (Bundesinstitut für Risikobewertung, Berlin, Germany) and T. Pitt (Laboratory of Health Care Associated Infection, Specialist and Reference Microbiology Division, Health Protection Agency, London, UK). Bacteria were grown on standard media as per the conditions recommended by the respective bacterial collection centres.
| Strain | Source | Geographical origina | Species | B. pm PCR |
|---|---|---|---|---|
| Melioidosis | ||||
| K96243 | Human | Thailand | B. pseudomallei | + |
| ATCC 23343T | ND | ND | B. pseudomallei | + |
| NCTC 4845 | Monkey | Singapore | B. pseudomallei | + |
| Soil 1977 | Soil | Madagascar | B. pseudomallei | + |
| D4899/303 | Environment | Venezuela | B. pseudomallei | + |
| 7894 | Human | Ecuador | B. pseudomallei | + |
| SID4350 | Human | N Thailand | B. pseudomallei | + |
| SID4352 | Human | N Thailand | B. pseudomallei | + |
| SID5752 | Human | UK/Thailandb | B. pseudomallei | + |
| SID2889 | Human | UK/Bangladeshb | B. pseudomallei | + |
| SID 4717 | Human | Bangladesh | B. pseudomallei | + |
| HumA | Human | Australia | B. pseudomallei | + |
| Heckeshorn | Human | Germany/Thailandb | B. pseudomallei | + |
| SID 3477 | Human | Italy | B. pseudomallei | + |
| Mal6 | Museum strain | ND | B. pseudomallei | + |
| E38 | Museum strain | ND | B. pseudomallei | + |
| 603a | Museum strain | ND | B. pseudomallei | + |
| Hainan 4 | Environment | China | B. pseudomallei | + |
| Hainan 55 | Environment | China | B. pseudomallei | + |
| NT 08 | Environment | Niger | B. pseudomallei | + |
| Glanders and farcy | ||||
| ATCC 23344T | Human | China | B. mallei | − |
| ATCC 15310 | Horse | Hungary | B. mallei | − |
| ATCC 10399 | Horse | China | B. mallei | − |
| NCTC 00120 | ND | London/Lister | B. mallei | − |
| NCTC 03708 | Mule | India | B. mallei | − |
| NCTC 03709 | Horse | India | B. mallei | − |
| NCTC 10229 | Horse | Hungary | B. mallei | − |
| NCTC 10245 | Horse | China | B. mallei | − |
| NCTC 10247 | Human | Turkey | B. mallei | − |
| NCTC 10248 | Human | Turkey | B. mallei | − |
| NCTC 10260 | Human | Turkey | B. mallei | − |
| Mukteswar | Horse | India | B. mallei | − |
| Bogor | Horse | Indonesia | B. mallei | − |
| Zagreb | Horse | Yugoslavia | B. mallei | − |
| M2 | Un | Un | B. mallei | − |
| 32 | Un | Un | B. mallei | − |
| 235 | Un | Un | B. mallei | − |
| 237 | Un | Un | B. mallei | − |
| 242 | Un | Un | B. mallei | − |
| Dubai7 | Horse | UAE | B. mallei | − |
- N, North; S, South; NE, North-East; UK, United Kingdom; USA, United States of America; UAE, United Arab Emirates; B., Burkholderia; Un, strains with unknown history (obtained from the former reference laboratory for glanders, BgVV, Berlin, Germany); ND, unknown/not determined.
- aThe source of the isolate is given when available.
- bIsolated from patient after stay abroad.
DNA preparation from pure cultures
One distinct colony of each strain was transferred from an agar plate to 200 μl lysis buffer [5x buffer D (PCR Optimation Kit; Invitrogen, DeShelp, The Netherlands, 1:5 diluted in Aqua dest.); 0.5% Tween 20TM (ICI, American Limited, Merck, Germany); 2 mg/ml proteinase K (Roche Diagnostics, Germany)]. After incubation at 56°C for 1 h and inactivation for 10 min at 95°C, 2 μl of the cleared lysate were used as template in the PCR assays.
Clinical samples
Clinical samples were obtained from a dromedary with generalized melioidosis in the United Arab Emirates in 1996 (Wernery et al., 1997). Briefly, samples were taken from a 7-year-old male camel with generalized melioidosis. Postmortem analysis revealed generalized miliar abscesses in most organs. B. pseudomallei was cultivated from various organs in pure culture and was identified by biochemical and motility testing. Tissue samples of the lungs were directly placed into formalin (10% v/v) for simultaneous inactivation and fixation, and subsequently embedded into paraffin following standard protocols. Sections (5 μm) were cut from paraffin blocks to give 20 mg of tissue and de-paraffinized by xylene-extraction. The samples were incubated for 10 min in xylene at room temperature (1200 μl, 2x) and 100% ethanol (1200 μl, 3x) while gently agitating the tube. After subsequent centrifugation at 13 000 g for 10 min, the supernatant was removed and the samples were then air-dried. For digestion of the tissues, 200 μl of lysis buffer was added. The samples were then incubated for 1 h at 56°C in a rotatory shaker. A clear lysate was obtained and the reaction was stopped by boiling the sample for 10 min. DNA was further purified using QiaAmp Tissue Kit (Qiagen, Hilden, Germany) as recommended by the manufacturer. The eluates were air-dried and resuspended in 5 μl sterile water, and 2 μl were used for PCR.
Primer design and PCR
The entire genomic sequences of B. pseudomallei K96243 (Acc. nos NC_006350 and NC_006351) and B. mallei ATCC 23344T (Acc. nos NC_006348 and NC_006349) were determined by the Sanger Institute and The Institute of Genomic Research (TIGR) respectively. Primers were designed based on differences among B. pseudomallei, B. mallei and B. thailandensis at locus AF254803 on chromosome 2 of B. pseudomallei K96243 encoding a metalloprotease (Lee and Liu, 2000). The accession numbers used for B. mallei were Mallei (ATCC 23344) IS407A: CP000011 REGION: 81594..81851 on chromosome 2 and Mallei (ATCC 23344) EPS-partial, CP000011 REGION: 81995..84358 on chromosome 2. The primer pair Bpm-f: (5′-ACTGCTTCGTTCAAGGCGACCGTC-3′) and Bpm-r: (5′-TGACGGCCTGAACGTCCGCGC-3′) was used to amplify a 199-bp (position 2.696.900 to 2.696.768 of NC_006351) fragment. For specificity testing, a series of strains of closely related genera, including possible sample contaminants, bacteria provoking a similar clinical picture, and potential B-agents were used (Table 1). PCR reaction was performed in 50 μl ready-to-go mastermix (Eppendorf GmbH, Hamburg, Germany) using 15 pmol of each primer. Amplification was carried out in a GeneAmp2400 thermal cycler (Perkin-Elmer; Applied Biosystems, Foster City, CA, USA). A total of 35 cycles were completed, each consisting of 30 s denaturation at 94°C, 30 s annealing at 68°C and elongation at 72°C for 1 min. A final elongation step of 7 min at 72°C completed the run. Of each PCR reaction, 7 μl was analysed by agarose gel electrophoresis (1% w/v in TAE buffer) for the presence of a 199-bp PCR product. Sequencing of the amplicons was carried out on ABI 377 PRISMTM Dye Sequencing Apparatus using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction KitTM (Perkin Elmer Applied Biosystems, Weiterstadt, Germany) according to the manufacturer's instructions. For alignment studies, the program CLUSTAL W (available from http://align.genome.jp) was used. The detection limit of the assays was analysed using serial dilutions of purified DNA of B. pseudomallei quantified with a GeneQuantTM device (Pharmacia, Freiburg, Germany).
Results
The comparison of the available sequences of the metalloprotease gene (mprA) of B. pseudomallei K96243 (Lee and Liu, 2000) and B. mallei ATCC 23344T revealed that this open reading frame was destroyed by an insertion event involving parts of the EPS regulon and an IS407A element in the B. mallei genome. Sequence identity of mprA was only 92% when compared with the corresponding sequence in B. thailandensis. This genetic difference in B. pseudomallei, B. mallei and B. thailandensis was used to design specific primers for the detection of B. pseudomallei by PCR. In subsequent PCR analyses, template DNA of all B. pseudomallei strains investigated were amplified, whereas other bacteria including B. mallei and B. thailandensis tested negative (Tables 1 and 2).
| Strain | Source | Geographical origina | Species | B. pm PCR |
|---|---|---|---|---|
| Closely related bacteria | ||||
| ATCC 700388T | Soil | Thailand | B. thailandensis | – |
| UE11 | Soil | NE Thailand | B. thailandensis | − |
| UE17 | Soil | NE Thailand | B. thailandensis | − |
| UE29 | Soil | NE Thailand | B. thailandensis | − |
| E111 | Soil | S Thailand | B. thailandensis | − |
| E217 | Soil | S Thailand | B. thailandensis | − |
| S2 | Soil | Viet Nam | B. thailandensis | − |
| S3 | Soil | Viet Nam | B. thailandensis | − |
| L1 | Soil | Laos | B. thailandensis | − |
| L13 | Soil | Laos | B. thailandensis | − |
| IMB P399 | Human | Germany | B. cepacia | − |
| IMB P407 | Human | Germany | B. cepacia | − |
| IMB P414 | Human | Germany | B. cepacia | − |
| DSM 11319T | Soil | Viet Nam | B. vietnamiensis | − |
| LMG 16225 | Fungus | France | B. fungorum | − |
| Possible sample contaminations | ||||
| LMG 1267T | Freshwater | Malaya | C. violaceum | − |
| DSM 7216 | Water | Germany | O. anthropi | − |
| ATCC 10145T | ND | ND | Ps. aeruginosa | − |
| IMB P739 | Plant | Egypt | R. solanacearum | − |
| IMB P26 | Human | Germany | X. maltophilia | − |
| LMG 559 | Brassica napus | UK | X. campestris | − |
| Differential diagnosis: clinical presentation | ||||
| NCTC 9682T | Horse | ND | Str. equi subsp. equi | − |
| NCTC 4676T | Cattle | England | Str. zooepidemicus | − |
| ISK 1178 | Human | Austria | Str. viridans | − |
| DSM 20565T | ND | ND | Str. pyogenes | − |
| DSM 12643T | Human | ND | Str. mitis | − |
| ATCC 27294T | Human | ND | M. tuberculosis | − |
| DSM 5281 | ND | ND | P. multocida | − |
| ATCC 12600T | Human | ND | Sta. aureus | − |
| Differential diagnosis: potential B agents | ||||
| BH | Environment | Austria | Ba. anthracis | − |
| 3520 | Cattle | Austria | Ba. anthracis | − |
| 4675 | Cattle | Austria | Ba. anthracis | − |
| IMB 003-897b | Human | Germany | Br. melitensis | − |
| IMB 003-898b | Human | Germany | Br. melitensis | − |
| IMB 003-899b | Human | Germany | Br. melitensis | − |
| FSC 041 | Tick | Canada | F. tularensis subsp. tularensis | − |
| FSC 043 | Human | USA | F. tularensis subsp. tularensis | − |
| F 58b | Human | UK | F. novicida-like | − |
| EV76 | Human | Madagascar | Y. pestis var. orientalis | − |
| Exu 21 | Human | Brazil | Y. pestis var. orientalis | − |
| PKH4 | Human | Kurdistan | Y. pestis var. medievalis | − |
- N, North; S, South; NE, North-East; B., Burkholderia; Ba., Bacillus; Br., Brucella; C., Chromobacterium; F., Francisella; O., Ochrobactrum; P., Pasteurella; Ps., Pseudomonas; R., Ralstonia; Sta., Staphylococcus; Str., Streptococcus; X., Xanthomonas; Y., Yersinia.
- ND, unknown/not determined.
- aThe source of the isolate is given when available.
- bIsolated from patient after stay abroad.
The detection limit of the assay was 10 fg for purified B. pseudomallei DNA or two genome equivalents respectively (Fig. 1). To demonstrate the applicability of the test for clinical application lung samples of a culture-proven B. pseudomallei-infected camel (Wernery et al., 1997) were tested. In the PCR analysis, the B. pseudomallei specific DNA fragment was amplified from each tissue (Fig. 1) and the integrity of the amplicons was proven by subsequent DNA sequencing (data not shown).
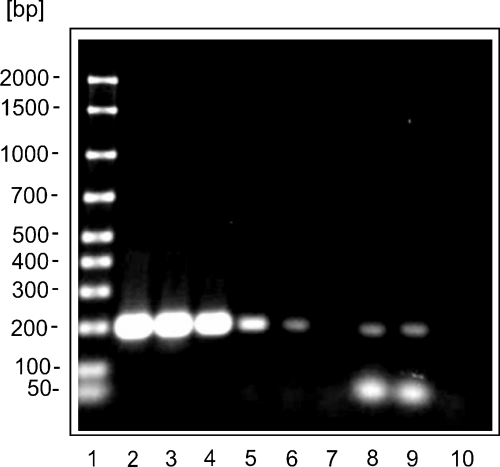
Electropherogram of amplicons (199 bp) of the Burkholderia pseudomallei metalloprotease gene (mprA) PCR assay (1% agarose gel, 8 V/cm, stained with ethidiumbromide). Lane 1: AmpliSize DNA Size Standard (50–2000 bp Ladder; BioRad, Munich, Germany); lanes 2 to 7: 100 pg, 10 pg, 1 pg, 100 fg, 10 fg, 1 fg template DNA B. pseudomallei Heckeshorn; lane 8 and 9: templates of lung samples of a camel died from melioidosis and lane 10: no template control.
Discussion and conclusions
Burkholderia pseudomallei, the causative agent of melioidosis in man and animals, is a saprophyte originating from South-east Asia. Because of the importation of clinically and subclinically infected animals and its extraordinary resistance, it has spread to regions on all continents with appropriate environmental conditions (Sprague and Neubauer, 2004). Nowadays, the regions of endemicity of B. pseudomallei (melioidosis) and B. mallei (glanders) overlap in Asia (Krishna et al., 1992; Bazargani et al., 1996; Al-Ani et al., 1998; Muhammad et al., 1998; Arun et al., 1999), America (Mota et al., 2000), and probably Eastern Europe. The clinical presentation of melioidosis in horses can easily be confused with glanders. Serological and molecular diagnostic tools are not discriminatory (Zysk et al., 2000). The reliable diagnosis is also hampered by the low sensitivity of bacterial cultivation and animal inoculation in clinical cases of both agents. In contrast to equine melioidosis, B. mallei infections in solipeds are notifiable to the Organisation Mondiale de la Santé Animal (OIE) and can cause severe restrictions in trading. The specific identification of B. pseudomallei and the exclusion of a B. mallei infection, especially in regions where both agents are endemic, are extremely important for the well timed onset of counter measures. The aim of this study was, therefore, to develop a robust but specific and sensitive PCR assay with a low detection limit to detect and to identify B. pseudomallei in pure cultures and clinical samples.
The mprA PCR assay had a sensitivity and specificity of 100%. We therefore assume that the metalloprotease gene is a stable genetic marker as it was detected in each of the B. pseudomallei strains examined and absent in all B. mallei strains we had at hand. The strains tested originated from environmental samples, i.e. soil and from warm-blooded animals including monkeys and humans. Neither the geographical origin (Europe, Asia, Africa, America or Australia) nor the time of isolation influenced the specificity of the assay as the strains were collected over a time period of more than 60 years. Moreover, the recombination which disrupted the metalloprotease gene in B. mallei was unlikely to revert spontaneously as it was present in all B. mallei strains investigated, including the oldest strain of our collection dating back to 1920, London (NCTC 00120) and a recent isolate from an outbreak in the United Arab Emirates in 2004.
Additionally, by using a point of recombination it is unlikely that mprA will be re-arranged in a B. mallei clone in such a way that an amplicon as in B. pseudomallei is produced. We believe that a specific conventional PCR assay targeting a single gene is superior to costly real-time PCR technology and complex multiplex PCR systems as PCR technology is also regularly available in many developing countries, i.e. the endemic region of melioidosis.
Despite the well known low bacterial load in infected tissues, the amplification was successful, demonstrating that the problems of cultivation and animal inoculation could be overcome by our PCR assay. The developed PCR assay can therefore be used to confirm a presumptive clinical diagnosis. That B. pseudomallei can even cause disease in unexpected and unusual geographical regions is furthermore demonstrated by the case described in this report. The climate in the UAE is extremely dry and sunny with a high UV light fraction and as such extremely adverse for B. pseudomallei. A case of melioidosis in this location is thus highly unlikely. In contrast to melioidosis, glanders in camelids has caused severe outbreaks in the past, as camelids are highly susceptible to B. mallei and are easily infected through the contact with glanderous horses (Sprague and Neubauer, 2004). However, the phenotypic diagnosis of B. pseudomallei was retrospectively confirmed by means of the developed PCR. By investigative epidemiology the probable source of infection was ascertained. The dromedary had been infected by contaminated alfalfa cultivated in Dubai on wet cow dung which had previously been imported from Pakistan. Alfalfa is an important diet of valuable racing camels. Additionally, it was also a very wet season with more rain.
In summary, the developed PCR assay provides a simple tool for the detection of B. pseudomallei in samples with low bacterial load as found in fresh clinical samples and formalin-fixed tissues. We therefore recommend the use of this assay for the diagnosis of melioidosis in natural outbreaks, especially in B. mallei-endemic regions.
Acknowledgement
We are grateful to C. Lodri for excellent technical assistance.




