Observed Prevalence of Tick-borne Pathogens in Domestic Animals in Sicily, Italy during 2003–2005
Summary
The objective of this study was to characterize the observed prevalence of tick-borne pathogens (TBP) in domestic animals in Sicily, Italy during 2003–2005. Serological (competitive ELISA and indirect immunofluorescence antibody, n = 3299) and DNA tests (polymerase chain reaction and reverse line blot, n = 2565) were conducted on horse, donkey, cattle, sheep, goat, pig and dog samples. Pathogens analysed included Anaplasma, Ehrlichia, Rickettsia, Babesia and Theileria species, and Coxiella burnetii. The most prevalent TBP were Anaplasma and Babesia species. The results reported herein suggested that cattle could serve as the major reservoir for Babesia and Theileria spp. while for Anaplasma spp. cattle, dogs, sheep and goats may be the most important reservoir species. These results expanded our knowledge about the prevalence of TBP in Sicily and provided information to understand the epidemiology of tick-borne diseases and may help to implement measures to diagnose, treat and control transmission to humans and animals in this region.
Introduction
Tick-borne diseases (TBD) impact human health and animal production and welfare. The development and implementation of control measures for TBD is dependent upon understanding the epidemiology of tick-borne pathogens (TBP) in a particular geographical region (Dehaumont, 2004).
Tick-borne pathogens such as Coxiella burnetti (Caracappa et al., 1986), Theileria spp. (Loria et al., 1999; Sparagano et al., 2000), Rickettsia spp. (Caracappa, 1989), Babesia spp. (Sparagano et al., 2000) and Anaplasma spp. (Caracappa, 1999) have been described in Sicily prior to 2000. However, after the creation of the Centro Nazionale di Referenza per Anaplasma, Babesia, Rickettsia e Theileria (C.R.A.Ba.R.T.) at the Instituto Zooprofilattico Sperimentale della Sicilia in 2003, the prevalence of TBP has been systematically evaluated.
Recent reports from our group have characterized Anaplasma spp. infections in Sicily (de la Fuente et al., 2005a,b,c). However, the updated analysis of the prevalence of other TBP in Sicily has not been reported. The current study was designed to summarize the results obtained by the C.R.A.Ba.R.T. for the characterization of the prevalence of TBP in domestic animals in Sicily.
Materials and Methods
Sample collection
Samples included in the study were supplied to C.R.A.Ba.R.T. for diagnosis or originated from random sampling conducted by the C.R.A.Ba.R.T. to monitor the prevalence of TBP in domestic animals in different provinces of Sicily (mainly in the province of Palermo).
Domestic animals sampled in the study were horses, donkeys, cattle, sheep, goats, pigs and dogs. Blood was collected in sterile tubes with and without anticoagulant (EDTA) and maintained at 4°C until arrival at the laboratory. Plasma and serum were then separated after centrifugation and stored at −20°C. Pathogens analysed were Anaplasma spp., A. marginale, A. phagocytophilum, Ehrlichia spp., E. canis, E. equi, Rickettsia rickettsii, R. conorii, Babesia spp., B. bigemina, B. bovis, B. caballi, B. canis, B. (Theileria) equi, B. microti, Babesia/Theileria spp., T. annulata and Coxiella burnetii.
Serological tests
Serum antibodies were determined for Anaplasma spp., A. phagocytophilum, E. equi, R. rickettsii, B. bigemina, B. bovis, B. caballi, B. canis, B. microti, B. (Theileria) equi and C. burnetii. The anaplasmosis competitive ELISA (cELISA; VMRD Inc., Pullman, WA, USA) was used to detect antibodies against Anaplasma spp. following the manufacturer's instructions. This assay specifically detects the presence of serum antibodies that target the MSP5 protein of Anaplasma spp. (Knowles et al., 1996). Percent inhibition values >30% were considered positive (de la Fuente et al., 2003, 2005c). Indirect immunofluorescence antibody (IFA) test kits for A. phagocytophilum, R. rickettsii, B. caballi, B. microti, B. (T.) equi and C. burnetii (Fuller Laboratories, Fullerton, CA, USA) were used following manufacturer's recommendations. The IFA tests for A. phagocytophilum, R. rickettsii, B. caballi, B. microti and B. (T.) equi use antigens derived from HL-60, Vero and erythrocyte cells infected with the HGE-1 isolate of A. phagocytophilum, the R strain of R. rickettsii and the corresponding Babesia spp., respectively, and samples were considered negative when no fluorescence was detected at 1 : 80, 1 : 50, 1 : 80, 1 : 64 and 1 : 80 dilution of the test serum, as recommended by the manufacturer. The test for C. burnetii use phase I and II antigens with a cut-off dilution of the test serum of 1 : 16. For B. bigemina and B. bovis, IFA tests from VMRD Inc. were used based on antigens derived from infected erythrocytes with cut-off dilution of 1 : 80 and 1 : 40 of the test serum, respectively, as recommended by the manufacturer. Antibodies to B. canis were detected by immunofluorescence using B. canis antigens kindly provided by Dr R. Böse (Institute of Parasitology, School of Veterinary Medicine, Hannover, Germany) with a cut-off dilution of 1 : 40 of the test serum. Fluorescein isothiocyanate-conjugated anti-host species immunoglobulin (Ig)G and/or IgM (Sigma, St Louis, MO, USA or Fuller Laboratories) were used as secondary antibodies.
DNA tests
DNA was extracted from blood samples using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma). The DNA was resuspended in sterile distilled water and stored at −20°C until used.
PCR analysis for Anaplasma spp. (de la Fuente et al., 2005c,d,e), A. marginale (de la Fuente et al., 2005c,e), A. phagocytophilum (Bown et al., 2003), Ehrlichia spp. (Munderloh et al., 1999), E. canis (Wen et al., 1997), R. rickettsii and R. conorii (Tzianabos et al., 1989), Babesia spp. and Babesia/Theileria spp. (Gubbels et al., 1999; Georges et al., 2001; Nagore et al., 2004), B. bigemina and B. bovis (Figueroa et al., 1993), B. caballi and B. (T.) equi (Battsetseg et al., 2002), B. canis (Carret et al., 1999; Caccio et al., 2002), T. annulata (D'Oliveira et al., 1995) and C. burnetii (To et al., 1996) were conducted as previously described. PCRs were performed with 1 μl (0.1–10 ng) DNA using 10 pmol of each primer and the Ready-To-Go PCR beads (Amersham, Piscataway, NJ, USA). Reactions were performed in an automated DNA thermal cycler for 35 cycles. PCR products were electrophoresed on 1% agarose gels to check the size of amplified fragments by comparison with a DNA molecular weight marker (1 Kb DNA Ladder; Promega Madison, WI, USA). Control reactions were done without the addition of DNA to the reaction to rule out contaminations during PCR.
Reverse line blot (RLB) was used for detection of Babesia/Theileria spp. as previously described (Gubbels et al., 1999; Georges et al., 2001; Nagore et al., 2004; Schnittger et al., 2004).
Statistical analysis
Within-host and between-host comparisons of TBP prevalence obtained with similar diagnostic techniques (serological or DNA tests respectively) were performed by chi-squared tests (spss 13.0 statistical program; SPSS Inc., Chicago, IL, USA). The sampling for B. (T.) equi and B. caballi in horses across the year allowed the statistical estimation of the probability for positive serological, PCR or RLB test results by means of logistic regression. For this purpose, diagnostic entries (n = 65) were grouped in bi-monthly periods as ‘explanatory categorical variable’. The differences were considered statistically significant when P ≤ 0.05.
Results
Horses
Serological (n = 551) and DNA (PCR or RLB, n = 358) tests were done for TBP on horse samples (Table 1). For both serological and DNA tests, the observed prevalence of A. phagocytophilum was lower (χ2 = 90.15, P = 0.00 and χ2 = 89.63, P = 0.00 respectively) than of B. caballi and B. (T.) equi. The latter did not differ in prevalence (χ2 = 0.01, P = 0.91). A tendency to seasonality was observed for prevalence of A. phagocytophilum, B. caballi and B. (T.) equi (Figs 1a and b). However, statistically higher (P < 0.05) seasonal prevalence was detected during spring for B. caballi only (Fig. 1b).
| Pathogen | Method | Positive | Negative | n | Prevalence (%) | SE |
|---|---|---|---|---|---|---|
| Horses | ||||||
| A. phagocytophilum | IFA | 12 | 142 | 154 | 7.79 | 2.16 |
| A. phagocytophilum | PCR | 0 | 111 | 111 | 0.00 | 0.00 |
| B. caballi | IFA | 109 | 83 | 192 | 56.77 | 3.58 |
| B. caballi | PCR | 29 | 91 | 120 | 24.17 | 3.91 |
| B. (T.) equi | IFA | 113 | 88 | 201 | 56.22 | 3.50 |
| B. (T.) equi | PCR | 40 | 87 | 127 | 31.50 | 4.12 |
| E. equi | IFA | 3 | 1 | 4 | 75.00 | 21.65 |
| Donkeys | ||||||
| A. phagocytophilum | IFA | 14 | 60 | 74 | 18.92 | 4.55 |
| A. phagocytophilum | PCR | 0 | 73 | 73 | 0.00 | 0.00 |
| B. caballi | IFA | 57 | 24 | 81 | 70.37 | 5.07 |
| B. caballi | PCR | 0 | 43 | 43 | 0.00 | 0.00 |
| B. (T.) equi | IFA | 30 | 51 | 81 | 37.04 | 5.37 |
| B. (T.) equi | PCR | 1 | 42 | 43 | 2.33 | 2.30 |
| Babesia/Theileria spp. | RLB | 9 | 31 | 40 | 22.50 | 6.60 |
| T. annulata | PCR | 0 | 33 | 33 | 0.00 | 0.00 |
| Cattle | ||||||
| Anaplasma spp. | cELISA | 627 | 470 | 1097 | 57.16 | 1.49 |
| A. marginale | PCR | 14 | 128 | 142 | 9.86 | 2.50 |
| A. phagocytophilum | PCR | 13 | 65 | 78 | 16.67 | 4.22 |
| A. phagocytophilum | IFA | 27 | 141 | 168 | 16.07 | 2.83 |
| B. bigemina | PCR | 1 | 12 | 13 | 7.69 | 7.39 |
| B. bigemina | IFA | 58 | 153 | 211 | 27.49 | 3.07 |
| B. bovis | PCR | 2 | 57 | 59 | 3.39 | 2.36 |
| B. bovis | IFA | 72 | 239 | 311 | 23.15 | 2.39 |
| Babesia/Theileria spp. | RLB | 138 | 216 | 354 | 38.98 | 2.59 |
| Babesia/Theileria spp. | PCR | 4 | 18 | 22 | 18.18 | 8.22 |
| C. burnetii | PCR | 0 | 9 | 9 | 0.00 | 0.00 |
| T. annulata | PCR | 12 | 82 | 94 | 12.77 | 3.44 |
| Sheep | ||||||
| Anaplasma spp. | cELISA | 151 | 66 | 217 | 69.59 | 3.12 |
| Anaplasma spp. | PCR | 14 | 48 | 62 | 22.58 | 5.31 |
| A. marginale | PCR | 3 | 88 | 91 | 3.30 | 1.87 |
| A. phagocytophilum | PCR | 3 | 87 | 90 | 3.33 | 1.89 |
| A. phagocytophilum | IFA | 17 | 136 | 153 | 11.11 | 2.54 |
| Babesia spp. | PCR | 2 | 14 | 16 | 12.50 | 8.27 |
| Babesia/Theileria spp. | RLB | 57 | 141 | 198 | 28.79 | 3.22 |
| Babesia/Theileria spp. | PCR | 4 | 37 | 41 | 9.76 | 4.63 |
| C. burnetii | PCR | 1 | 17 | 18 | 5.56 | 5.40 |
| C. burnetii | IFA | 0 | 37 | 37 | 0.00 | 0.00 |
| T. annulata | PCR | 0 | 21 | 21 | 0.00 | 0.00 |
| Goats | ||||||
| Anaplasma spp. | cELISA | 10 | 12 | 22 | 45.45 | 10.62 |
| A. marginale | PCR | 3 | 13 | 16 | 18.75 | 9.76 |
| A. phagocytophilum | PCR | 0 | 48 | 48 | 0.00 | 0.00 |
| A. phagocytophilum | IFA | 0 | 13 | 13 | 0.00 | 0.00 |
| Babesia spp. | PCR | 1 | 12 | 13 | 7.69 | 7.39 |
| Babesia/Theileria spp. | RLB | 3 | 24 | 27 | 11.11 | 6.05 |
| Babesia/Theileria spp. | PCR | 18 | 5 | 23 | 78.26 | 8.60 |
| C. burnetii | PCR | 0 | 7 | 7 | 0.00 | 0.00 |
| T. annulata | PCR | 0 | 49 | 49 | 0.00 | 0.00 |
| Pigs | ||||||
| Anaplasma spp. | PCR | 1 | 27 | 28 | 3.57 | 3.51 |
| Ehrlichia spp. | PCR | 0 | 97 | 97 | 0.00 | 0.00 |
| B. bigemina | PCR | 0 | 18 | 18 | 0.00 | 0.00 |
| Babesia spp. | PCR | 0 | 23 | 23 | 0.00 | 0.00 |
| Babesia/Theileria spp. | RLB | 0 | 8 | 8 | 0.00 | 0.00 |
| Babesia/Theileria spp. | PCR | 0 | 194 | 194 | 0.00 | 0.00 |
| T. annulata | PCR | 0 | 20 | 20 | 0.00 | 0.00 |
| Dogs | ||||||
| A. phagocytophilum | IFA | 39 | 48 | 87 | 44.83 | 5.33 |
| A. phagocytophilum | PCR | 0 | 2 | 2 | 0.00 | 0.00 |
| Anaplasma spp. | PCR | 12 | 18 | 30 | 40.00 | 8.94 |
| B. canis | IFA | 16 | 112 | 128 | 12.50 | 2.92 |
| B. canis | PCR | 5 | 2 | 7 | 71.43 | 17.07 |
| B. microti | IFA | 0 | 8 | 8 | 0.00 | 0.00 |
| B. canis | IFA | 1 | 2 | 3 | 33.33 | 27.22 |
| Babesia/Theileria spp. | PCR | 0 | 8 | 8 | 0.00 | 0.00 |
| C. burnetii | IFA | 2 | 23 | 25 | 8.00 | 5.43 |
| E. canis | PCR | 0 | 51 | 51 | 0.00 | 0.00 |
| Ehrlichia spp. | PCR | 1 | 4 | 5 | 20.00 | 17.89 |
| R. conorii | PCR | 7 | 44 | 51 | 13.73 | 4.82 |
| R. ricketsii | IFA | 1 | 31 | 32 | 3.13 | 3.08 |
| R. ricketsii | PCR | 0 | 4 | 4 | 0.00 | 0.00 |
- IFA, indirect immunofluorescence antibody; PCR, polymerase chain reaction; RLB, reverse line blot; cELISA, competitive ELISA.
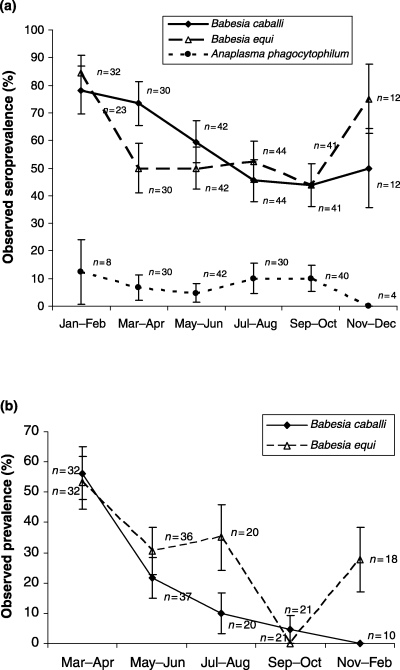
Seasonal prevalence of Anaplasma phagocytophilum, Babesia caballi and B. (T.) equi in horses in Sicily. (a) Seroprevalence determined by indirect immunofluorescence antibody. (b) Prevalence determined by PCR. Values represent mean ± SE.
Donkeys
A similar number of serological (n = 236) and DNA (n = 232) tests were conducted on donkey samples (Table 1). Similar to results obtained for horses, the seroprevalence was higher for B. caballi (χ2 = 41.24, P = 0.00) and B. equi (χ2 = 6.24, P = 0.01) than for A. phagocytophilum. However, in donkeys the seroprevalence was higher for B. caballi than for B. (T.) equi (χ2 = 18.10, P = 0.00).
Cattle
Cattle are the most abundant domestic species in Sicily. Therefore, the number of serological (n = 1787) and DNA (n = 771) tests was comparatively high (Table 1). Anaplasma spp. presented higher (P < 0.05) seropre-valence than other TBP analysed. However, Babesia/Theileria spp. had the highest prevalence using DNA tests (χ2 = 35.46, P = 0.00). By PCR, the prevalence of A. phagocytophilum and T. annulata was higher than that of B. bovis (χ2 = 6.07, P = 0.01 and χ2 = 3.83, P = 0.05 respectively).
Sheep
A total number of 407 and 537 serological and DNA tests, respectively, were conducted with sheep samples (Table 1). By PCR, Anaplasma spp. displayed higher prevalence than A. marginale (χ2 = 13.88, P = 0.00) and A. phagocytophilum (χ2 = 13.69, P = 0.00). The seroprevalence of Anaplasma spp. was also higher than that of A. phagocytophilum (χ2 = 68.93, P = 0.00).
Goats
In 183 DNA tests (Table 1), the prevalence of Babesia/Theileria spp. was higher than that of Anaplasma spp. (χ2 = 12.81, P = 0.00). The prevalence of A. marginale was higher than that of A. phagocytophilum (χ2 = 9.44, P = 0.00). In the 35 serological tests conducted (Table 1), the seroprevalence of Anaplasma spp. was higher than that of A. phagocytophilum (χ2 = 7.34, P = 0.00).
Pigs
Only DNA tests (n = 388) were done on pig samples (Table 1). Of them, a single positive result was obtained for Anaplasma spp. without significant differences for prevalence when compared with other TBP analysed.
Dogs
Serological (n = 283) and DNA (n = 158) tests were done for TBP on dog samples (Table 1). By PCR, the prevalence of R. conorii was higher than that of E. canis (χ2 = 6.51, P = 0.01). Serologically, the prevalence of A. phagocytophilum was higher than that of R. ricketsii (χ2 = 18.23, P = 0.00), B. canis (χ2 = 28.43, P = 0.01) and C. burnetti (χ2 = 10.16, P = 0.00) respectively.
Pathogens
The analysis of pathogen species was done for those that were analysed in most domestic animals (Fig. 2). The most notable results were that (a) the seroprevalence of Anaplasma spp. was higher in sheep, followed by cattle and goats and it was zero in donkeys, (2) the highest seroprevalence of A. phagocytophilum was found in dogs and (3) the highest prevalence of A. phagocytophilum, Babesia/Theileria spp. and T. annulata was found in cattle.
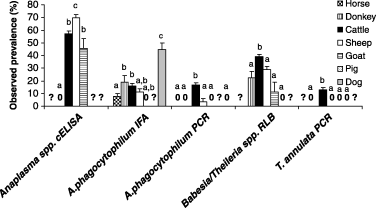
Prevalence for tick-borne pathogens that were detected in at least four domestic animal species. Different letters for the same analysis denote statistically significant differences (P < 0.05). Symbols: ‘?’, not analysed; ‘0’, zero observed prevalence.
Discussion
Tick-borne pathogens are endemic in Sicily as well as in other regions of Italy and Europe (Cringoli et al., 2002; Tassi et al., 2002; Parola, 2004; Sambri et al., 2004; Bernabeu-Wittel and Segura-Porta, 2005; Stanek, 2005). The observed prevalence was generally lower when estimated by DNA tests than with serological tests. Similar differences have been found in other studies and may reflect the absence of detectable levels of bacteremia in some animals (de la Fuente et al., 2005b).
The results of the observed prevalence reported herein suggest that cattle could serve as the major reservoir for Babesia and Theileria spp. while for Anaplasma spp., cattle, dogs, sheep and goats may be the most important reservoir species. We have previously shown that in Sicily infections with Anaplasma spp. occur in cattle and sheep (de la Fuente et al., 2005a,b, 2005c). The Anaplasma cELISA used in this study detects antibodies to A. marginale, A. phagocytophilum and A. ovis (Dreher et al., 2005). The PCR products were not sequenced in this study but it is known that the A. marginale PCR also detects the presence of A. ovis DNA (de la Fuente et al., 2005b). Therefore, it is likely that cattle were the major reservoir for A. marginale, dogs for A. phagocytophilum and sheep and goats for A. ovis. This possibility agrees with the difference between observed seroprevalence of Anaplasma spp. and A. phagocytophilum in cattle, sheep and goats and the host range reported for A. marginale, A. phagocytophilum and A. ovis (Kocan et al., 2004).
The most prevalent TBP in our study were Anaplasma and Babesia species. Anaplasma phagocytophilum can infect a wide range of vertebrate species including domesticated and wild animals and humans with an impact on animal and human health (Hofmann-Lehmann et al., 2004; Dumler et al., 2005; de la Fuente et al., 2005a,d, 2005e; Naranjo et al., 2006). Evidence of infection with A. phagocytophilum were found in all domestic animals studied, except for goats and pigs. However, the PCR sample positive for Anaplasma spp. in one pig could correspond to A. phagocytophilum. Infections with A. phagocytophilum have been reported in wild boar in Europe (Hulinska et al., 2004). Anaplasma marginale, which is endemic in Sicily where it causes economic loss to the cattle industry (Caracappa, 1999), had a high observed seroprevalence in cattle confirming previous reports in Sicily (de la Fuente et al., 2005b,c). Babesia spp. could also alter animal health and affect cattle production (Bock et al., 2004). As reviewed for other regions of the world (Kocan et al., 2003; Bock et al., 2004; Dumler et al., 2005; Stanek, 2005), our results suggest an increased risk for animal and human health for the most prevalent TBP identified in Sicily, Anaplasma and Babesia species.
The tick vectors for the TBP analysed herein are present in Sicily and have been found infected with these pathogens (Loria et al., 1999; Sparagano et al., 2000; Georges et al., 2001; de la Fuente et al., 2005b; Torina et al., 2006). The higher prevalence of B. caballi during spring in horses agrees with the seasonal abundance of the tick vectors Rhipicephalus spp. and Hyalomma m. marginatum (Torina et al., 2006).
The results reported herein expand our knowledge about the prevalence of TBP in Sicily. The knowledge of prevalence of TBP in domestic animals is important to understand the epidemiology of TBD and may help to implement measures to diagnose, treat and control transmission to humans and animals (Dehaumont, 2004; Bratton and Corey, 2005).
Acknowledgements
This research was funded by The Ministry of Health, Italy. Dr R. Böse (Institute of Parasitology, School of Veterinary Medicine, Hannover, Germany) is acknowledged for providing B. canis antigens for IFA test.




