Organ culture, but not hypothermic storage, facilitates the repair of the corneal endothelium following mechanical damage
Abstract.
Purpose: To evaluate the reparative capacity of the mechanically injured endothelium of corneas stored under organ culture (OC) or hypothermic conditions.
Methods: The central endothelium of 12 pairs of human corneas with similar endothelial parameters was damaged to create a 1 mm2 lesion. One cornea from each pair was stored under OC and one under hypothermic conditions. The endothelial cell density (ECD), coefficient of variation, hexagonality and percentage of dead cells were assessed before and after damage and on days 7, 14, 21 and 28 of storage.
Results: The mean ECD of corneas subsequently stored under OC or hypothermic conditions was 2764/mm2. Immediately after damage, a denuded Descemet’s membrane with a few remaining dead cells was observed at the injured area. After 7 days of storage under OC conditions, almost no dead cells were observed at the place of injury. A non-significant worsening of the qualitative parameters (polymegatism and pleomorphism) was found. After 14 days, ECD was 1933/mm2 and 2478/mm2 centrally and pericentrally, respectively. Similar values were found after 21 and 28 days of storage. The lesions with remnant dead cells persisted throughout hypothermic preservation. From day 14 the corneas became cloudy and in poor condition, while the pericentral ECD was 2523/mm2.
Conclusion: The reparative capacity of the cornea is maintained under OC but not under hypothermic conditions. For corneas containing dead endothelial cells, OC is therefore the method of choice because it may improve the quality of the stored tissue.
Introduction
Two approaches to cornea storage for subsequent grafting are in common use: hypothermic and organ culture (OC) storage (Maas-Reijs et al. 1997; Jeng 2006).
Under hypothermic storage, the cornea is generally maintained in a commercially available medium at 4–8°C. All of these media contain, in addition to nutrients, osmotically active substances to maintain the normal physiological thickness and clarity of the tissue (Pels et al. 1999). The cornea is maintained in a closed system from preparation to grafting, including during corneal assessment using slit-lamp and specular microscopy (Chu 2000). The aim of this approach is to minimize or even inhibit cell metabolism in order to preserve the original condition of the cornea for as long as possible. Although the maximum recommended storage time is theoretically 14 days, the actual average storage time is 4 days (Wilhelmus et al. 1995; EEBA 2007).
Corneal storage under OC conditions is more technically difficult and time-consuming. After microscopic assessment the cornea is maintained at 31–37°C in media based on minimal essential medium with the addition of foetal calf serum (Pels et al. 1999; Jeng 2006). The main aim of OC is to support cellular (especially endothelial) metabolism and viability as closely as possible to that observed under physiological conditions. OC corneas can be stored for up to 35 days, which enables the creation of better transplantation schedules, matching between donor cornea and recipient, and microbiological control (Armitage & Easty 1997). A few days before transplantation, the cornea is transferred into transport medium, in which an osmotically active substance (routinely 5% Dextran T 500) leads to deswelling of the cornea. Subsequently, a second assessment should be performed to detect potential latent endothelial cell damage and to determine the condition of the cornea shortly before grafting (Pels et al. 1999; Builles et al. 2006).
An intact functional endothelium with a high endothelial cell density (ECD) is critical for long-term tissue survival (Bourne 2001), although it should be noted that successful grafts using corneas organ-cultured for more than 4 weeks have been described (Frueh & Böhnke 1995). It is well known that the proliferative capacity of the corneal endothelium is inhibited soon after birth (Murphy et al. 1984). The ECD then decreases with age at a rate of approximately 0.6% per year (Laule et al. 1978; Murphy et al. 1984; Bourne et al. 1997). Thus, at age 85, the endothelium has a density of 2300 cells/mm2 (Yee et al. 1985). The lowest permissible ECD generally accepted for corneas intended for grafting is 2000–2300 cells/mm2 (Pels et al. 1999; Ehlers 2002).
The aim of this study was to compare the extent and speed of repair of the mechanically damaged endothelium of corneas stored under OC or hypothermic conditions. When dead endothelial cells are desquamed from the endothelial monolayer, cell-free areas are repaired by a shift of the surrounding endothelial cells and by their enlargement (Hoppenreijs et al. 1994). The assessment of dead endothelial cells is one of the crucial aspects of corneal evaluation. The incorrect assessment of dead cells (DC) as viable cells in the corneal endothelium may lead to falsely elevated ECD values. Typically, the number of DC is not assessed in a closed hypothermic system. In an open system in which the cornea is prepared under sterile conditions in a biohazard hood, trypan blue staining of DC is usually performed and the percentage of DC is calculated using a computer analysis system (Thuret et al. 2005). The percentage of DC in corneas intended for grafting should not exceed 5% (EEBA 2007). Corneal ECD decreases during both OC and hypothermic storage (Pels & Schuchard 1983; Komuro et al. 1999). The cell loss may not exceed a maximum of 20% during 30 days of storage because an abnormal cell loss of more than 20% may indicate latent damage to the endothelial cells that was not visible before culturing (Pels & Schuchard 1983).
Because endothelial lesions are present in some donor corneas, wound healing that occurs during storage should facilitate the re-establishment of an endothelial monolayer and thus enable the grafting of corneas with healed lesions.
Materials and Methods
Repair of corneas under OC and hypothermic conditions
The use of human corneas adhered to the tenets of the Declaration of Helsinki. Twelve corneal pairs unsuitable for transplantation (reasons other than insufficient endothelial quantity or quality) were used. The whole bulbi were obtained from donors (mean age 67 ± 12 years, range 47–81 years) less than 24 hrs after death. After decontamination, the bulbi were examined by slit lamp and then corneoscleral rings were obtained by trephination under sterile conditions in a biohazard hood. Only corneas with an ECD of live cells (ECDA) higher than 2000 cells/mm2 were used. Between the paired corneas, differences in ECD were no more than 10%, the percentage of DC was no more than 1.6% and the number of Descemet’s folds was no more than three.
The assessment was performed after the corneal endothelium was treated with 0.12% trypan blue (Sigma-Aldrich, Steinhaim, Germany) in phosphate-buffered saline (PBS) and 0.9% sucrose in water using light microscopy (CKX41; Olympus, Tokyo, Japan) and an Olympus C-3040 camera (Olympus). The centre of the cornea was located microscopically by counting the number of fields per cornea at its widest part and then moving back towards the centre by half of this number of fields. One central and four non-overlapping pericentral-phase contrast photographs (0.3 mm2, magnification of 200 ×) were taken. Similarly, one central and four pericentral bright-field photographs were taken at a magnification of 100 × to include a larger area for assessing the percentage of DC (Fig. 1).

A schema illustrating how the photographs were taken. One central and four non-overlapping pericentral bright-field photographs were taken at a magnification of 100 ×. The blue area indicates the endothelial lesion.
After the initial assessment, during which the cornea was placed endothelial-side-up in a Petri dish, the cornea was transferred to a biohazard hood and centred on a drop of PBS. The visually determined central part of each cornea was damaged mechanically using a special metal rod. The flat end of the round rod (diameter: 0.5 mm) was pressed gently with constant pressure for 15 seconds onto the central part of the endothelial surface. The lesions of all corneas were induced by one experienced person (KJ). Subsequently, the damaged cornea was treated with trypan blue and sucrose, and photographs were taken again. Data regarding the position of the lesion were recorded and used for subsequent assessments.
Subsequently, one cornea of each pair was randomly assigned to hypothermic conditions (Optisol-GSl; Bausch & Lomb Inc., Rochester, New York, USA) and stored at 4°C. The second cornea was cultured at 31°C in E-Minimum Essential Medium (MEM with Earle’s salts), L-Glutamine, 25 mm HEPES (AppliChem, GmbH, Darmstadt, Germany), NaHCO3 (2.24 g/l), an antibiotic–antimycotic solution of penicillin G (100 U/ml), streptomycin sulphate (100 μg/ml) and amphotericin B (Fungizone), and 2% foetal bovine serum (both from Invitrogen/Gibco, Glasgow, UK).
Corneas of both groups were assessed again on days 7, 14 and 21; OC corneas were also assessed on day 28. The presence of cell debris, observed using bright-field microscopy after 7 days’ storage in OC, indicated the location of the previously induced lesions in the central endothelium. Taken together, the data recorded at the time of lesioning regarding the position of the lesion and the alterations in endothelial morphology observed using phase-contrast microscopy enabled the precise localization of the lesion site during subsequent assessments. All corneas were deswelled for approximately 12 hrs at room temperature in OC medium containing 5% dextran (Sigma-Aldrich, Buchs, Switzerland) prior to their assessment.
The photographs were processed by a semi-automated Lucia computer analysis system (Laboratory Imaging, Prague, Czech Republic) to obtain average values of the ECD, ECDA, percentage of DC, the coefficient of variation of the cell area (CV) and the percentage of hexagonal cells (6A). At least 100 cells were assessed separately from one central and four pericentral-phase contrast photographs using the variable frame principle. The centres of neighbouring cells were marked manually in each photograph before the automatic system calculated the required parameters. Solitary DC or areas of DC were marked manually on bright-field photographs, and then the images were processed by the Lucia computer analysis system to calculate the percentage of DC.
Four additional corneas were used to confirm the induction of corneal damage. Immediately after the injury, the endothelium was stained with 0.25% trypan blue in 0.9% saline and consequently with alizarin red S (0.2% in 0.9% saline), pH 4.2 (Sigma, Steinhaim, Germany), to visualize the denuded Descemet’s membrane and any remaining DC (Fig. 2) (Sperling 1977; Taylor & Hunt 1981).
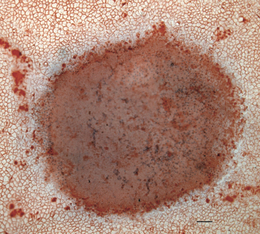
Bright-field micrograph of the corneal endothelium immediately after the induction of the lesion. The damage was complete over the entire injured surface. Some dead cells (blue nuclei) remain on the surface of the lesion. Trypan blue and Alizarin red S staining. Scale bar represents 100 μm.
Speed of repair of corneas stored under OC conditions
Seven additional corneas were damaged mechanically (as described earlier), and the surface of the injury was examined in bright-field photographs (magnification of 100 ×) taken after trypan blue staining. The same procedure was performed 1–6 days after damage. Blue spots, indicating the extent of injury, were measured (automatic calculation of manually demarked areas) using a Lucia computer analysis system.
Statistics
Results are expressed as mean ± standard deviation (SD). Data analysis was carried out using statistica software (StatSoft, Prague, Czech Republic). A Wilcoxon matched-pairs test was used to compare the quantitative variables (between the ECD before damage and the ECD after different time periods post-injury) in both groups (hypothermic and OC). The different time periods required for repair were compared by a paired t-test. Statistical significance was considered to be a p-value of 0.05 or less.
Results
Repair of corneas under OC and hypothermic conditions
Prior to injury, the values of ECD, DC, CV and 6A of corneas subsequently stored under OC or under hypothermic conditions did not differ significantly. The mean ECD in the pericentral area was higher than the ECD in the central area for corneas later stored under OC or hypothermic conditions.
The mean values of ECD (± SD), DC, CV and 6A in the central and pericentral endothelium of fresh corneas and those stored under OC or hypothermic conditions for different time periods are presented in Tables 1 and 2.
| Storage (days) | ECD (mm2) | ECDA (mm2) | DC (%) | CV | 6A |
|---|---|---|---|---|---|
| Organ culture | |||||
| Before lesion | 2765 ± 349 | 2756 ± 346 | 0.3 ± 0.5 | 17.1 ± 3.6 | 57.7 ± 6.8 |
| After lesion | |||||
| 0 | N | N | 100 ± 0** | N | N |
| 7 | 1762 ± 267** | 1755 ± 265** | 0.4 ± 0.5 | 19.6 ± 3.6 | 45.4 ± 7.6** |
| 14 | 1933 ± 354** | 1928 ± 349** | 0.2 ± 0.2 | 20.1 ± 3.0 | 48.0 ± 10.6 |
| 21 | 2162 ± 417** | 2159 ± 417** | 0.1 ± 0.3 | 20.1 ± 3.4 | 45.4 ± 6.4** |
| 28 | 1889 ± 595* | 1879 ± 593* | 0.5 ± 0.5 | 20.1 ± 5.9 | 49.8 ± 8.2*** |
| Hypothermic storage | |||||
| Before lesion | 2697 ± 404 | 2686 ± 408 | 0.4 ± 0.9 | 18.2 ± 4.4 | 50.8 ± 11.4 |
| After lesion | |||||
| 0, 7, 14, 21 | N | N | 100 ± 0** | N | N |
- Mean ± standard deviation.
- ECD, endothelial cell density; ECDA, ECD of live cells; DC, dead cells; CV, coefficient of variation of the cell area; 6A, percentage of hexagonal cells; N, not possible to assess.
- * p < 0.05, ** p < 0.01, *** p < 0.001.
| Storage (days) | ECD (mm2) | ECDA (mm2) | DC (%) | CV | 6A |
|---|---|---|---|---|---|
| Organ culture | |||||
| Before lesion | 2834 ± 381 | 2818 ± 376 | 0.6 ± 0.6 | 16.8 ± 2.8 | 53.8 ± 5.1 |
| After lesion | |||||
| 0 | 2803 ± 407 | 2738 ± 467 | 2.6 ± 5.5 | 17.6 ± 2.6 | 53.5 ± 7.5 |
| 7 | 2522 ± 366** | 2520 ± 366** | 0.1 ± 0.1* | 19.2 ± 1.5 | 45.0 ± 5.8** |
| 14 | 2478 ± 425** | 2476 ± 424** | 0.1 ± 0.1* | 19.6 ± 2.8 | 48.8 ± 6.5* |
| 21 | 2389 ± 424** | 2389 ± 424** | 0.0 ± 0.0** | 19.1 ± 2.3 | 48.7 ± 4.2** |
| 28 | 2327 ± 636* | 2327 ± 635* | 0.0 ± 0.0* | 19.4 ± 5.3 | 47.5 ± 7.5* |
| Hypothermic storage | |||||
| Before lesion | 2761 ± 389 | 2752 ± 395 | 0.4 ± 0.5 | 17.2 ± 2.0 | 52.7 ± 8.1 |
| After lesion | |||||
| 0 | 2795 ± 418 | 2777 ± 431 | 0.7 ± 0.9 | 17.5 ± 1.9 | 54.5 ± 7.6 |
| 7 | 2635 ± 388* | 2618 ± 389* | 0.7 ± 0.6* | 18.0 ± 2.2 | 46.6 ± 9.2 |
| 14 | 2523 ± 363* | 2488 ± 381* | 1.5 ± 2.4 | 16.6 ± 2.4 | 49.0 ± 9.3 |
| 21 | 2505 ± 465 | 2332 ± 654 | 8.4 ± 11.4* | 18.0 ± 2.2 | 46.7 ± 5.1 |
- Mean ± standard deviation.
- ECD, endothelial cell density; ECDA, ECD of live cells; DC, dead cells; CV, coefficient of variation of the cell area; 6A, percentage of hexagonal cells.
- * p < 0.05, ** p < 0.01.
Immediately after inducing an injury of the central cornea, a bluish denuded Descemet’s membrane with remnant dead endothelial cells was observed, with little difference between corneas. As was confirmed on two corneal pairs, the extent of damage was complete over the entire surface that was touched by the flat end of the metal rod. No live endothelial cells remained at the site of injury (Fig. 4). The size of the wounds was 1.12 and 1.16 mm2 for corneas later stored under hypothermic or tissue-culture conditions, respectively. In the region adjacent to the wound (pericentral area), the mean ECD was not changed significantly. In this area the percentage of DC increased to 2.6% or 0.7% in corneas destined for storage under OC or hypothermic conditions, respectively. In some of the corneas, bluish spots of different shapes, containing no DC, were observed in the areas adjacent to the wound.
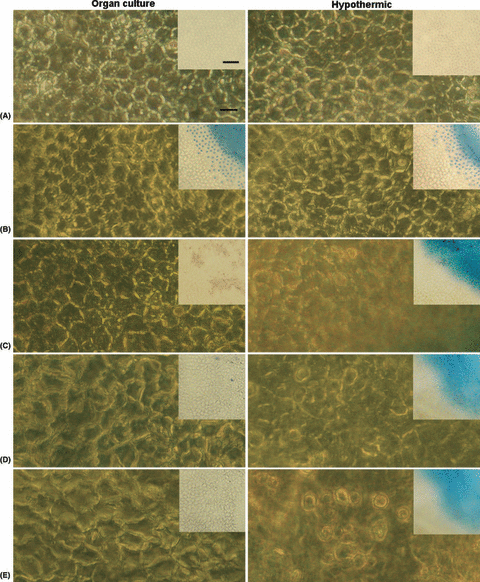
Phase contrast micrograph of the pericentral endothelium of fresh corneas and corneas stored under organ culture (ORGAN CULTURE) or hypothermic conditions (HYPOTHERMIC). (A) Before lesion, (B) immediately after induction of the lesion, (C) 7, (D) 14 (E) and 21 days after the induction of the lesion. The dead cells released from the endothelium of corneas stored under hypothermic conditions are visible on days 14 and 21. Scale bar represents 10 μm. Small bright-field micrographs demonstrate the central part of the endothelium at the same time-points. Dead cells are stained by trypan blue. Scale bar represents 50 μm.
The presence of cell debris, observed using bright field microscopy after 7 days of storage in OC, indicated the location of the previously induced lesions in the central endothelium. No DC or few DC were present in such areas or in adjacent ones. The original damaged area was repaired fully by enlarged cells of irregular shape. In this central part ECD was significantly decreased, as was 6A, while CV was non-significantly increased (Fig. 3A). A number of elongated cells were observed shifting centripetally to the site of the former lesion (Fig. 3B). The endothelial morphology of fresh and stored corneas is shown in Fig. 4.
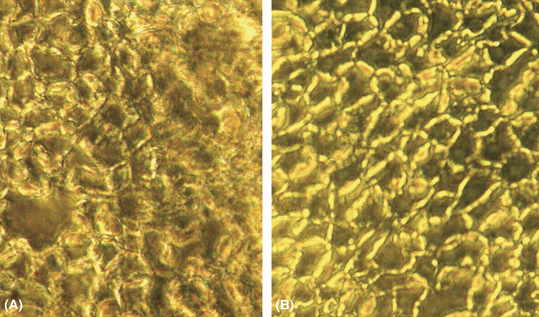
Phase contrast micrograph of the corneal endothelium 7 days after the induction of the lesion: irregular cell mosaic. (A) Polymegatism and pleomorphism are clearly visible in the central as well as the pericentral areas. (B) Elongated cells are shifting centripetally to the site of the lesion. Scale bar represents 10 μm.
Seven days of storage did not lead to repair in corneas stored under hypothermic conditions. The bluish-coloured wounds of the corneal endothelium, indicating the presence of DC, were clearly visible, with no changes in their extent compared to their state immediately after the injury. The area adjacent to the wound was cloudy, but the endothelial cell mosaic was relatively unchanged compared to that before and just after damage. One hundred per cent and 0.7% of DC were present centrally and pericentrally, respectively.
After 14 days, no signs (presence of DC or cell debris) of the injury were observed in OC-maintained corneas. The locations of the original wounds were identifiable only by the presence of larger and irregularly shaped cells. ECD in the central part increased compared to day 7, while it decreased slightly in the pericentral area. The percentage of DC did not exceed 0.2%.
The central part of corneas preserved for 14 days in hypothermic storage was unchanged compared to its state immediately after lesioning, although these corneas became cloudy because of oedema. The pericentral ECD could be assessed in 10 of 12 corneas. The percentage of DC in the pericentral area was markedly higher than before lesioning (1.5%).
After 21 days in OC storage, the mean ECD was elevated in the central endothelium and decreased pericentrally compared to day 14. A similar condition of the corneas with slightly increased ECD could be observed 28 days after wounding.
The corneas stored for 21 days under hypothermic conditions were cloudy, oedematous and in poor condition. The pericentral ECD could be assessed in five corneas (2505 ± 465/mm2). The extent of the central injury was unchanged compared to the previous assessments, with blue spots indicating the endothelial lesion (100% of DC). Pericentrally 8.4% of DC were detected.
Speed of repair of corneas stored under OC conditions
The mean surface area of the damaged region was 1.14 ± 0.1 mm2 (range 1.03–1.25 mm2) immediately after the injury. The extent of the injury decreased significantly to 84%, 43% and 11% of original values after 24, 48 and 72 hrs, respectively (Table 3). A spot with DC was observed in one cornea (0.04 mm2, i.e. 3.2% of the original injury) after 4 days in OC. No DC or residual cell debris was present at the location of the former wound in the other six corneas stored under OC conditions. The centres were repaired fully by enlarged cells of irregular morphology. No signs of damage were observed in any of the corneas assessed at days 5 and 6.
| Extent of the lesion at different time-points (mm2) | ||||||
|---|---|---|---|---|---|---|
| Day | Immediately after lesion | 1 | 2 | 3 | 4 | 5 |
| Mean ± SD | 1.14 ± 0.1 | 0.96 ± 0.14 | 0.49 ± 0.11 | 0.13 ± 0.16 | 0.01 ± 0.02 | 0.00 ± 0.00 |
| p-value | 4.03 × 10−3** | p < 0.0001*** | p < 0.0001*** | p < 0.0001*** | p < 0.0001*** | |
- Mean ± standard deviation (SD).
- ** p < 0.01, *** p < 0.001.
Discussion
This study examined the repair capacity of corneas that underwent a mechanical injury of the endothelium and the influence of the length and type of storage. A corneal endothelial lesion with a mean surface area of 1.14 mm2 (approximately one hundredth of the whole endothelial surface) was restored completely within 5 days in OC, while no repair was observed under hypothermic conditions. The ECD in the wounded central endothelium reached the minimal density (2000 cells/mm2) that is considered acceptable for grafting (EEBA 2007) after 21 days of storage in OC medium, but not at earlier time-points.
Two processes occurring in the corneal endothelium probably influence the ECD values: firstly, the shift of the cells located around the injury to the wounded area, which leads to an increased ECD at the site of former damage and a decrease in the ECD in areas adjacent to the lesion; and secondly, an overall decrease of ECD dependent on the length of storage. A decrease in the mean ECD in the pericentral area to 90%, 88% and 85% of the original values was observed under OC conditions on days 7, 14 and 21 after injury, respectively. This 10–15% decrease is slightly greater than the decrease seen in undamaged corneas, in which decreases of 5% or 10% of the original values were found after one or two weeks of OC storage (Pels & Schuchard 1983). This represents a decrease of approximately 1% per day, as also reported by Thuret et al. (2005).
The medium that was used for deswelling of the OC corneas on days 7, 14, 21 and 28 contained 5% dextran. Although it was shown that the presence of dextran in deswelling medium may induce endothelial alterations, especially after periods longer than 4 days (Borderie et al. 1997), we do not expect that the 12 hrs time-period used in our experiments leads to toxic effects on the corneal endothelium, although the repeated transfer of corneas between medium with dextran and dextran-free medium may represent some stress for the tissue.
The lesions in the endothelium of corneas stored under hypothermic conditions did not change in either their extent or in the presence of DC at the injury site. The mean ECD at locations adjacent to the wound was relatively stable, decreasing to 95%, 93% and 90% of original values after 7, 14 and 21 days of preservation, respectively. A slightly greater (9.5–16%) decrease in ECD, inevitably caused by cell death in the endothelium, was observed by Means et al. (1996) after 4–21 days in hypothermic storage. The impossibility of calculating the number of DC at the lesion site and, after longer storage periods, also in the pericentral area was caused by corneal oedema, which may arise from an insufficient endothelial capacity after lesion induction in which the replacement of DC is hindered by the inhibition of cellular metabolism at the low temperatures typical of hypothermic storage.
The assessment of DC is performed by most banks that prefer OC storage (EEBA 2007). In contrast, under hypothermic storage the percentage of DC is not calculated because the staining of DC cannot be performed in the closed system in which the corneas are kept from excision up to grafting (Bourne 2001; Jeng 2006). The presence of non-disclosed assessed endothelial lesions may increase the risk of transplanting corneas with high numbers of DC. An improvement in the endothelial parameters was also shown in corneas that were first preserved under hypothermic conditions and then transferred for several days to OC (Camposampiero et al. 2003). Despite these facts, there is no obvious superiority of one of the storage systems over the other, based on the similar clinical results achieved with grafts prepared by both methods (Rijneveld et al. 1992; Frueh & Böhnke 2000).
The size of the lesion that had to be repaired influenced the ECD negatively. However, in cases in which DC are located in smaller areas or in folds of Descemet’s membrane or are diffusely present in the endothelium, the shifting and enlargement of cells to repair such damage may be relatively quick (Sperling 1978) and lead to a relatively smaller decline in ECD. The human cornea has an increased ECD in the pericentral and peripheral regions compared to the central region (Schimmelpfennig 1984). Therefore, the peripheral endothelium may be the area of continual cellular supply used to maintain the central ECD at a relatively constant level throughout life (Amann et al. 2003). The stimulative effect of OC on the repair of endothelial cells is supported by the proliferative capacity (expressed by the presence of proliferative cell nuclear antigen) detected in the peripheral corneal endothelium after cornea storage in OC, but is not observed before culturing (Gan et al. 1998).
Our results indicate that the reparative capacity of corneas should be maintained in organ culture but not under hypothermic conditions. We have shown that corneas with a relatively high percentage of DC or an endothelial lesion together with a high ECD may be stored and then checked by a second assessment, the results of which will indicate whether the cornea is suitable for grafting or not.
Acknowledgements
This work was supported by a grant from the Internal Grant Agency of the Czech Republic NR8340-3 and the Czech Ministry of Education, Youth and Sports research project 0021620806/20610011.




