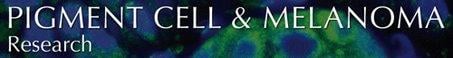Positive crosstalk between ERK and p38 in melanoma stimulates migration and in vivo proliferation
Summary
Melanoma is one of the most therapy-resistant cancers. Activating mutations in BRAF and NRAS are the source of extracellular signal regulated protein kinase (ERK) pathway activation. We show that melanoma cell lines, originating in different metastatic sites, with BRAF or NRAS mutations, in addition to active mitogen activated protein kinase (MAPK)–ERK, also have highly activated stress activated protein kinase (SAPK)-p38. This is in direct contrast to carcinoma cells in which the activity of the two kinases appears to be mutually exclusive; high level of p38 activity inhibits, through a negative feedback, ERK activity and prevents tumorigenesis. Melanomas are insensitive to ERK inhibition by p38 and utilize p38-signaling pathway for migration and growth in vivo. We found a positive functional loop linking the high ERK activity to surface expression of αVβ3-integrin. This integrin, by interacting with vitronectin, induces p38 activity and increases IL-8 production, enhancing cell migration. Because the negative loop from p38 to ERK is lost, the two kinases can remain simultaneously activated. Inhibition of ERK and p38 activities is more effective in blocking in vivo growth than inhibition of each kinase individually. Future therapies might have to consider targeting of both pathways.
Significance
In most cancer cells activation of ERK is linked to proliferation while activation of p38 is growth-arresting or proapoptotic. Also, in most cases activation of the two pathways is inversely regulated, and high p38 strongly inhibits ERK activity. In contrast, we found that in melanoma, both pathways are activated simultaneously, and more importantly, they maintain a positive feedback loop from active ERK to p38. We show that both pathways are required for melanoma growth in vivo. Thus, unlike other cancers, in which combining of ERK inhibitors with p38 activators might be beneficial in melanoma both pathways might have to be inhibited for optimal growth inhibition. This unique scenario might be partially responsible for melanoma resistance to some therapies.
Introduction
We have recently described a paradigm that, from in vitro measurements, predicts the in vivo behavior of carcinoma cells derived from different cancers. It utilizes a balance between two signaling pathways, a growth promoting-ERK, and a growth inhibitory and/or apoptosis inducing-SAPK, p38. We determined that high P-ERK/P-p38 ratio predicts for progressive in vivo growth, while the inverse ratio predicts for cancer dormancy. We have identified three surface receptors, uPAR, α5β1-integrin, and epidermal growth factor receptor (EGFR) that interact with each other and with extracellular matrix (ECM) protein, fibronectin to generate a fully active ERK pathway. This indicates that cell lines derived from highly malignant tumors retain a degree of dependence on ECM for their proliferative stimuli, thus opening a therapeutic possibility.
While each of the tested cell lines had either high ERK or high p38 activity, but not both, a single melanoma (M24met), had simultaneously high ERK and p38, yet it produced tumors when inoculated in vivo (Aguirre-Ghiso et al., 2003). We wondered whether this was an ‘aberrant’ behavior of a single cell line, or a behavior representative of other melanomas. A screen of eight melanoma cell lines showed that seven of eight had simultaneous activation of both pathways. This observation raised a number of questions, such as the source of signals that activate ERK and p38, the mechanism(s) that allows both kinases to remain active and the role, if any, that high p38 activity may play in the malignant phenotype of melanoma.
We have previously identified the source of ERK activation and p38 inhibition in carcinoma cells to involve interaction of uPA/uPAR with α5β1-integrin and recruitment and ligand-independent activation of EGFR (Liu et al., 2002). Activated α5β1-integrins form fibronectin fibril leading to inhibition of p38 activity (Aguirre-Ghiso et al., 2000). We found that this mechanism was not functional in most melanomas examined (Y. Estrada and L. Ossowski, unpublished results). We concluded that different mechanisms must be responsible for generation of signals to ERK and p38 in melanoma and that the crosstalk between the pathways must be altered to allow for simultaneous activation of both.
We have now identified the source of signals responsible for pathway activation, and determined that the negative feedback that exists between the pathways in other cancer cells (Aguirre-Ghiso et al., 2003) is lost in melanomas. Moreover, we identified a positive feedback loop between ERK and p38 activities, mediated at least in part, by αVβ3 integrin. Unlike the established pro-apoptotic and growth inhibitory role of p38 (Bradham and McClay, 2006; Olson and Hallahan, 2004), we show here that melanomas depend on p38 for migration and for growth in vivo and that the p38 induced migration is mediated by IL-8.
Results
Concurrent activation of ERK and p38 in melanoma
We previously found that melanoma, M24 was the only exception to the ‘high ERK low p38 activity rule’ as a predictor of tumorigenicity, and that in these cells both pathways were activated, yet M24 was tumorigenic in vivo. We now examined P-ERK and P-p38 in other melanomas, derived either from primary human skin melanoma (MeWo), non-skin metastases (A375, A2058, UCT-2) or uvea (SP6.5) or M24 of unknown derivation. With the exception of MeWo, all melanomas tested had high P-ERK to ERK ratio (Figure 1A, results not shown and (Aguirre-Ghiso et al., 2003). Unlike in the T-HEP3, a head and neck carcinoma, in which both ERK1 and two were equally phosphorylated and coordinately regulated (Aguirre-Ghiso et al., 2003), in melanomas the predominant phosphorylated species was ERK2.
Basal ERK and p38 activities and in vivo growth. (A) P-ERK level determination. Cells were serum-starved overnight, lysed, and 20 μg of cell protein was analyzed by Western blotting for P-ERK (top panel) and total ERK1/2 (bottom panel). The bands were scanned and quantified by Image J. The experiment was repeated 3 times with similar results. (B) P-p38 level determination. Cells were treated as in A, except that 50 μg of protein was analyzed for P-p38 (top panel) or total p38 (lower panel). The experiment was repeated twice with similar results. (C) In vivo growth of melanomas. D-HEp3 cell line (dormant in vivo), M24met, A2058, and A375 cells were inoculated on CAMs at 5 × 105/CAM of 10-day-old chick embryos and after 5 days of incubation the tumors were excised, enzymatically dissociated and tumor cells were counted. Data represent two individual experiments with 8 samples per group.
We previously established that in carcinoma and fibrosarcoma cells, interaction of three receptors, uPAR, α5β1-integrin and EGFR, and the level of uPAR expression, or its interaction with the integrin, regulates ERK activity (Aguirre-Ghiso et al., 2000; Chaurasia et al., 2006). However, most melanomas do not express uPAR and only some express EGFR, (results not shown), yet with the exception of MeWo, all have high P-ERK to ERK ratio (3.4–8.3), (Figure 1A and results not shown). As published by others (Gray-Schopfer et al., 2007), mutations in oncogenes are responsible for the persistently active ERK pathway. We confirmed this by sequencing of both strands of the PCR-amplified products of BRAF exon 11 and exon 15, and NRAS exon 2 and exon 3. With the exception of M24met, all of the melanomas with high ERK activity harbored BRAF (V600E) mutation; the M24met had an NRAS (Q61R) mutation (Table 1), while MeWo cells were wild type for both BRAF and NRAS and had low endogenous level of P-ERK, confirming a link between BRAF mutation and persistent activation of the ERK pathway (Gray-Schopfer et al., 2007). A375 cells had no wt allele while in the rest of the cell lines tested the mutant allele predominated.
| Cell line | Mutation |
|---|---|
| M24met | NRAS Q61R |
| A375 | BRAF V600E |
| A2058 | BRAF V600E |
| UCT-2 | BRAF V600E |
| SP6.5 | BRAF V600E |
| MeWo | BRAF wt |
Regardless of their site of derivation, all melanomas tested using Western blotting had high P-p38 levels greater than the dormant form of T-HEp3, the D-HEp3 cell line (Figure 1B, and results not shown). Thus, coincident with high ERK activity (Figure 1A) all the melanomas had also very high p38 kinase activity (Figure 1B and results not shown).
Melanomas with high P-ERK, but also high P-p38, can grow in vivo
We previously established that a high P-ERK/P-p38 balance is required for in vivo growth of carcinomas (Aguirre-Ghiso et al., 2003). T-HEp3 cells have low level of P-p38, high ERK activity (Figure 1A, B) and grow rapidly in vivo, while their dormant counterpart, D-HEp3 cells have higher level of P-p38, much lower P-ERK (Figure 1A, B) and are growth arrested (dormant) when inoculated in vivo (Aguirre-Ghiso et al., 2003, 2004). We next examined the effect the simultaneous activation of ERK and p38 pathways exerts on in vivo growth of melanomas. Suspensions of 5 × 105 cells of A2058, A375 and M24met cultures, as well as D-HEp3 cells, which served as negative control, were inoculated on each of five chorioallantoic membranes (CAMs) of 10-day-old chick embryos, as previously described (Ossowski and Reich, 1980). Following 5 days of incubation the tumors were excised, dissociated, and tumor cells counted. While the number of D-HEp3 cells recovered from the CAMs was almost equal to the inoculum, the number of cells in each of the three melanomas rose three to fourfold (Figure 1C). Thus, the high relative p38 activity in these cells, in presence of high ERK activation, does not lead to growth arrest in vivo.
Positive loop between active ERK, αvβ3-integrin expression and p38 activation.
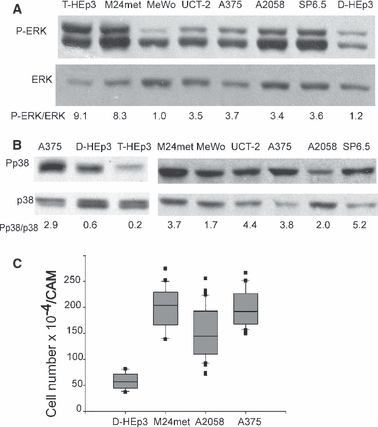
Because αVβ3 integrin is associated with advanced, vertical phase of melanoma growth (Albelda et al., 1990; Seftor, 1998), and because p38 in an invasive breast cancer cell line can be activated through αV(13), we examined whether αVβ3 interaction with its main ligand, vitronectin, might contribute to p38 activation and to melanoma progression. Fluorescence-activated cell sorter (FACS) analysis indicates that all melanomas in our series express αVβ3-integrin (Figure 2A and results not shown). To test if the integrins affect p38 activation we plated cells, kept in suspension for 30 min, on dishes pre-coated with vitronectin, allowed them to attach for 20 and 40 min and tested them for P-p38 level. Adhesion to vitronectin increased the ratio of P-p38/p38 from a basal state of <1.0, to a ratio of 2.2, 5.2 and 12.3 in A2058, M24met and A375, respectively. Plating of A375 cells on laminin did not affect P-p38 (Figure 2B, right panel). In breast carcinoma MDA 231, the ratio rose from 0.1 to only 1.2, but the induction was more sustained (Figure 2B). To further implicate αvβ3-integrin in p38 activation, we incubated A375, A2058 and M24met melanoma cells with blocking anti-αv-integrin antibody and lysed the cells when first signs of cell rounding (4–6 h) were detected. This treatment strongly reduced the P-p38 level, without affecting the total p38, (Figure 2C).
Regulation of αvβ3-integrin expression and its role in p38 activation. (A) Surface expression of αVβ3. Surface integrin expression was analyzed by FACS using anti-αVβ3 antibody (Clone LM609, 10 μg/ml) as described in Materials and Methods, isotype-specific IgG served as negative control. (B) Activation of p38 in cells on vitronectin. Cells were serum-starved overnight, detached with EDTA, kept in suspension (5 × 105 cells/ml of serum-free medium) for 30 min, plated on vitronectin-coated (1 μg/ml) plates and lysed after 20 and 40 min. The lysates were analyzed for P-p38 content by Western blotting and for total p38 after membrane stripping. As a negative control, A375 cells were inoculated on a laminin (1 μg/ml) coated dish and after 20 min processed as above. (C) A blocking anti-αV antibody inhibits p38 activity. Cells in a monolayer were treated with 1.5 μg/ml of blocking anti αV, (AV-1) antibody for 4–6 h, lysed and analyzed by Western blotting for P-p38 and p38 content. The bands were scanned, quantified by Image J, ratios between P-p38 and p38 determined and the inhibitory effect of antibodies expressed as percent of ratio of the isotype matched Ig (IgG1). (D) Active ERK is required for β3-surface expression. A2058 and A375 cells were incubated for 40 h with 50 μM PD98059, or 5 μM U0126 or 5 μM U0124 (as negative control) and analyzed by FACS for surface β3-integrin expression as described in Materials and Methods. Cells in parallel dishes were lysed and 20 μg of protein of each was tested for P-ERK and ERK content by Western blotting.
To test whether the αVβ3-integrin is part of a positive feedback loop between high ERK and p38 activities, cells were treated with two different MEK (MAPK/ERK kinase) inhibitors, PD98059 and U0126, and with an inactive compound, U0124, as negative control. After 40 h of treatment parallel cultures were tested using FACS for β3 expression and others were lysed and tested for P-ERK level by Western blotting. Both compounds strongly inhibited P-ERK and this inhibition was linked to a very potent (approximately fivefold) down-modulation of surface expression of β3-integrin in A375 and A2058 cells (Figure 2D). A weaker effect on β3 (and on ERK inhibition) was observed in M24met cells (results not shown). PD98059 treatment also reduced the αVβ3 (results not shown). Thus, persistently high ERK activity maintained by BRAF mutation supports high expression of αVβ3, an integrin involved in p38 activation in melanoma cells (Figure 2B, C).
Melanomas lack negative feedback from p38 to ERK
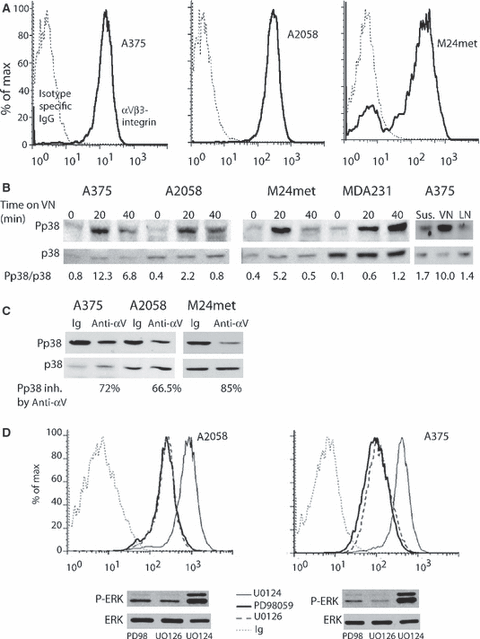
We previously found that carcinoma cell lines display a tight inverse balance between ERK and p38 and in these cells p38 inhibition predictably resulted in enhanced ERK activity and ability to grow in vivo (Aguirre-Ghiso et al., 2003) and Figure 3A, left panel. To test for this interaction in melanoma, we treated MeWo with wild type BRAF and A375 and A2058 with V699E mutation melanomas for 30 min and 24 h with a pharmacological inhibitor (SB203580) of p38. Inhibition of p38 slightly increased ERK activity in MeWo but strongly inhibited it in A375 and A2058 (Figure 3A). A second generation inhibitor, SB239063, considered to be more p38 specific (Underwood et al., 2000), produced a 3.5-fold stimulation of P-ERK in D-HEp3 cells, had a weaker stimulatory effect on MeWo cells, and none, or slightly inhibitory, effect on A375, A2058 and UCT-2 cells (Figure 3A). The loss of negative feedback from p38 to ERK in A375 melanoma was confirmed also by a genetic approach; P-ERK was not increased by expression of DN-p38 in A375 cells, while the same treatment increased P-ERK level in MeWo cells (Figure 3B). Thus, ERK activity generated downstream of mutant BRAF (A375, A2058 and UCT-2) or NRAS (M24met, Table 1) is not increased when p38 is inhibited, suggesting loss of negative crosstalk between these two pathways. The lack of response is not due to the inability of SB203580 or SB239063 to inhibit p38 as both compounds inhibit p38 kinase activity (74–96%) (Figure 3C). The residual activity was not due to the presence of p38 γ or/and p38δ, which are not inhibited by SB203580, as neither was detectable in melanoma cell lines using Western blotting (results not shown).
Lack of negative crosstalk between p38 and ERK. (A) Pharmacologic inhibition of p38 activity. Cells were serum-starved overnight, treated with 5 μM SB203580 or 10 μM SB239063 for 30 min or 24 h, lysed and analyzed for P-ERK and ERK by Western blotting. The bands of the scanned Western blots were quantified by Image J. Control (untreated cells) was arbitrarily set as 1and the rest of the values related to controls. (B) Inhibition of p38 activity by a dominant negative p38α. Cells were transfected with 2 μg p38-DN-FLAG plasmid and after 48 h were lysed and analyzed for p38-DN expression using anti-flag antibody or P-ERK with anti-P-ERK antibodies, or ERK with anti-ERK antibodies, respectively (insert). Bars represent bands scanned, quantified using NIH image and corrected for total ERK. (C) Cells were treated with 50 μM arsenic trioxide for 3 hrs, lysed, P-p38 was immunoprecipitated from 200 μg protein and the immunocomplex was incubated in a kinase reaction containing ATF-2 as substrate and either SB203580 or SB239063 or neither. The level of phospho-ATF-2, determined by Western blot was used to monitor the kinase reaction. In the left panel the inhibitors were used at 1 and 10 μM, and in right panel only at 10 μM. The bands were scanned and quantified using NIH image, the intensity of the bands produced by the untreated cells was set as 100%.
Published data suggest that at least in some cells, acute activation of ERK leads to increased MKP-1, a phosphatase with predominant p38 specificity (Camps et al., 2000) and that the effect of ERK on MKP-1 might be both transcriptional (Brondello et al., 1997; Ryser et al., 2004) and post-translational (protection from proteasomal degradation) (Brondello et al., 1999). We reasoned that melanoma might have lost this molecular connection and as a result can simultaneously express high ERK and p38. However, we could not confirm the ERK effect on MKP-1 either at transcriptional or post-translational level, even when tested in carcinoma cells with high ERK and low p38 activity (results not shown). Similarly, we attempted to explore the reason for lost sensitivity of ERK to inhibition by high-level of active p38. A phosphatase, PP2A that can be activated by p38 has been shown to inactivate MEK. We expected to find reduced level of this phosphatase in cells with high p38 activity but Western blots of the PP2A catalytic domain showed no such difference (results not shown). It is still possible that, as previously shown (Yang et al., 2001) PP2A activity, and not expression, is down regulated in melanomas contributing to loss of ERK inhibition by p38.
Activated p38 contributes to cell migration and in vivo growth of melanoma
Convincing evidence indicates that mutated BRAF or NRAS, by activating the ERK pathway, contributes to the malignant phenotype of melanoma (Dhomen and Marais, 2007; Gray-Schopfer et al., 2005). Published evidence also suggests that activation of p38 induces expression of matrix metalloproteinases (MMPs) (Simon et al., 2001) and that active p38 increases the stability of uPA- and other selected RNAs (Dean et al., 2003; Montero and Nagamine, 1999). In our hands, knockdown of p38 by siRNA in melanoma cell lines did not affect MMPs; conditioned media of control and p38-siRNA treated melanomas, tested by zymography, contained similarly high levels of MMP-2 and minor bands, corresponding to MMP-9 and MMP-1 (results not shown). Of the melanoma tested only A2058 had detectable levels of uPA and, in these cells, no reduction was observed when p38 was knocked down (results not shown).
We next tested whether p38 activation played a role in melanoma cell migration. M24met and UCT-2 cells were pre-treated with SB239063 for 48 h and their migration was tested in a modified Boyden chamber. As shown in Figure 4A (left and right panels), inhibition of p38 activity significantly reduced the number of migrating cells.
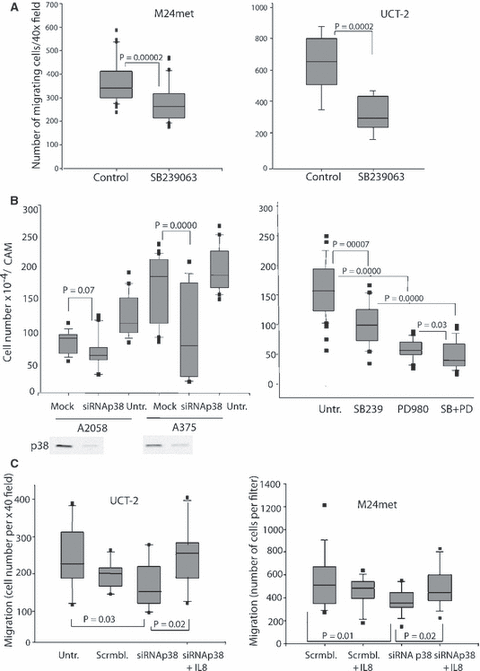
Active p38 is required for cell migration and both kinases are required for in vivo growth. (A) Active p38 is required for cell migration. Cells were treated with 10 μg/ml of SB239063 in serum-free medium and inoculated into the upper chamber of a modified Boyden chamber at 1 × 105 cells per well with or without SB. Fibronectin served as chemoattractant and migration was quantified after 8 h as described in Materials and Methods. The results shown are from two experiments, eight filters per group. (B) Active ERK and p38 are required for in vivo growth of melanoma. A2058 and A375 cells were transfected with 25 nM p38α-siRNA for 24 h using SiQuest transfection reagent. Cells were then detached, inoculated (at 5 × 105 cells/CAM) onto CAMS and after 5 days of incubation the tumors were excised, minced, enzymatically dissociated and tumor cells were counted (left panel). The bars represent two experiments, 16–20 replicates each. Alternatively, (right panel) A375 cells were treated with SB239063 (10 μM), or PD98059 (20 μM) or both, or kept in medium alone for 24 h and then processed as in left panel. The results shown are mean of five tumors and four individual cell counts per sample. This experiment was repeated twice with similar results. (Untr.-untreated). (C) p38 induced cell migration is mediated through IL-8. UCT-2 and M24met cells were transfected with p38α or scrambled siRNA mix (1 μg siRNA/1.5 × 106 cells) and 48 h later 105 cells were inoculated into Boyden chambers in serum-free medium alone or supplemented with 100 ng/ml IL-8 and fibronectin. Migrating cells were counted as in Figure 4A. (Scrmbl.-scrambled siRNA)
We also tested the role of p38 in melanoma growth in vivo by using two approaches to p38 inhibition. In the first, p38α was reduced with specific siRNA and 24 h after transfection the cells were inoculated on CAMs, and incubated for 5 days. As shown in Figure 4B (left panel), p38-siRNA, which substantially reduced p38α expression, significantly inhibited the growth of A375 cells; the mock transfection had no effect. Growth inhibition in A2058 cells, although reproducible in four individual experiments, did not reach significance in three of the four experiments, most likely because of sensitivity of these cells to several transfection agents, including the one shown. (A comparison to the untreated control showed significant inhibition). In another series of experiments A375 cells were pre-incubated with 10 μM SB239063, or with PD98059 (20 μM), or the combination of the two inhibitors for 24 h and then tested for growth on CAMs. As shown in Figure 4B (right panel), treatment with either SB239063 or PD98059 produced a significant reduction in cell number in in vivo tumors, and the combination of the two drugs showed further significant inhibition. SB239063 treatment also significantly reduced the in vivo growth of M24met cells (results not shown). In a parallel experiment, A375 pretreated with PD98059, SB239063 or both or neither, were grown on CAMs and after 6 days the tumors were weighed, fixed, sectioned through the largest circumference and stained with H&E staining. As before, the treatments reduced the total tumor mass; the control tumors coalesced to form a contiguous mass of tightly packed cells, some showing more mesenchymal features. The PD/SB-treated tumors formed very small nests of tumor cells surrounded by the host stroma (results not shown).
Pharmacologic inhibition of p38 activity or knockdown of p38 reduces IL-8 release by melanoma cells; effect on migration
The SAPK-p38 has been shown, through NF-κB activation, to mediate IL-8 expression (Na et al., 2001; Ueda and Richmond, 2006) and the pro-tumorigenic role of IL-8 in melanomas is most convincingly documented (Bar-Eli, 1999; Schadendorf et al., 1993; Singh and Varney, 2000). We therefore postulated that the loss of migration and proliferation we observed upon p38 inhibition might be mediated through IL-8. We incubated A375, A2058, UCT-2 and M24met cells in medium alone or with 10 μM SB239063 for 48 h and collected and analyzed the conditioned media for IL-8 content. The level of IL8 was strongly reduced by SB239063 or by p38α-siRNA (Table 2).
| Cell type | Control (IL8 ng/ml) | SB treatment (% inhibition) | p38α-siRNA (% inhibition) |
|---|---|---|---|
| A375 | 9.9 | 88 | 75 |
| A2058 | 4.5 | 87 | 67 |
| UCT-2 | 10.8 | 54 | 67 |
| M24met | 7.6 | 88 | 50 |
- For SB treatment, the values, calculated from a standard curve, are the mean of six determinations from two individual experiments; for p38α-siRNA, the values are mean of three determinations, one experiment.
To test whether this pathway contributed to the reduced migration in cells in which p38 was inhibited or knocked down, we transfected UCT-2 and M24met cells with p38-siRNA and tested whether exogenous IL-8 will restore their ability to migrate. The results (Figure 4D) confirm that p38 knockdown inhibits migration and that IL-8 rescues this inhibition. Thus, IL-8, downstream of p38, affects cell migration.
Discussion
Our results allow us to reach an unexpected conclusion that, in contrast to most cancer cells in which persistent ERK and p38 activation appear to be mutually exclusive, in melanoma cells both pathways are simultaneously activated. More importantly, in melanoma, active p38 appears to be necessary for cell migration and proliferation. This finding implies that melanoma cells are capable of bypassing the negative crosstalk from p38 to ERK that exists in other cancer cells and that the therapeutic interventions might have to be directed simultaneously to both pathways. Others have found rewired ERK–JNK signaling pathways in melanoma (Lopez-Bergami et al., 2007) and oncogenic, as opposed to tumor-suppressing, functions of the ATF-2 transcription factor (Bhoumik and Ronai, 2008). We confirmed that mutated BRAF or NRAS, and not surface receptors and their interaction with matrix, are responsible for most of the ERK activating signaling in melanoma. However, at least part of the p38 activating signal is derived from receptor/matrix interaction, namely the interaction of αVβ3 with vitronectin. Our data can be organized within the context of the published evidence into the following novel working model (Figure 5): mutated BRAF, or NRAS, maintains a persistent state of ERK activation, which in turn is responsible for high surface expression of αVβ3. Interaction of αVβ3 with vitronectin (and possibly other matrix proteins) stimulates p38 activity. Because the negative feedback from p38 to ERK is lost, both pathways remain active. We found that IL-8 is downstream of p38 and that it is important for cell migration.
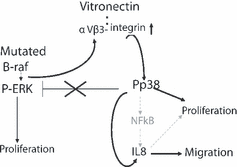
Working model of the feedback-loops in melanoma.
Predominance of evidence links an increased p38 activity with apoptosis or differentiation, and p38 has been classified as tumor or metastasis suppressor (Bradham and McClay, 2006; Hickson et al., 2006). Some evidence, however, links it with malignant progression, especially in connection with proteases and invasion (Han et al., 2002) and/or proliferation (Recio and Merlino, 2002). In addition to the classical pathway of p38 activation, p38 can autophosphorylate and self activate through an interaction with TAB1 (Ge et al., 2002). The autophosphorylation, but not the MKK3/MKK6 phosphorylation of p38 is sensitive to SB203580 inhibition (Ge et al., 2002). We found that in melanoma cells the SB239063 treatment did not inhibit phosphorylation of p38 (results not shown), implicating the classical signaling cascade in its activation. We focused on αVβ3 integrin as a possible source of p38 activation because both p38 and the integrin have been linked to tumor progression. Also, integrin-blocking antibodies were shown to reduce melanoma growth in nude mice (Mitjans et al., 2000) and transfection of a melanoma cell line with radial phase characteristics with a β3-integrin subunit, converted it into an invasive, vertical phase phenotype (Hsu et al., 1998). Indeed, we found that plating melanoma cells on vitronectin, a preferred substrate for αVβ3 integrin, strongly increased p38 activation (Figure 2) while treatment of cells with αV integrin blocking antibody, produced a strong reduction in the basal P-p38 level. Similar observations were reported previously in an invasive breast cancer cell line and a melanoma cell line transfected to express αVβ3 integrin (Chen et al., 2001). These authors concluded that it is the cytoplasmic domain of αv that is responsible for p38 activation and that increased uPA level might mediate the increase in invasiveness, a finding we could not confirm.
It is possible that engagement of αVβ3 with vitronectin activates Rac or cdc42, leading to p38 activation (for review (Hall, 2005). Another possibility is that a crosstalk between αVβ3 and α5β1 integrins, both expressed on the surface of melanoma cell lines (Figure 2A, and results not shown) activates p38. FN-fibril formation by α5β1 depends on the interaction of the integrin with RGD (Arginine, Glycine, Aspartic acid) and a synergy site in FN (Mao and Schwarzbauer, 2006). When the synergy site in fibronectin is not accessible, the interaction of αVβ3 with FN predominates over that of α5β1, switching to a less efficient FN fibril generation. Melanoma express high level of αVβ3, at least partially fueled by persistent ERK activation (Woods et al., 2001 and Fig. 2C), possibly because the β3-promoter contains an element that binds a transcription factor of the ets family, a substrate for ERK (Villa-Garcia et al., 1994). In a mouse melanoma, blocking of αVβ3 integrin leads to an increase in fibronectin fibril formation (Yang et al., 2003). We tested and found that none of the melanoma with BRAF mutation (total of seven cell lines) had fibronectin fibrils on their surface (results not shown). Since we (3) and others (Bourdoulous et al., 1998) have shown that disruption or reduction in FN-fibrils leads to p38 activation, possibly through cdc42 activation, it is conceivable that the persistent expression of αvβ3 and inhibition of fibril formation yields the high p38 activity.
In our hands, simultaneous activation of both ERK and p38 pathways appears to be unique to melanoma; we showed the existence of a negative feedback from p38 to ERK in cancer cell lines derived from breast, prostate, head and neck, and fibrosarcoma (Aguirre-Ghiso et al., 2003). Other authors (Junttila et al., 2007; Li et al., 2003) showed absence of p38-mediated inhibition of the ERK pathway in head and neck tumor cell lines, but inspection of their results confirms that, similarly to our results, at least under basal conditions, high level of P-ERK is accompanied by very low P-p38 levels (Li et al., 2003). We clearly showed that inhibition of p38 activity with SB203580 or a more specific inhibitor, SB239063 (Lee et al., 1999), or expression of DN-p38α, did not increase the level of P-ERK in melanoma (Figure 3). The SB203580 treatment inhibited ERK activity, while SB239063 had no effect on ERK. The specificity of these inhibitors for p38α and β is defined by the presence of a non-bulky amino acid residue (threonine 106) in their ATP-binding pocket (Wilson et al., 1997). C-Raf contains a threonine residue in an equivalent position to that of p38 and is also inhibited by SB239058 in vitro (Hall-Jackson et al., 1999). The ATP-binding pocket of B-raf is similar and it might also be inhibited by SB203580 leading to a reduced ERK activity. Although, SB203580 induces a potent, ras independent in vivo activation of c-Raf, this activation does not translate to a downstream effect on ERK (Hall-Jackson et al., 1999). Regardless of the effect on Raf, our results, using a more specific p38 inhibitor and expression of the DN-p38, which show induction of ERK activity in carcinoma cells, but not in melanomas, provide strong support for the conclusion that the negative feedback from p38 to ERK is lost in melanoma.
Our results also strongly point to an important role of p38 in both in vivo growth and migration. We hypothesized that p38 activation might represent one of the upstream signals for NF-κB activation and induction of IL-8, a chemokines tightly linked to melanoma progression (Bar-Eli, 1999; Huang et al., 2000; Ueda and Richmond, 2006). Extensive published evidence connects NF-κB activation to IL-8 induction (Amiri and Richmond, 2005; Huang et al., 2000; Na et al., 2001; Shimizu et al., 2007). The role of p38 in NF-κB activation is less well studied but several reports confirm that p38 is in the pathway of NF-κB and IL-8 (Dai et al., 2004; Mainiero et al., 2000). Importantly, we show that the inhibition of p38 activation or knockdown of p38α protein both lead to a reduced IL-8 production and reduced proliferation and migration. Reduced migration can be restored by exogenous IL-8, linking p38 to IL-8 and its biological activity.
Overall, our results highlight a novel and unexpected chain of signaling events that set ERK and p38 activities in a positive feedback loop. Judged by our previous results this appears to be unique to melanoma and might call for therapeutic approaches that will have to consider both ERK and p38 as simultaneous targets.
Materials and methods
Reagents and antibodies
Dimethyl sulfoxide, Triton X-100, Na-orthovanadate. NaFl, protease inhibitors, bovine serum albumin (BSA), collagenase Type 1A, human fibronectinwere from Sigma Chemical Co. (St. Louis, MO, USA). Aprotinin and trypsin were from MP Biomedicals (Aurora, OH, USA). PD98059, SB203580, and SB239063, U0124 and U0126 were from Calbiochem (San Diego, CA, USA). Dulbecco’s Eagle’s minimum essential medium (DMEM) and antibiotics were from GIBCO Laboratories (Grand Island, NY, USA). Fetal bovine serum (FBS) was from JRH Biosciences (Lenexa, KS, USA). COFAL-negative embryonated eggs were from Specific Pathogen-Free Avian Supply (SPAFAS) (North Franklin, CT, USA). Purified human vitronectin and laminin were from Chemicon (Temecula, CA, USA). p38α-siRNA mix and scrambled siRNA mix were from New England Biolabs (Ipswich, MA, USA). Antibodies: Anti-phospho ERK 1/2 (anti phospho-Tyr 204; clone E4) and anti-p38 were from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA); anti-ERK from BD Transduction Laboratories (Lexington, KY), anti-Pp38 and p38 MAPK assay kit were from Cell Signaling (Beverly, MA, USA). Anti αV (Clone AV-1), anti β3 (Clone PM6/13), and anti αVβ3 (clone LM609) were from Chemicon (Temecula, CA, USA). Alexa 646 was from Molecular Probes (Carlsband, CA, USA). Normal mouse IgG was from Sigma. Nucleofector and nucleofector kits were from AMAXA (Gaithersburg, MD, USA). SiQUEST transfection reagent was from Mirus (Madison, WI, USA). Puregene Cell and Tissue Kit were from Gentra (Minneapolis, MN, USA). Taq DNA Polymerase was from Invitrogen (Carlsbad, CA, USA). QIAquick PCR Purification Kit was from Qiagen (Valencia, CA, USA).
Cell lines and cell culture conditions
Human epidermoid carcinoma HEp3 (T-HEp3), serially passaged on CAMS of chick embryos, was used as a source of tumorigenic cells; spontaneous dormant tumor cells (D-HEp3) were T-HEp3 cells passaged in culture 120–170 times. MeWo, A375, and A2058 were obtained from the American Type Culture Collection (Manassas, VA, USA). M24met cells were provided by Dr. B. Muller from Scripps Institute (La Jolla, CA, USA), UCT-2 by Dr. E.L. Wilson from NYU (New York, USA) and SP6.5 cells were provided by Dr. S. Guérin from Centre Hospitalier Universitaire de Quebec (Saint-Foy, QC, Canada). T-HEp3, D-HEp3, A375, A2058, SP6.5, were cultured in DMEM; UCT-2, MeWo, and M24met in RPMI, all with 10% heat-inactivated FBS.
BRAF and NRAS mutation analysis
Genomic DNA was extracted and purified from cultured cells using Puregene Cell and Tissue Kit. BRAF exons 11 and 15, and NRAS exons 2 and 3 were PCR amplified using forward and reverse primer sequences as described previously (Davies et al., 2002). PCR amplification was carried as described previously (Davies et al., 2002). PCR amplicons were purified using QIAquick PCR Purification Kit and sequenced on both strands using a Prism Model 3700 Capillary Array Sequencer, Applied Biosystems, (Foster City, CA). The relative level of mutant and wild type form of BRAF was determined.
ERK and p38 activity, activation and inhibition
Cells were serum-starved for 24 h, lysed with radioimmuno precipitation assay (RIPA) buffer (1% Triton X-100, 140 mM of NaCl, 10 mM of Tris, 0.02% sodium azide, 0.1% SDS, 0.5% deoxycholate, 1 mM of orthovanadate, 1 mM of NaFl, 200 KIU/ml of aprotinin, 1 μg/ml of leupeptin, 1 μg/ml of pepstatin, 1 mM of phenylmethylsulfonyl fluoride) and extracted on ice for 30 min, and 20 μg of protein (P-ERK), and 50 μg (p38) were analyzed using standard Western blotting. For p38 inhibition effect on ERK activity, cells were serum starved overnight and treated with either 5 μM SB203580 or 10 μM SB239063 for 30 min and 24 h. MeWo and A375 cells were transfected with 2 μg p38-DN-FLAG plasmid, incubated for 40 h in DMEM with serum and for the last 8 h in serum-free medium, lysed with RIPA buffer and 20 μg of protein was analyzed by immunoblotting for P-ERK and total ERK content. Anti-FLAG antibody (Sigma Chemical Co.) was used to determine DN-p38 expression. For effect of vitronectin or laminin on p38 activation, cells were detached and incubated in suspension for 30 min in serum-free medium, plated on vitronectin-coated plates (1 μg/ml) for either 20 or 40 min, or on laminin for 20 min, lysed with RIPA buffer and 50 μg of protein was analyzed by immunoblotting with an anti-Pp38 antibody (Cell Signaling) and for total p38 with anti-p38 antibody (Santa Cruz). Cells in suspension served as a negative control. The bands were scanned and quantified using Image J (a public domain image processing program from the National Institutes of Health). For inhibition by αVβ3 of p38 activation, cells in monolayer were treated with a αV blocking antibody (AV-1, Chemicon) for 4–6 h (just before they showed signs of detachment), lysed with RIPA buffer and examined using Western immunoblotting using anti-Pp38 antibody (Cell Signaling), and after membrane stripping, with anti-p38 antibody.
p38-SAPK activity
p38 activity was analyzed using the p38 MAP Kinase Assay Kit (Cell Signaling). Briefly, p38 was immunoprecipitated overnight from 200 μg protein, the complex washed three times, and used in a kinase assay as per manufacturer instructions. (Some kinase reactions also included 1–10 μM SB239063). The proteins were analyzed by Western blotting with a phospho-specific anti-ATF-2 antibody.
Migration assay
Melanoma cells were treated with 10 μM SB239063 for 48 h or transfected with 1 μg/1.5 × 106 cells of a mixture of p38α-siRNAs, or scrambled RNA, for 48 h prior to migration assay. Cells (1 × 105) were inoculated into modified Boyden chamber in serum-free medium without or with 10 μM SB239063, with 10 μg/ml human FN in the lower chamber, incubated at 37°C for 8 h, and cells that migrated were fixed and stained for 10 min in a phosphate-buffered saline (PBS) containing 1% formalin and 0.5% crystal violet, photographed and 5–40× fields were counted. In some experiments, the migrated cells were detached by trypsinization, allowed to adhere to l-poly-lysine coated wells, fixed, stained and counted.
FACS analysis
Cells were detached with 2 mM EDTA in PBS and resuspended in cold PBS with 1%BSA. Anti-αVβ3 antibody (Clone LM609), β3 (clone PM6/13), or isotype matched IgG were added to 1 × 106 cells at 10 μg/ml, incubated at 4°C for 30 min, and after two washes, incubated for 30 min with Alexa 647 antibody (1 μg/ml). The cells were analyzed in FACS Cantor Beckton Dickinson (San Diego, CA, USA). To examine the effect of ERK inhibition on β3-expression, melanoma cells were pre-incubated with 50 μM PD98059, or 5 μM of U0124 (negative control) or U0126 for 40 hrs and processed as above using anti-β3-integrin antibody.
Growth of tumor cells on CAMs
Subconfluent cell monolayers were treated with 5 μM SB203580, 10 μM SB239063, 50 μM PD98059, or left untreated for 48 hrs. In some experiments (A2058), cells were transfected with p38-siRNA (25 nM) using SiQUEST transfection reagent (Mirus), according to manufacturer’s instructions, and after 24 hrs detached, washed, inoculated on the CAMS (5 × 105 cell per CAM) of 10-day-old chick embryos and after 5 days the tumors were excised, minced, dissociated into single cell suspensions with type 1A collagenase and the tumor cells were counted. In one experiment following treatment as above, the tumors were excised, weighed then fixed, sectioned and stained with H&E.
IL-8 measurement
Cells were treated for 48 h with 10 μM p38 inhibitor, SB239063, in medium supplemented with 2% heat-inactivated FBS. For some experiments, cells (1 × 106) were transfected with 1 μg of p38-siRNA using AMAXA nucleofector and after 48 h conditioned media were analyzed in triplicates for IL-8 content using human specific CXL-8 ELISA kit from R&D Systems (Minneapolis, MN, USA).
Acknowledgements
This work was supported by USPHS Research Grant CA-40578, the Samuel Waxman Cancer Research Foundation and the Peter J. Sharp Foundation. The gift of the following cells lines: Drs. Barbara Mueller (Scripps Muller, Scripps Institute (La Jolla, CA, USA), M24met cells, Dr. Lyn Wilson, NYU Medical Center, NY, UCT-2 cells, and Dr. Sylvian Guérin from Centre Hospitalier Universitaire de Quebec (Saint-Foy, QC, Canada), SP6.5 cells, is gratefully acknowledged.