Exercise Training Improves Heart Rate Variability in Older Patients With Heart Failure: A Randomized, Controlled, Single-Blinded Trial
Abstract
Congest Heart Fail. ****;**:**–**. ©2012 Wiley Periodicals, Inc.
Reduced heart rate variability (HRV) in older patients with heart failure (HF) is common and indicates poor prognosis. Exercise training (ET) has been shown to improve HRV in younger patients with HF. However, the effect of ET on HRV in older patients with HF is not known. Sixty-six participants (36% men), aged 69±5 years, with HF and both preserved ejection fraction (HFPEF) and reduced ejection fraction (HFREF), were randomly assigned to 16 weeks of supervised ET (ET group) vs attention-control (AC group). Two HRV parameters (the standard deviation of all normal RR intervals [SDNN] and the root mean square of successive differences in normal RR intervals [RMSSD]) were measured at baseline and after completion of the study. When compared with the AC group, the ET group had a significantly greater increase in both SDNN (15.46±5.02 ms in ET vs 2.37±2.13 ms in AC, P=.016) and RMSSD (17.53±7.83 ms in ET vs 1.69±2.63 ms in AC, P=.003). This increase was seen in both sexes and HF categories. ET improved HRV in older patients with both HFREF and HFPEF.
Heart failure (HF) is a highly prevalent disease that affects 5.8 million adults in the United States, with 600,000 new cases diagnosed each year.1 HF has become a disorder of the elderly, with more than 80% of patients older than 65.1 The majority of older patients with HF have a preserved ejection fraction (HFPEF).2 Older patients with HF, especially those with HFPEF, have not been well represented in clinical trials, which constitute a major clinical challenge since it has been shown that these patients respond differently to novel therapies than younger patients with HF, especially those with reduced ejection fraction (HFREF).3
Heart rate variability (HRV) is defined as beat-to-beat variations in heart rate of individuals in sinus rhythm. HRV is a noninvasive, reproducible, and easy-to-obtain measurement of cardiac autonomic nervous system function and its response to environmental changes.4,5 Reduced HRV generally indicates either attenuation in the autonomic regulation of the sinoatrial node or failure of the sinoatrial node to respond to such regulation.4 Since first described by Hon and Lee in 1965,6 reduced HRV has been seen with normal aging7 and sedentary lifestyle8 and in a number of neurological, metabolic, inflammatory, and cardiovascular disorders.4 In most of these disorders, reduced HRV has been shown to be an independent predictor of death.4,9 Patients with HF in particular often have markedly reduced HRV.4,10–12 Reduced HRV in patients with HF predicts poor prognosis.13 Specifically, impairment in ≥1 HRV parameter has been found to be a significant predictor of sudden cardiac death,14–16 progression of HF and HF death,17 and total mortality.13,18–21
Physical exercise and endurance exercise training (ET) have been shown to improve HRV in various populations, including healthy athletes,8 individuals with sedentary lifestyle,22 survivors of acute myocardial infarction,23 and patients with coronary artery disease who have undergone coronary artery bypass grafting surgery or percutaneous coronary intervention.24 Several studies have examined the impact of ET on HRV in patients with HF showing mostly favorable results.25–30 These studies, however, included only patients who are relatively young (age range: 55–67) and have exclusively HFREF (mean left ventricular ejection fraction range: 29%–36%). The ET applied to the intervention groups in these studies varied in mode, intensity, and duration. Some of these studies did not use control groups, only one study used randomization, and none used any method of blinding. Therefore, the objective of the present study was to examine the effect of supervised endurance ET on HRV in older patients with both HFPEF and HFREF in a randomized, controlled, and single-blinded design.
Methods
Study Population and Design
Our patients were participants in a National Institute of Health–funded clinical trial of ET in patients 65 years or older with both HFPEF and HFREF. The primary outcomes of this trial, exercise capacity and quality of life, have been previously reported.31,32 Neurohormonal activation and autonomic function were among the secondary outcomes of this trial. In this study, we report the impact of ET on HRV as a measure of autonomic function in older patients with HF. All participants who had HRV measured at entry and exit of the trial were included in this study.
The protocol was approved by the Wake Forest University Baptist Medical Center’s institutional review board. During a screening visit, patients were familiarized with the testing environment and procedures and written informed consents were obtained. During a subsequent baseline visit, the outcome measures, including HRV measurements, were obtained. All tests, including HRV, were performed in the morning before participants had any oral intake since midnight, including medications, except for water. After obtaining baseline measurements, participants were randomized to either 16 weeks of an aerobic exercise training group (ET) or an attention control group (AC). Measurements of HRV were repeated after 16 weeks of ET. All testing was performed and analyzed by individuals blinded to participant’s group allocation.
Heart Rate Variability
To measure HRV, each participant was connected to a cardiac monitor and asked to lie down in a quiet room with dimmed lights for 15 minutes. Then, a supine, resting 10-minute electrocardiographic (ECG) recording was obtained and used to calculate time-domain HRV parameters using commercially available software (PREDICT II HRVECG; Arrhythmia Research Technology, Inc, Austin, TX). The ECG recordings were examined by an electrophysiologist who was blinded to participant group allocation. Participants with nonsinus rhythm (including atrial fibrillation and paced rhythm) were excluded because HRV could not be measured in these individuals. ECG recordings with normal sinus rhythm were further examined for nonsinus ectopic beats. All ectopic beats and their subsequent sinus beats were filtered out prior to calculating HRV parameters. Because of the short duration of ECG recording, only the following 2 time-domain HRV parameters could be obtained and used in the analysis: the standard deviation of all normal RR intervals (SDNN) and the root mean square of successive differences in normal RR intervals (RMSSD).
Exercise Training
Participants randomized to ET exercised 3 times per week for 16 weeks under medical supervision for a total of 48 sessions in a dedicated facility.33 Each session lasted 1 hour and consisted of warm-up, stimulus, and cool-down phases. The stimulus phase consisted of walking on a track as well as lower extremity cycling on a Schwinn Air dyne (Nautilus, Inc, Vancouver, WA). Data from the baseline exercise test were used to generate an individualized initial exercise prescription according to standard methods in HF patients. During the initial period of training, participants exercised at 40% to 50% of heart rate reserve (HRR) for the first 2 weeks while the duration of the exercise was gradually increased on each of the 2 modes of exercise (walking and cycling). Over the following several weeks, the intensity of exercise was gradually increased to 60% to 70% HRR and the exercise duration was increased to 15 to 20 minutes on each training mode. The exercise prescription (intensity and duration) was adjusted as needed based on medical considerations and clinical responses.33,34 Missed exercise sessions were made up so that each patient completed no less than 40 of the 48 (>80%) ET sessions.
Participants randomized to the AC group received phone calls every 2 weeks throughout the 16-week follow-up. This served to provide attention and interaction with the study staff. The telephone conversations focused on adherence with the study protocol, reminders and encouragement to keep upcoming study visits, and to ascertain whether there were any new medical events since the prior contact. The telephone conversations intentionally did not address exercise behaviors.
Statistical Analysis
Baseline characteristics were compared between the 2 study groups using 2-sample t test for continuous variables and Fisher exact test for categoric variables. The changes in HRV between baseline and follow-up were calculated for all participants. Comparisons of change in HRV between groups were made using analysis of covariance (ANCOVA) procedure. To maximize precision and control for random differences between study groups, we adjusted for baseline values of HRV, age, sex, pulse pressure, HF category (HFREF vs HFPEF), and baseline measures of exercise capacity and heart rate recovery. Both HRV parameters (SDNN and RMSSD) were non-normally distributed and logarithmic transformation was applied prior to analysis. In order to evaluate whether the effect of ET on HRV differs by age, sex, or HF category, we repeated the ANCOVA procedure while testing for interaction between ET and each one of these variables separately. Two-sided level of significance was set at P<.05 for all analyses. SAS enterprise 9.1 software (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Study Population and Baseline Characteristics
There were 101 participants randomized into the study (50 AC and 51 ET). Of those, 21 participants (10 AC and 11 ET) missed either baseline or follow-up ECG recordings and were excluded from analysis. Among those who had both baseline and follow-up ECG recordings, 14 participants (6 AC and 8 ET) had nonsinus rhythm (atrial fibrillation or permanent pacemaker with paced rhythm) and were excluded from analysis. The remaining 66 participants (35 AC and 31 ET) were included in the analysis. Baseline characteristics of these participants are described in Table I and were compared between the two study groups. Generally, these participants were elderly and representative of patients with HF in the community in age, sex distribution, and the proportion of HFPEF. These participants, however, had randomly higher-than-usual systolic blood pressure and pulse pressure, and lower-than-usual rates of usage of certain medications including β-blockers and angiotensin-converting enzyme (ACE) inhibitors. As evident from Table I, ET and AC groups did not differ across a broad range of demographics, comorbid conditions, heart failure characteristics, medication use, and measures of exercise capacity and HRV, all indicating an effective randomization. The only exception was the random difference in pulse pressure, which was higher in the AC group than in the ET group (Table I).
| Characteristics at Baseline | AC Group (n=35) | ET Group (n=31) | P Value |
|---|---|---|---|
| Age, y | 70.1±5.6 | 68.0±4.8 | .099 |
| Men | 13 (37.1) | 11 (35.5) | >.9 |
| Caucasians | 28 (80) | 27 (87) | .521 |
| Body weight, kg | 77.7±14.6 | 81.9±14.7 | .242 |
| Body mass index, kg/m2 | 28.6±6.1 | 29.4±5.4 | .555 |
| Body surface area, m2 | 1.87±0.20 | 1.93±0.19 | .231 |
| Heart rate, beats per min | 71.9±10.5 | 76.7±14.5 | .132 |
| Systolic blood pressure, mm Hg | 150.6±19.9 | 143.0±18.3 | .116 |
| Diastolic blood pressure, mm Hg | 78.7±8.3 | 81.5±9.0 | .208 |
| Pulse pressure, mm Hg | 71.9±16.2 | 61.5±16.2 | .013 |
| History of diabetes | 10 (28.6) | 5 (16.1) | .256 |
| History of pulmonary edema | 15 (42.9) | 8 (25.8) | .198 |
| β-Blocker use | 4 (11.4) | 6 (19.3) | .497 |
| Calcium channel blockers use | 12 (34.3) | 9 (29.0) | .792 |
| Digoxin use | 12 (34.3) | 9 (29.0) | .792 |
| ACE inhibitor use | 17 (48.6) | 14 (45.2) | .810 |
| Exercise peak oxygen uptake, mL/kg/min | 12.9±2.6 | 14.0±2.7 | .11 |
| Exercise peak work load, W | 47.9±16.5 | 53.6±18.6 | .189 |
| Exercise time, min | 6.3±2.8 | 7.1±3.2 | .172 |
| HRR at 1 min, beats per min | 20.0±11.8 | 26.3±13.6 | .065 |
| SDNN, ms | 30.2±17.7 | 31.6±21.0 | .776 |
| RMSSD, ms | 20.4±15.5 | 24.7±24.7 | .406 |
| Left ventricular ejection fraction >40% | 20 (57.1) | 17 (54.8) | 1.0 |
| NYHA class II | 21 (60) | 19 (61.3) | >.9 |
| NYHA class III | 14 (37.1) | 12 (38.7) | >.9 |
- Abbreviations: AC, attention control; ACE, angiotensin-converting enzyme; ET, exercise training; HRV, heart rate variability; NYHA, New York Heart Association; RMSSD, root mean square of successive differences in normal RR intervals; SDNN, the standard deviation of all normal RR intervals. Values are expressed as mean±standard deviation or number (percentage).
Participants randomized to ET adhered well to the intervention, with an average number of ET sessions attended being 44.2±6.7 and a median compliance (number of sessions attended divided by number of sessions offered for each participant) being 89.1% (interquartile range, 84.6–93.3). Missed sessions were often due to acute illness or hospitalization.
Effect of ET on HRV
Following the 16-week intervention, the ET group had a significantly greater increase in HRV compared with that in the AC group (Table II). The mean increase in SDNN was 15.46±5.02 ms in the ET group vs 2.37±2.13 ms in the AC group (P=.016), and the mean increase in RMSSD was 17.53±7.83 ms in the ET group vs 1.69±2.63 ms in the AC group (P=.003) (Figure 1). This effect of ET on HRV did not differ by sex or HF category (all interaction P values >.05). There was, however, a trend toward greater improvement in SDNN in HFREF than in HFPEF (interaction P=.06) (Figure 2). The increase in HRV measures did not significantly correlate with different measures of exercise capacity, including peak exercise oxygen consumption, peak exercise work load, or exercise time. There was, however, a positive correlation between the increase in HRV and the number of ET sessions attended, although such correlation did not reach statistical significance (r=0.23 [P=0.22] for change in SDDN, and r=0.25 [P=0.16] for MSSD).
| HRV | AC Group | ET Group | P Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Change | Baseline | Follow-Up | Change | ||
| SDNN | 30.2±17.7 | 32.6±15.4 | 2.4±2.13 | 31.6±21.0 | 47.0±36.5 | 15.5±5.02 | .016 |
| RMSSD | 20.4±15.5 | 22.1±15.9 | 1.7±2.62 | 24.7±24.7 | 42.3±48.3 | 17.5±7.85 | .003 |
- Abbreviations: AC, attention control; ET, exercise training; RMSSD, the root mean square of successive differences in normal RR intervals; SDNN, the standard deviation of all normal RR intervals. Data are expressed as mean±standard deviation. P Values represent comparison of least-squared means of the change in heart rate variability (HRV) following adjustment for age, sex, heart rate, pulse pressure, β-blocker usage, baseline exercise capacity (peak exercise oxygen uptake, peak work load, exercise time, and heart rate recovery during baseline exercise stress testing), baseline HRV values, and heart failure category (preserved vs reduced ejection fraction).
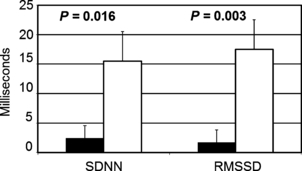
Raw, unadjusted mean changes (±standard deviation) in heart rate variability (HRV) parameters in the two study groups. P values represent comparison of the transformed data after adjusting for age, sex, pulse pressure, heart failure category (preserved vs reduced ejection fraction), and baseline values of HRV and exercise capacity. Black boxes represent the attention control group; white boxes represent the exercise training group; SDNN indicates the standard deviation of all normal RR intervals; RMSSD, root mean square of successive differences in normal RR intervals.
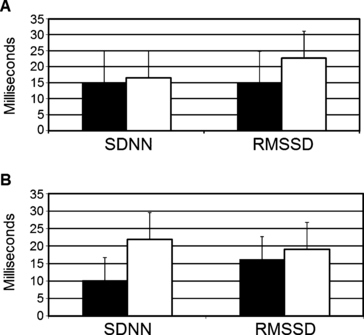
Raw, unadjusted mean changes (±standard deviation) in heart rate variability parameters in the exercise training group stratified by sex (panel A: black boxes represent men; white boxes represent women) and by heart failure categories (panel B: black boxes represent heart failure and preserved ejection fraction; white boxes represent heart failure and reduced ejection fraction). SDNN indicates the standard deviation of all normal RR intervals; RMSSD, root mean square of successive differences in normal RR intervals.
Discussion
In this study, we showed that endurance ET significantly improved HRV in older patients with HF regardless of age, sex, or HF category (HFREF or HFPEF). This improvement in HRV is possibly dose-dependant and did not correlate with measures of exercise capacity. To our knowledge, this is the first randomized, controlled, and single-blinded study to examine the impact of ET on HRV in older adults with both HFPEF and HFREF. In spite of the challenges in adherence that usually face outpatient exercise studies, we were able to achieve close to a 90% compliance rate. Our findings are in agreement with those from earlier studies that have examined the impact of ET on HRV in relatively younger populations who exclusively had HFREF.25–30 Therefore, this study supports the contention that the favorable effect of ET on HRV is robust in a variety of HF populations, including the elderly and those with HFPEF, who were not targeted in earlier studies.
Patients with HF often have cardiac autonomic dysfunction manifested with reduced HRV.4,10 This is thought to be related to neurohormonal activation and attenuation of cardiac vagal tone.11,12 Elderly patients with HF have even more impaired HRV that is explained in part by age-related autonomic dysfunction7 and by their associated comorbidities such as hypertension, diabetes mellitus, atherosclerotic heart disease, and inflammatory conditions.4 In addition, elderly patients with HF are more often women, have HFPEF, and have a higher burden of noncardiac comorbidities. They have repeatedly shown to respond differently to various drug therapies and other interventions, including ET. Therefore, it was reasonable to expect that impaired HRV in older patients with HF, especially HFPEF, might respond differently to ET than in younger patients with HFREF. With the rising incidence of HF among the elderly, especially HFPEF, it was important to ascertain the benefit of ET on cardiac autonomic dysfunction in this patient population.
The mechanism by which ET improves HRV is not well understood. It is believed, however, that ET increases vagal tone and reduces sympathetic cardiac influence, resulting in improvement in HRV.9,35 At least 2 mediators are thought to play a role in increasing cardiac vagal tone in response to ET: nitric oxide (NO) and angiotensin II. NO is thought to have a direct effect on cardiac vagal tone and an indirect effect on sympathetic cardiac influence.36 ET has been shown to improve NO bioavailability and endothelial function.37 The second mediator, angiotensin II, is a known inhibitor of cardiac vagal activity.38 Athletes and physically trained individuals have been shown to have lower levels of plasma renin activity, and therefore lower angiotensin II levels, than nonathletes and untrained individuals.39 Nevertheless, determining the exact mechanism by which ET improves HRV and autonomic cardiac regulation requires additional research studies.
Although it is well-established that impaired HRV in patients with HF is a significant predictor of morbidity and mortality,13,14,17,19,20 the clinical implication, namely improving exercise capacity and survival, of improving HRV in this patient population is still not established. In this study, we did not find any significant correlation between improvement in HRV and changes in exercise capacity. Such a correlation was reported by Ponikowski and colleagues,40 however, who demonstrated a significant correlation between improvement in HRV parameters and improvement in ventilatory response to ET. Therefore, it remains unclear whether improvement in autonomic dysfunction is a mediator to the improvement in exercise capacity in patients with HF. Our study was not designed to examine survival, so we were unable to examine the correlation between change in HRV and survival. However, survival benefit of improving HRV in response to ET was demonstrated in a small pilot study by Larsen and colleagues,25 who reported a trend toward better survival in patients with HF who showed improvement in HRV in response to ET. Future studies are needed to determine whether improvement in HRV indicates improvement in exercise capacity and survival.
Study Limitations
Although our study participants resemble the general population with HF in several aspects including age, sex distribution, and proportion of HFPEF,1 because this study was conducted several years ago,31,32 the rates of usage of certain medications including β-blockers and ACE inhibitors (5% and 16%, respectively) were lower than current rates. It is unknown whether greater use of these medications would modify the effect of ET on HRV. Considering the relatively small sample size and the low usage of these medications, we were unable to confidently test whether these medications altered the impact of ET on HRV. The high systolic blood pressure and pulse pressure in our study sample could also have altered their response to ET. These high values of systolic and pulse pressures could partially be explained by the high proportion of elderly women with HFPEF among our participants, and partially by the fact that these participants were recruited several years ago before the implementation of newer guidelines that advocate more aggressive management of hypertension. In addition, 2 patient groups were excluded from our study by design, which may affect the generalizability of our results. The first group includes those with atrial fibrillation and permanent pacemaker with paced rhythm for whom HRV could not be measured. It is known that patients with atrial fibrillation often have significant autonomic dysfunction.41 However, it remains unknown how ET would impact autonomic dysfunction in these patients. The second group includes those who were unable to undergo exhaustive exercise testing and outpatient supervised ET (those who are nonambulatory, residing in a nursing home, or recently hospitalized). Therefore, it is not clear whether lower-intensity exercise or unsupervised home-based exercise tailored for these patients in particular would prove beneficial.
We used a relatively short-term recording of HRV (10 minutes). While this method of HRV measurement has been validated,4 long-term recording (24-hour) remains the gold standard. Since these measurements were done in resting conditions during morning hours, we do not know whether the improvement seen in HRV is sustained during adrenergic surges throughout the day. Lastly, in this study, we only used time-domain parameters. While time-domain measurements correlate well with frequency-domain measurements of HRV, such correlation is weaker with shorter recording times.4
Although this study was completed a number of years ago,31,32 its findings are still significant. It fills a critical gap in our knowledge about the impact of ET on HRV in a growing population of older patients with HF, especially those with HFPEF. It shows that autonomic dysfunction in older patients with HF can be safely improved by ET.
Conclusions
ET improves HRV in older patients with HF. This effect is equally significant in men and in women, and may be greater in patients with HFREF than in HFPEF. Further studies are needed to determine the clinical implication of these findings.
Acknowledgments
Acknowledgements and disclosures: National Institutes of Health (NIH) R37AG18915 and RO1AG12257; The Claude D. Pepper Older Americans Independence Center of Wake Forest University. NIH P30AG21332 and NIH MO1RR07122; General Clinical Research Center of the Wake Forest University School of Medicine. NIH 5T32HL087730-02; Training Grant in Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. The sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Khalil Murad, MD: none; Peter H. Brubaker, PhD: Boston Scientific CRM>10K; David M. Fitzgerald, MD: none; Timothy M. Morgan, PhD: none; David C. Goff, Jr., MD, PhD: has served as a consultant for (<$10K) and received grant support from Merck (<$10K), and as a DSMB member for a trial funded by Takeda (<$10K). Elsayed Z. Soliman, MD, MS, MSc: none; Joel D. Eggebeen, MS: none; Dalane W. Kitzman, MD: Has served as consultant for and/or received grant support from Synvista (>$10K), Bristol-Meyers-Squibb (>$10K), Novartis (>$10K), Boston Scientific (>$10K), Relypsa (>$10K), Forest Laboratories, and Medtronic.




