A Comparison of 16 Weeks of Continuous vs Intermittent Exercise Training in Chronic Heart Failure Patients
Abstract
The authors compared the effects of continuous (CON) and intermittent (INT) exercise training programs on functional capacity, quality of life (QOL), and cardiac function in 23 congestive heart failure patients. Patients were randomized to CON exercise training (n=13; aged 66±7 years; peak oxygen consumption [VO2], 12.4±2.5 mL/kg/min; weight, 83±12 kg; left ventricular ejection fraction [LVEF], 29.5%±7.2%) or INT exercise training (n=10; aged 59±11 years; VO2, 12.2±6.5 mL/kg/min; weight, 87±24 kg; LVEF 27%±7.9%). These groups completed 16 weeks of stationary cycling at 70% VO2 thrice weekly for 30 minutes continuously or 60 minutes (60 seconds work:60 seconds rest) intermittently; both groups completed the same absolute volume of work. Three QOL questionnaire responses, VO2, LVEF, and regional tissue Doppler were quantified. After exercise training, VO2 increased by 13% in the CON group (P=.12) and significantly by 21% in the INT group (P=.03), although not significantly between the groups (P=.72). In the CON group, Minnesota Living With Heart Failure score improved at 16 weeks (P=.02), while in the INT group, Hare–Davis scores improved (P=.02). Cardiac volumes, resting and peak LVEF, contractile reserve, and tissue velocities were all unchanged from baseline. Intermittent exercise may improve functional capacity to a greater extent than continuous exercise. QOL changes were variable between groups. Congest Heart Fail.
In patients with stable heart failure (HF) taking optimal cardiac medication, exercise training is usually recommended 3 to 5 days per week for 20 to 60 minutes.1 Aside from the nature of the training activity, the effects of training may vary with different dose parameters, specifically program length, session duration and frequency, and workload or intensity.2 The exercise specialist should attempt to progress the patient by first increasing the session duration and subsequently increasing exercise intensity. In the most severely impaired patients, with initial exercise intolerance, sessions may begin at a duration of 3 to 5 minutes with 3 or 4 sessions completed during the course of the day,3 although practically, residential or home exercise are more suited to administration of such a program.
Continuous vs Intermittent Exercise Training
Aerobic training (eg, walking for up to 2 hours a day and bicycle exercise at 70% to 80% peak capacity for 40 minutes on 4 days per week during 2 months) has been shown to produce major (23%) improvement of functional capacity in congestive heart failure (CHF) patients.3 Sustained workloads at this level are unsuitable for patients with severe HF, whose functional exercise capacity is very low. In these patients, aerobic exercise training at low workload has been shown to increase peak aerobic capacity, with improvements in skeletal muscle and vascular flow capacity of the lower limb while exposing the left ventricle to lower wall stress.4 Alternatively, high workloads may be feasible for repeated intermittent periods, compared with a standard workload for 15 minutes at 30-second bursts at higher workloads (separated by 60-second recovery periods at virtually unloaded resistance) has demonstrated an equivalent of about 75% of peak aerobic capacity and lower increase of rate-pressure product, but a greater increment of blood lactate (implying greater peripheral muscle stress).5 This approach has been shown to be efficacious in HF patients,5 and interval stress has been shown to be as effective as continuous workloads in older, healthy, and post–coronary artery bypass surgery populations.6 In a systematic review of 81 HF exercise training studies, only 7 used an intermittent exercise training protocol and only 2 of these reported peak oxygen update (VO2) changes of 10%7 and 20%,5 respectively, compared with a 16.5% overall change in continuous studies.2
Central vs Peripheral Adaptations
In a review of more than 80 HF exercise training studies, only a handful revealed evidence of improved left ventricular function following training.8–10 In contrast, numerous reports have demonstrated improved endothelial function after exercise training in HF cohorts.11,12
The underlying theory is that higher-intensity, intermittent stress is more likely to promote peripheral adaptations and produce concurrent improvements in functional capacity and quality of life.
We therefore sought to determine whether intermittent exercise training, at the same absolute volume would produce similar adaptations to a continuous exercise program in patients with chronic HF.
Methods
Patient selection. We studied a total of 23 CHF patients undergoing exercise training. Patients were defined by left ventricular ejection fraction (LVEF) <35% and at least 2 minor and 1 major Framingham criteria.14 Local ethical committee approval was obtained. Patients were randomly assigned to an intermittent (INT) or continuous (CON) exercise group, and patients were stratified for baseline peak VO2 as this is a strong predictor of prognosis.15 Patients were excluded if they demonstrated primary valvular disease, unstable angina, or an inability to exercise. All patients had been taking stable medical management for at least 1 month prior to baseline testing. Table I lists the clinical characteristics of the patients, including Framingham HF criteria, comorbid diseases, and drug therapy. Most patients were in New York Heart Association (NYHA) functional class III.
| Demographic | INT | CON | P Value |
|---|---|---|---|
| Age, y | 59.1±11 | 62.9±9.3 | .38 |
| Women, No. (%) | 2 (20) | 0 (0) | .15 |
| Body mass index, kg/m2 | 28.9±6.1 | 28.1±3.0 | .44 |
| Peak VO2, mL/kg/min | 12.2±6.5 | 12.4±2.5 | .91 |
| Ejection fraction, % | 27±8 | 29.5±7.2 | .47 |
| Clinical | |||
| Framingham heart failure criteria, No. (%) | 10 (100) | 10 (100) | 1.00 |
| NYHA functional class II/III | 4/6 | 4/6 | 1.00 |
| Diabetes, No. (%) | 4 (40) | 2 (20) | .36 |
| Atrial fibrillation, No. (%) | 4 (40) | 4 (40) | 1.00 |
| Previous MI, No. (%) | 5 (50) | 7 (70) | .39 |
| Smoking, No. (%) | 2 (20) | 5 (50) | .18 |
| Shortness of breath, No. (%) | 9 (90) | 10 (100) | .33 |
| Edema, No. (%) | 8 (80) | 9 (90) | .56 |
| Medication, No. (%) | |||
| β-Blockade | 10 (100) | 8 (80) | .15 |
| ACE inhibitor | 10 (100) | 9 (90) | .33 |
| Aspirin | 7 (70) | 5 (50) | .39 |
| Statin | 8 (80) | 6 (60) | .36 |
- Abbreviations: ACE, angiotensin-converting enzyme; CON, continuous exercise group; INT, intermittent exercise group; MI, myocardial infarction; NYHA, New York Heart Association; VO2, peak oxygen consumption.
Exercise Testing. All patients underwent cardiopulmonary exercise testing on a cycle ergometer, using a 10-W/min stepped protocol. The electrocardiogram was continuously monitored for ST-segment changes and arrhythmias, blood pressure, and 12-lead electrocardiograms were recorded before exercise, every 2 minutes during the test, and during the recovery period after exercise. Tests were symptom-limited, with the usual end points being dyspnea and leg fatigue. Peak heart rate and workload were recorded immediately upon cessation of the exercise test, and these values were used to generate the initial training workload. VO2 was obtained by breath-by-breath analysis of expired gas (V29C Sensormedics, Yorba Linda, CA), averaged over 20-second intervals. Every 3 sequential measurements were averaged and VO2 was defined as the greatest mean value during exercise.
Exercise Echocardiography. Before and immediately after the exercise VO2 test, 2-dimensional echocardiography was recorded with commercially available equipment (System FiVe, General Electric-Vingmed, Milwaukee, WI) with the patient in the supine left lateral decubitus position. Images were obtained using a 3.5-MHz transducer at 16-cm depth in 5 standard views at rest and after exercise. Contractile reserve (CR) was defined as the increment between resting and peak exercise LVEF.
Myocardial Doppler Analysis. Digital images were acquired with a commercially available ultrasound system (Vivid 7, General Electric-Vingmed), at a depth of 16 cm in the 3 standard apical views. Three cardiac cycles from each 2-dimensional echo view were analyzed using standard software (Echopac, General Electric-Vingmed) by readers blinded to visual wall motion analysis.
Peak long-axis systolic velocities within each segment16 were obtained by locating the sample volume in the middle of each segment at rest. To overcome regional variations, the lateral systolic velocity and septal systolic velocity segments were analyzed separately, and early diastolic velocities were also measured at the septal and lateral aspects of the mitral annulus. The mean of systolic and early diastolic velocities was also analyzed. Although not site-specific, tissue Doppler is robust and reproducible, and is predictive of outcome.17,18 Our group has previously assessed the reproducibility of systolic velocity (Sm) by tissue Doppler analysis, and the intra-observer variability for Sm was 0.4±0.4 cm/s (coefficient of variation 8%) and the interobserver variation is 0.6±0.5 cm/s (coefficient of variation 5%) in our institution.16,19
Myocardial strain-rate analysis was derived by analysis of tissue Doppler data using standard software (Echopac Version 6.2; General Electric-Vingmed). Strain rate data were measured as the slope of the regression line based on all the velocity estimates between 2 points in the myocardium separated by distance r (in this study, 12 mm), corresponding to the region that is used to estimate local contraction.20 The midsegment from the septal and inferior walls was assessed.
Endothelial Function.
Flow-Mediated Dilatation. Longitudinal ultrasound scans of the brachial artery were performed using an ATL HDI5000 ultrasound system (Philips/ATL, Bothell, WA) with a 12-MHz imaging probe. Focus was set just beyond the posterior arterial wall.
Patients were allowed to rest for approximately 5 to 10 minutes before the scan. A resting scan was performed with approximately 10 seconds of pulsed Doppler flow at 60°C to 70°C to the artery. The artery was then occluded with a pneumatic cuff for 4.5 minutes. The cuff was placed on the forearm for brachial artery scans and inflated to 250 mm Hg. After the allowed time, the cuff was released and pulsed Doppler was again recorded for approximately 10 seconds. Beginning at 60 seconds post–cuff deflation, which allows for release of nitric oxide and a vasoactive response, a second scan was acquired. Using HDILab software, the percentage change in dilatation was measured.21
Intima–Media Thickness. The carotid arteries were scanned using high frequency ultrasound in a supine position with the head directed away from the side of interest and the neck extended slightly. Both left and right arteries were scanned in the anterior, lateral, and posterior planes, and gated digital cine-loops of 2 cardiac cycles were acquired for offline analysis as described by Fathi and Marwick.21 Measurements were performed on a PC using the IMT v3.17 plug-in for HDILab software v1.91c (Philips/ATL). The software calculates the mean and standard deviation for the intima–media thickness in the region of interest box, which again is measured on the R wave of the electrocardiogram. The mean of the 3 planes in both the left and right carotid arteries were recorded for comparison between groups.21
QOL Measures. Three previously validated questionnaires assessing QOL were completed by all patients at baseline and at 16 weeks. The Minnesota Living With Heart Failure Questionnaire (MLWHF)22 is specific to HF; its 21 questions give a total score and also a physical and emotional dimension score. The Hare–Davis Cardiac Depression Scale23 is a general tool administered to the cardiac patient population. As with the MLWHF questionnaire, lower scores on Hare–Davis Cardiac Depression Scale indicate that patients perceive their health to be improving. Finally, we followed the 8 dimensions of the 36-Question Short-Form (SF-36) General Health Questionnaire.24
Exercise Training. All patients undertook 16 weeks of supervised cycle ergometer exercise training at 60 RPM, at a frequency of three 30-minute CON or 60-minute INT sessions per week, and at a workload corresponding to an initial intensity of 60% to 70% peak VO2 from the cardiopulmonary exercise test. These groups completed 16 weeks of stationary cycling at 70% peak VO2, thrice weekly for 30-minute CON or 60-minute (60 seconds of work:60 seconds of rest) INT sessions; both groups completed the same absolute volume of work. Exercise intensity was uptitrated by 2 to 5 W/week, provided that patients were tolerating the cycle training. In patients in paced rhythm or experiencing frequent ectopy, rate of perceived exertion (RPE) was also used to guide exercise intensity, using a target RPE of 3 to 5 (moderate to hard) on the modified Borg scale.25 In patients who were most limited by shortness of breath, a respiratory rate <30/min was used to adjust exercise intensity.
Sample Size Calculation. Based on the 16.8%±8% change in VO2 from the studies included in the meta-analysis of HF exercise training studies,2 we calculated with 80% power and 5% significance that 9 patients per group were required.
Statistical Analysis. Results are reported as mean±standard deviation. Correlations with change in VO2 were performed with clinical variables, resting and peak LVEF, contractile reserve, hemodynamic responses to stress, peak systolic and early diastolic tissue velocities, and other measures of cardiac function. Repeated-measures analysis of variance with Bonferroni correction were used to determine changes in primary end points with time. Statistical analyses were performed with SPSS software (IBM Corporation, Armonk, NY).
Results
Baseline. Table I shows that there were no significant differences between baseline clinical, demographic, and pharmacologic characteristics of patients in both groups. All patients completed the training and there were no adverse events or hospitalizations during the study.
Functional Capacity. After exercise training, VO2 increased by 13% in the CON group (P=.12) and significantly by 21% in the INT group (P=.03). The margin of improvement between groups was unremarkable at baseline and 16 weeks (P=.49). The minute ventilation-carbon dioxide production slope was reduced after training in the INT group (P=.03) and ventilatory threshold (VT) was improved in the CON (P=.01) and INT groups (P=.02). Other cardiopulmonary variables were unchanged. Table II shows this and other cardiopulmonary variables at baseline and 16 weeks in both groups. Five of 13 CON patients (38%) and 6 of 10 INT patients (60%) demonstrated >5% improvement in VO2.
| Cardiopulmonary Characteristic | INT | INT, 16 Weeks | P Value Between INT Groups | CON | CON, 16 Weeks | P Value Between CON Groups | P Value INT vs CON, Baseline | P Value INT vs CON, 16 Weeks |
|---|---|---|---|---|---|---|---|---|
| VO2, mL/kg/min | 12.2±6.5 | 14.7±4.5 | .03 | 12.4±2.5 | 14±4 | .12 | .91 | .72 |
| VT, mL/kg/min | 7.3±1.6 | 10.2±3.9 | .02 | 7.4±1.4 | 9.2±2.0 | .01 | .95 | .53 |
| VE/VCO2 slope | 35.5±6.4 | 30.2±4.4 | .03 | 32±4.5 | 30.3±5.6 | .26 | .18 | .96 |
| Peak RQ | 1.07±0.08 | 1.13±0.12 | .07 | 1.09±0.05 | 1.11±0.04 | .37 | .59 | .67 |
| Peak HR | 98±35 | 107±30 | .17 | 115±26 | 118±28 | .62 | .24 | .39 |
- Abbreviations: CON, continuous exercise group; HR, heart rate; INT, intermittent exercise group; RQ, respiratory quotient; VE/VCO2, minute ventilation-carbon dioxide production; VO2, peak oxygen consumption; VT, ventilatory threshold.
Cardiac Function.
Global Cardiac Function. Resting LVEF (P=.53) and immediate post-exercise (P=.93) LVEF was unchanged in the INT group after 16 weeks of exercise training. The same was true of LVEF in the CON group at rest (P=.41) and post-exercise (P=.23). Table III shows resting and peak LVEF values at baseline and post-exercise in both groups.
| INT | INT, 16 Weeks | P Value Between INT | CON | CON, 16 Weeks | P Value Between CON | P Value Between Groups, Baseline | P Value Between Groups, 16 Weeks | |
|---|---|---|---|---|---|---|---|---|
| Resting ESV, mL | 132±26 | 119±47 | .35 | 123±44 | 115±49 | .74 | .61 | .88 |
| Resting EDV, mL | 184±34 | 168±55 | .42 | 169±49 | 166±55 | .34 | .41 | .95 |
| Resting LVEF | 27±7.9 | 32.8±9.7 | .53 | 29.5±7.2 | 29.3±12.2 | .41 | .47 | .49 |
| Post-exercise LVEF | 33.7±11.2 | 34.2±13.2 | .93 | 30.8±9.2 | 34.2±13.3 | .23 | .54 | .39 |
| Contractile reserve | 1.3±8.5 | 6.1±4.7 | .97 | 5.7±10.4 | 5.8±6.6 | .08 | .31 | .91 |
- Abbreviations: CON, continuous exercise group; EDV, end-diastolic volume; ESV, end-systolic volume; INT, intermittent exercise group; LVEF, left ventricular ejection fraction.
Regional Cardiac Function. Tissue Doppler velocities and strain/rate: Both systolic and diastolic tissue velocity (cm/s) showed unremarkable differences between groups and between timeframes. Both systolic and diastolic tissue velocities and strain and strain rate values at baseline and 16 weeks reflected impaired regional cardiac function. Table IV demonstrates tissue velocity, strain, and strain rate values.
| INT | INT, 16 Weeks | P Value Between INT | CON | CON, 16 Weeks | P Value Between CON | P Value Between Groups, Baseline | P Value Between Groups, 16 Weeks | |
|---|---|---|---|---|---|---|---|---|
| Systolic velocity, cm/s | 3.53±1.6 | 3.4±1.7 | .84 | 3.75±1.25 | 3.6±1.11 | .42 | .74 | .70 |
| Diastolic velocity, cm/s | 3.92±1.8 | 3.03±1.6 | .16 | 4.04±1.71 | 4.2±1.6 | .77 | .89 | .26 |
| Strain rate | −0.65±0.54 | −0.65±0.51 | .96 | −0.92±0.93 | −0.72±0.39 | .58 | .95 | .74 |
| Strain, % | −11.5±9.5 | −11.3±7.9 | .96 | −11±6.7 | −14.5±9 | .38 | .89 | .41 |
- Abbreviations: CON, continuous exercise group; INT, intermittent control group.
Quality of Life.
Minnesota Living With Heart Failure. Table V shows MLWHF QOL scores. In the INT group, MLWHF score was unchanged after exercise training (P=.11). In the CON group, MLWHF total score and emotional dimension improved after exercise training (both P=.02). There were no significant differences between groups.
| INT | INT, 16 Weeks | P Value Between INT | CON | CON, 16 Weeks | P Value Between CON | P Value Between Groups, Baseline | P Value Between Groups, 16 Weeks | |
|---|---|---|---|---|---|---|---|---|
| Minnesota total score | 41.9±21.4 | 30.1±17.3 | .11 | 47.2±14.1 | 34.6±19.5 | .02 | .53 | .59 |
| Minnesota physical | 14.2±8.2 | 11.2±8.2 | .19 | 15.4±8.7 | 10.1±7.1 | .17 | .74 | .75 |
| Minnesota emotional | 9.9±6.2 | 6.7±5.3 | .05 | 11.4±5.5 | 5.2±3.0 | .02 | .80 | .51 |
| Hare–Davis Cardiac Scale | 102±12.4 | 84±27 | .02 | 77.3±21.1 | 74±12.4 | .20 | .03 | .27 |
- Abbreviations: CON, continuous exercise group; INT, intermittent control group.
Hare–Davis Cardiac Depression Scale. In the CON and INT groups, Hare–Davis score improved (P=.05 and P=.02, respectively) and the difference between groups at baseline was better (lower) in the CON group (P=.03) (Table V).
Short-Form 36. The 8 dimensions of the SF-36 questionnaire are illustrated in Figure 1 and Figure 2. In the CON group, only vitality (VT) showed significant improvements at 16 weeks (P=.03). In the INT group, only emotional role (RETS) showed a significant improvement at 16 weeks (P=.05). There were no significant differences between groups in any of the 8 dimensions both at baseline and 16 weeks.
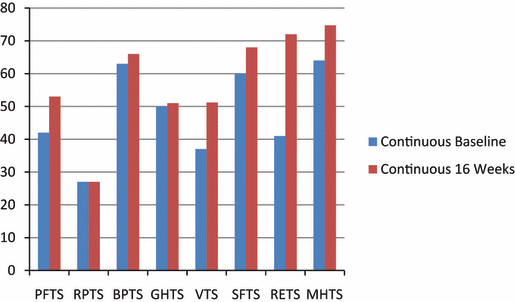
Baseline and 16-week SF-36 dimensions for the continuous exercise training group.
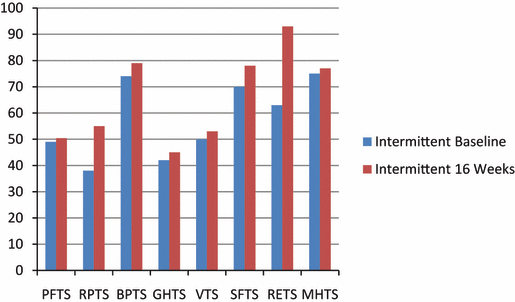
Baseline and 16-week SF-36 dimensions for the intermittent exercise training group.
Endothelial Function.
Flow-Mediated Dilatation. Flow-mediated dilatation (FMD) values in the CON group demonstrated a mean change of 8%±5.2% at baseline and 8.3%±5.4% at 16 weeks (P=.79). The INT group’s corresponding values were 7.4%±5.5% at baseline and 9.2%±3.4% at 16 weeks (P=.14). There were no differences in FMD values between groups at baseline (P=.79) or at 16 weeks (P=.25).
Intima–Media Thickness. FMD values in the CON group demonstrated a mean change of 0.66±0.1 at baseline and 0.66±0.14 at 16 weeks (P=.39). The INT group’s corresponding values were 0.69±0.11 at baseline and 0.72±0.10 at 16 weeks (P=.30). There were no differences in IMT values between groups at baseline (P=.58) or at 16 weeks (P=.79).
Discussion
There was a greater magnitude of change in functional capacity in the INT group compared with the CON group. The magnitude of change for CON and INT (13% and 21%, respectively) was consistent with similar studies of 16-week duration.2 Similarly, measures of central adaptations, namely global (LVEF) and regional measures of cardiac function, such as systolic and diastolic tissue velocities, strain, and strain rate values at baseline and 16 weeks, all remained unchanged after exercise training. Measures of peripheral or endothelial function such as FMD and IMT reflected poor hyperemia-induced dilatation and mild to moderate thickening of the media-adventitia, respectively, and both FMD and IMT were essentially unchanged after exercise training. There was some evidence of improved QOL measures in both groups after exercise training, which is consistent with previous work.26 The intervention had good adherence, with no patient withdrawal or adverse events, and was therefore considered safe.
Previous work from our group has shown that this exercise prescription delivered during a 4-month period is sufficient time to elicit changes in VO2 and QOL.27 Our work also demonstrates that INT exercise training is at least as effective as CON exercise training in chronic HF patients, provided the same absolute volume of work is completed. It should also be considered that, in this patient cohort, 16 weeks is perhaps sufficient time to accomplish optimal (>15%) functional capacity improvements as suggested in a systematic review.2 Unpublished data from this review suggest that studies of 8-weeks’ duration yielded only a 13% increment in VO2, while a 16-week program is likely to yield a 21% VO2 increase. An exception to this timescale may be residential programs with multiple daily exercise training sessions such as that of Dubach,3 who achieved a 23% increase in peak VO2 in 8 weeks. In this same review, QOL improvements were consistent with a systematic review in HF patients.26 It may be that exercise intensity, in this case, 60% to 70% VO2, was insufficient to elicit changes in endothelial function, and previous work has shown high-intensity (90% VO2) to be superior to moderate-intensity training.28
The implications for this work are greatest for the moderate to severe classification of HF patients (NYHA class III). These patients are more likely to tolerate an intermittent rather than a CON exercise training program. Furthermore, previous analyses of exercise training in this patient group suggest that older, least fit, lower NYHA class, lower LVEF patients and patients with ischemic cardiomyopathy are most likely to benefit in terms of mortality and morbidity from an exercise training program.29 Previous work has shown that a 4-month period may be insufficient time to elicit changes in LVEF.30
From a mechanistic standpoint, it is unclear why adaptations to exercise training occurred in our patients. One may postulate that although the same volume of work was completed, metabolism, and specifically the cardiovascular system, were taxed for a longer duration (60 vs 30 minutes). This may explain the greater changes in the intermittent group. Significant VO2 changes CON (13%) and INT (21%), respectively, are likely to have a noticeable effect in the patients themselves. Nevertheless, these functional capacity changes were not associated with significant alterations in cardiac or endothelial measures. This supports the notion that a term longer than 16 weeks may be necessary for a mechanism of adaptation to be identified as a result of exercise training in HF cohorts.
Limitations. This study was limited by the small sample size consisting of relatively young patients (mean age, 59 years [INT] and 63 years [CON]); the age difference is nearly 4 years and this likely explains the nonsignificant (13%) change in VO2 of the CON group. Moreover, the results may not be applicable to elderly patients. We also calculated sample size based on a 16.8% change in VO2. Other limitations were that only 2 of 23 patients were women and the study was a single-center trial.
Conclusions
INT exercise training should be considered at least an equally valuable tool for severe HF patients unable to complete continuous exercise, provided a similar volume of work is completed.
Financial Disclosures: Neil Smart received a scholarship from the National Heart Foundation of Australia and a grant from Mutual Benefit Fund, Australia.




