Des-gamma-carboxyprothrombin in patients with hepatocellular carcinoma and liver cirrhosis
Conflict of interest: none.
Abstract
OBJECTIVES: To ascertain serum and tissue expression of des-gamma-carboxyprothrombin (DCP) in patients with hepatocellular carcinoma (HCC) and liver cirrhosis and clarify the relationship between DCP expression and prognosis.
METHODS: Expression of DCP in tissues was evaluated with immunohistochemical staining using anti-DCP antibody in 74 patients with a single primary HCC nodule and liver cirrhosis. Their serum DCP levels were determined using an enzyme immunoassay with a double antibody sandwich system.
RESULTS: Positive DCP expression in cancerous and non-cancerous tissues was related to a worse prognosis for patients with HCC and liver cirrhosis. The combined evaluation of tissue DCP expression and serum DCP level showed that prognosis was the worst for patients with positive tissue DCP expression and a high serum DCP level. Univariate analysis indicated that a lower 5-year survival rate was significantly correlated with positive tissue DCP expression, a high serum DCP level and the combined factor of positive tissue DCP expression and a high serum DCP level. Multivariate analysis indicated that the combined factor of positive tissue DCP expression and a high serum DCP level was a significant prognostic factor.
CONCLUSION: The combined evaluation of tissue DCP expression and serum DCP level is more useful than either factor alone in predicting prognosis for patients with HCC and liver cirrhosis.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the commonest malignancies and is especially prevalent in Asia. Recent epidemiological studies have suggested that HCC is responsible for 500 000 deaths globally every year, and the incidence of HCC is increasing worldwide.1,2 Surgical resection is the most effective method for curing this disease, but few patients are eligible for surgery because of their intrahepatic or distant metastases at the time of diagnosis. Therefore, early detection of HCC and its recurrence is crucial.3 Although alpha-fetoprotein has served as a representative tumor marker of HCC for more than 40 years, its reported sensitivity and specificity are not satisfactory for early diagnosis of HCC.4 Since the first description by Liebman et al. in 1984,5 des-gamma-carboxyprothrombin (DCP), also referred to as protein induced by vitamin K absence or antagonist-II (PIVKA-II), has been found to be another useful tumor marker of HCC.6–12
Hepatocarcinogenesis is closely linked to chronic liver damage but rarely develops in a healthy liver during normal aging.1 Liver cirrhosis is an important risk factor for HCC since more than 80% HCC cases feature a background of cirrhosis. Hepatic resections for HCC in the cirrhotic liver are characterized by early recurrence and poor survival.13,14 Earlier detection of HCC in an increasing number of cirrhotic patients and the development of new therapeutic techniques have been vital to improve survival for this group of patients.15 Since vitamin K is crucial to the production of DCP, patients with decompensated cirrhosis may have higher DCP levels because of alterations in vitamin K production secondary to cholestasis, malnutrition, renal failure or use of medications that alter gut flora.16 Furthermore, a previous study by the current authors showed that DCP expression in cancerous and in non-cancerous tissues proved useful in determining the prognosis of patients with HCC.17 These studies suggested that DCP might be related to the malignant behavior of those liver diseases and might help in the diagnosis of patients with HCC and chronic liver diseases. In order to clarify the relationship between DCP expression and patients' prognosis, the present study detected the serum and tissue expression of DCP in cirrhotic livers with a single primary HCC nodule.
MATERIALS AND METHODS
Patients
Serum and liver tissue samples were collected from 74 patients with a single primary HCC nodule. These patients underwent surgical resection at the Hepato-Biliary-Pancreatic Surgery Division, Department of Surgery, Graduate School of Medicine, The University of Tokyo from February 1995 to December 1999. This study group consisted of 51 men and 23 women aged from 35 to 81 years (mean age 61 years). All patients were found to have underlying liver cirrhosis. Their clinicopathological characteristics were evaluated according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer.18 The TNM system developed by the International Union against Cancer was used for tumor staging.19 Non-HCC liver tissue samples taken from 29 patients with no underlying chronic hepatitis and cirrhosis served as controls in order to create the staining index. In addition, 26 needle biopsy liver tissue samples (14 of which were found to have underlying chronic hepatitis and 12 of which had cirrhosis) were also used as a comparative study group to evaluate DCP staining in non-cancerous liver tissues. The study was approved by the Ethics Committee of The University of Tokyo.
Immunohistochemical staining
Sections (4-µm thick) were obtained from archival formalin fixed, paraffin-embedded tissue blocks, deparaffinized by xylene solution and dehydrated through a graded series of ethanol solutions. Endogenous peroxidase was inactivated through administration of 0.3% hydrogen peroxide/methanol for 30 min. After microwave irradiation, the slides were incubated with blocking serum at room temperature for 30 min. The sections were then incubated with the primary anti-DCP monoclonal antibody (MU-3, 1:900 dilution; Eisai, Tokyo, Japan) for 60 min at room temperature. After the sections were incubated with biotinylated secondary antibody for 60 min, detection of DCP was achieved by the biotin-streptavidin-peroxidase complex method using a commercial kit (Histofine SAB-PO kit; Nichirei, Tokyo, Japan). 3,3′-diaminobenzidine was used as the chromogen and hematoxylin was used as a counterstain. Sections that had not been subjected to primary antibody incubation served as a negative control to monitor background staining. To evaluate the level of DCP expression in each sample, the DCP staining index was defined as the percentage of DCP stained cells in the total tissue cell population in 10 randomly selected microscopic fields, or in the entire section if the section consisted of fewer than 10 fields. The positive staining threshold for detection was then obtained based on the percentage of DCP stained cells in these 74 patients (more than 15%, mean + 2 × standard deviation [SD]).
Enzyme immunoassay
Determination of serum DCP level was performed using an enzyme immunoassay with a double antibody sandwich system (Eitest PIVKA-II; Eisai, Tokyo, Japan). DCP was expressed in arbitrary units (AU), with 1 AU being equivalent to 1 µg of purified prothrombin in accordance with previous studies.20 Cut-off values were set at 62.5 mAU/mL before 1998 and 40 mAU/mL since 1998, based on previous studies.21,22
Statistical analysis
Statview 5.0 (SAS Institute, Cary, NC, USA) and JMP 9.0.0 (SAS Institute, Cary, NC, USA) were used for data analysis. A χ2 test was used to evaluate the relationship between DCP expression levels and the clinicopathological parameters of patients with HCC. Survival curves were calculated using the Kaplan–Meier method and compared to the results of the log–rank test. All variables that had P values of less than 0.05 in univariate analysis were selected for multivariate analysis, which was performed using Cox's proportional-hazards model. P < 0.05 was considered as statistically significant.
RESULTS
Tissue DCP expression and its relationship to clinicopathological characteristics
In the analyzed patients, hepatitis viral infection was found in 66 patients (66/74, 89.19%). Of these, 14 were positive for hepatitis B surface antigen, 48 were positive for hepatitis C virus antibody and 4 were positive for both markers. The other 8 patients were negative for both hepatitis markers. The sizes of the tumors varied from 1.0 cm to 14.0 cm (mean ± SD 4.0 ± 2.6). The 74 tumors consisted of 14 HCC that were well-differentiated, 52 moderately differentiated, and 8 poorly differentiated. Vascular invasion was found in 20 patients (20/74, 27.03%) and intrahepatic metastatic lesions were found in 22 (22/74, 29.73%).
Immunohistochemical staining for DCP indicated that 56 patients (56/74, 75.68%) had positive DCP staining in cancerous tissues. Of these 56 patients, 13 had positive DCP staining in cancerous tissues as well as in non-cancerous tissues (Fig. 1a). And 6 had positive staining in non-cancerous tissues only (Fig. 1b). The percentage of DCP stained cells in cancerous and non-cancerous tissues from HCC patients was significantly higher than in resected or needle biopsied non-HCC tissues21. These observations allowed patients to be divided into four categories depending on the DCP staining characteristics in cancerous and non-cancerous tissues.
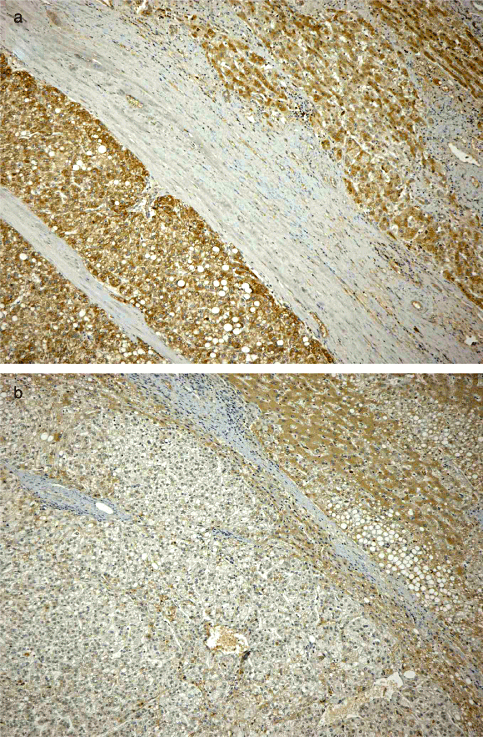
Typical example of des-gamma-carboxy prothrombin (DCP) staining in cancerous and non-cancerous tissues of patients with hepatocellular carcinoma (HCC) and liver cirrhosis showing (a) positive DCP staining in both cancerous and non-cancerous tissues; (b) positive staining in non-cancerous tissues only. Original magnification: ×100.
The relationship between tissue DCP staining and clinicopathological characteristics is shown in Table 1. Positive DCP staining in non-cancerous tissues was more frequent in patients with poor differentiation (poor vs well or moderate: 62.50% vs 14.29% or 23.08%, P = 0.0330), vascular invasion (present vs absent: 45.00% vs 18.52%, P = 0.0249) and a more advanced TNM stage (III + IV vs I + II: 44.00% vs 16.33%, P = 0.0114). In contrast to non-cancerous tissues, positive DCP staining in cancerous tissues was more frequent in patients without capsule formation than those with noted capsule formation (absent vs present: 93.75% vs 70.69%, P = 0.0347). Significant relationships among other clinicopathological characteristics (e.g. age, gender, Child–Pugh score) and positive DCP staining were not found (P > 0.05).
| Clinicopathological factors (n) | H (%) | P value | N+ (%) | P value | Ca+ (%) | P value |
|---|---|---|---|---|---|---|
| Tumor differentiation | NS | 0.0330 | NS | |||
| Well (14) | 3 (21.43) | 2 (14.29) | 9 (64.29) | |||
| Moderate (52) | 26 (50.00) | 12 (23.08) | 41 (78.85) | |||
| Poor (8) | 2 (25.00) | 5 (62.50) | 6 (75.00) | |||
| Capsule formation | NS | NS | 0.0347 | |||
| Present (58) | 27 (46.55) | 16 (27.59) | 41 (70.69) | |||
| Absent (16) | 4 (25.00) | 3 (18.75) | 15 (93.75) | |||
| Capsule infiltration | 0.0008 | NS | NS | |||
| Present (52) | 28 (53.85) | 14 (26.92) | 39 (75.00) | |||
| Absent (22) | 3 (13.64) | 5 (22.73) | 17 (77.27) | |||
| Vascular invasion | 0.0144 | 0.0249 | NS | |||
| Present (20) | 13 (65.00) | 9 (45.00) | 17 (85.00) | |||
| Absent (54) | 18 (33.33) | 10 (18.52) | 39 (72.22) | |||
| Intrahepatic metastases | 0.0028 | NS | NS | |||
| Present (22) | 15 (68.18) | 8 (36.36) | 18 (81.82) | |||
| Absent (52) | 16 (30.77) | 11 (21.15) | 38 (73.08) | |||
| TNM stage | 0.0011 | 0.0114 | NS | |||
| III + IV (25) | 17 (68.00) | 11 (44.00) | 19 (76.00) | |||
| I + II (49) | 14 (28.57) | 8 (16.33) | 37 (75.51) | |||
| Tumor size | <0.0001 | NS | NS | |||
| >20 mm (47) | 28 (59.57) | 14 (29.79) | 36 (76.60) | |||
| ≤20 mm (27) | 3 (11.11) | 5 (18.52) | 20 (74.07) | |||
| Recurrence | 0.0001 | NS | NS | |||
| Recurrence (36) | 23 (63.89) | 8 (22.22) | 27 (75.00) | |||
| No recurrence (38) | 8 (21.05) | 11 (28.95) | 29 (76.32) |
- H, High serum DCP levels; N+, positive DCP staining in non-cancerous tissues; Ca+, positive DCP staining in cancerous tissues; NS, not significant.
Serum DCP level and its relationship to clinicopathological characteristics
The serum DCP level was determined in all 74 patients with HCC and liver cirrhosis. According to the cut-off values (62.5 mAU/mL before 1998 and 40 mAU/mL since 1998), 31 patients (31/74, 41.89%) had a high serum DCP level. As shown in Table 1, the serum DCP level was significantly associated with capsule infiltration (present vs absent: 53.85% vs 13.64%; P = 0.0008), vascular invasion (present vs absent: 65.00% vs 33.33%, P = 0.0144), intrahepatic metastases (present vs absent: 68.18% vs 30.77%, P = 0.0028), TNM stage (III + IV vs I + II: 68.00% vs 28.57%, P = 0.0011), tumor size (>20 mm vs≤20 mm: 59.57% vs 11.11%, P < 0.0001), and tumor recurrence (recurrence vs no recurrence: 63.89% vs 21.05%, P = 0.0001). Other clinicopathological characteristics such as age, gender, Child–Pugh score, tumor differentiation, growth type and capsule formation around the tumors were not significantly associated with the serum DCP level.
The relationship between DCP staining characteristics and survival
The relationship between the DCP staining characteristics and patient survival was analyzed. When classified according to tissue DCP staining characteristics as described above, the overall survival rates for patients with HCC and liver cirrhosis (n = 74) were determined by the Kaplan–Meier method as shown in Figure 2a. The patients were followed up for longer than 60 months. Patients with positive DCP staining in both cancerous and non-cancerous tissues (n = 13, 5-year survival rate = 12.59%) had a significantly worse prognosis than those with negative DCP staining in both cancerous and non-cancerous tissues (n = 12, 5-year survival rate = 83.33%; P = 0.0028) and those with negative DCP staining in cancerous or non-cancerous tissues, or both (P = 0.0361). Patients with positive DCP staining in cancerous tissues but not in non-cancerous tissues (n = 43, 5-year survival rate = 41.91%) and patients with positive DCP staining in non-cancerous tissues but not in cancerous tissues (n = 6, 5-year survival rate = 33.33%) had a significantly lower survival rate than those with negative DCP staining in both cancerous and non-cancerous tissues. In addition, patients with positive DCP staining in cancerous (n = 43 + 13) or non-cancerous (n = 6 + 13) tissues had a worse prognosis than those with negative DCP staining (data not shown). Patients with positive DCP staining in non-cancerous tissues (n = 19, 5-year survival rate = 20.90%) had a significantly worse 5-year survival rate than those with negative DCP staining in non-cancerous tissues (P = 0.0271).

Kaplan–Meier curves for overall survival (OS) analyses showing (a) OS of patients with hepatocellular carcinoma (HCC) and liver cirrhosis as a function of tissue des-gamma-carboxy prothrombin (DCP) staining characteristics in cancerous (Ca) and non-cancerous (N) tissues. Bold solid line, N(+)/Ca(+); dashed line, N(+)/Ca(−); thin solid line, N(−)/Ca(+); dotted line, N(−)/Ca(−). (b) OS of patients with HCC and liver cirrhosis as a function of tissue DCP staining characteristics. Solid line, positive staining in cancerous or non-cancerous tissues, or both; dotted line, negative staining in both cancerous and non-cancerous tissues. (c) OS of patients with HCC and liver cirrhosis as a function of serum DCP levels. Solid line, a high serum DCP level; dotted line, a low serum DCP level. (d) OS of patients with HCC and liver cirrhosis as a function of both serum (S) and tissue (T) DCP. Bold solid line, S(High)/T(+); dashed line, S(Low)/T(+); thin solid line, S(High)/T(−); dotted line, S(Low)/T(−).
These results suggest that positive DCP expression was related to worse prognosis. Furthermore, a noteworthy finding is that DCP expression in non-cancerous tissues may also be related to prognosis. Therefore, in the following data analyses, patients were divided into two categories: those with positive DCP expression in cancerous or non-cancerous tissues, or both (positive tissue DCP) (n = 62) and those with negative DCP expression in both cancerous and non-cancerous tissues (negative tissue DCP) (n = 12). Patients with positive DCP expression had a significantly worse prognosis than those with negative DCP expression in both cancerous and non-cancerous tissues (Figure 2b, P = 0.0142).
The relationship between serum DCP level and survival
The relationship between serum DCP level and patient survival was also analyzed. As shown in Figure 2c, patients with a high serum DCP level (n = 31, 5-year survival rate = 25.95%) had a significantly worse prognosis than those with a low serum DCP level (n = 43, 5-year survival rate = 55.56%; P = 0.0022).
The relationship between combined tissue/serum DCP expression and survival
The significance of combined tissue and serum DCP expression was also analyzed. Depending on the level of tissue and serum DCP expression, patients were divided into four groups as shown in Table 2. Kaplan–Meier curves for overall survival rates of the four groups are shown in Figure 2d. Patients with positive tissue DCP expression and a high serum DCP level (n = 26, 5-year survival rate = 14.42%) had a significantly worse prognosis than patients with negative tissue DCP expression and a low serum DCP level (n = 7, 5-year survival rate = 85.71%), those with negative tissue DCP staining and a high serum DCP level (n = 5, 5-year survival rate = 80.00%) and those with positive tissue DCP staining and a low serum DCP level (n = 36, 5-year survival rate = 48.78%; P = 0.0087).
| Tissue DCP expression (%) | Serum DCP level | Total (%) | |
|---|---|---|---|
| High | Low | ||
| n (%) | n (%) | ||
| Positive | 26 (35.13) | 36 (48.65) | 62 (83.78) |
| Negative | 5 (6.76) | 7 (9.46) | 12 (16.22) |
| Total | 31 (41.89) | 43 (58.11) | 74 (100.00) |
Univariate and multivariate analyses
The results of univariate Cox regression survival are shown in Table 3. A lower 5-year survival rate was significantly correlated with positive tissue DCP expression (P = 0.0142), a high serum DCP level (P = 0.0022), combined factor of positive tissue staining and a high level of serum DCP expression (P < 0.0001), the presence of vascular invasion (P = 0.0009), the presence of intrahepatic metastases (P < 0.0001), the presence of biliary invasion (P = 0.0019), a more advanced TNM stage (P < 0.0001), a larger tumor size (P = 0.0322) and recurrence (P = 0.0003) (Table 3). Significant relationships between survival and other characteristics such as age, gender, hepatitis virus infection, tumor differentiation, growth type, capsule formation and capsule infiltration were not found.
| Variables | n | 5-year survival rates (%) | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Tissue DCP expression† | 0.0142 | |||
| Positive | 62 | 34.98 | 4.932 (1.184–20.542) | |
| Negative | 12 | 83.33 | 1 | |
| Serum DCP level | 0.0022 | |||
| High | 31 | 25.95 | 2.591 (1.368–4.908) | |
| Low | 43 | 55.56 | 1 | |
| Tissue/serum DCP expression | <0.0001 | |||
| Positive expression in tissue and a high serum level | 26 | 14.42 | 3.631 (1.902–6.934) | |
| Other groups | 48 | 58.52 | 1 | |
| Vascular invasion | 0.0009 | |||
| Present | 20 | 20.96 | 2.881 (1.488–5.579) | |
| Absent | 54 | 50.78 | 1 | |
| Intrahepatic metastases | <0.0001 | |||
| Present | 22 | 4.77 | 5.036 (2.653–9.559) | |
| Absent | 52 | 61.24 | 1 | |
| Biliary invasion | 0.0019 | |||
| Present | 6 | 0.00 | 3.680 (1.513–8.948) | |
| Absent | 68 | 47.60 | 1 | |
| TNM stage | <0.0001 | |||
| III+IV | 25 | 8.73 | 5.824 (3.037–11.170) | |
| I+II | 49 | 59.88 | 1 | |
| Tumor size | 0.0322 | |||
| >20 mm | 47 | 37.57 | 2.096 (1.041–4.221) | |
| ≤20 mm | 27 | 52.50 | 1 | |
| Recurrence | 0.0003 | |||
| Recurrence | 36 | 22.93 | 3.247 (1.639–6.435) | |
| No recurrence | 38 | 63.66 | 1 |
- † Patients were divided into two categories: those with positive des-gamma-carboxy prothrombin (DCP) expression in cancerous or non-cancerous tissues or both (positive tissue DCP) (n = 62) and those with negative DCP expression in both cancerous and non-cancerous tissues (negative tissue DCP) (n = 12). CI, confidence interval.
The relationship between combined tissue and serum DCP expression and prognosis was then examined by multivariate analysis according to Cox's regression hazards model. Pathological variables that were significant in univariate analysis were selected and included in multivariate analysis. Since the TNM stage includes various clinicopathological factors such as tumor size and infiltration, it was omitted from this analysis. As shown in Table 4, the combined factor of tissue DCP staining and serum DCP level was found to be a significant prognostic factor (hazard ratio: 2.242, P = 0.0133) along with intrahepatic metastases (hazard ratio: 3.785, P = 0.0002).
| Variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Tissue/serum DCP expression | ||
| Positive expression in tissue and a high serum level vs other groups | 2.242 (0.897–4.465) | 0.0133 |
| Intrahepatic metastases | ||
| Present vs absent | 3.785 (1.802–7.339) | 0.0002 |
- DCP, des-gamma-carboxy prothrombin; CI, confidence interval.
DISCUSSION
The pathophysiology of hepatocarcinogenesis is closely linked to the evolution of cirrhosis. Although the exact mechanism of DCP production in HCC tissues is yet to be clearly understood, DCP is reportedly produced by malignant hepatocytes that result from an acquired post-translational defect in the vitamin K-dependent carboxylase system.23–26 The absence of vitamin K or the presence of its antagonist inhibiting vitamin K-dependent carboxylase activity results in the secretion of immature prothrombin lacking gamma-carboxy-glutamic acid residues (that is DCP) into the blood. The production of DCP is not a specific event in HCC cells although it is now widely accepted as a tumor marker for HCC.27 Our previous studies using clinically resected HCC tissues showed DCP expression in cancerous tissues and in the surrounding non-cancerous tissues of patients with HCC.17,21 Therefore, non-cancerous hepatocytes in the midst of vitamin K insufficiency might produce DCP. In this study, DCP expression in non-cancerous tissues was also observed in the cirrhotic livers of patients with HCC (n = 19, 25.68%) and was significantly correlated with the presence of a poorly differentiated tumor, vascular invasion and a more advanced TNM stage. Furthermore, patients with positive DCP expression in cancerous or non-cancerous tissues, or both, had a significantly worse survival rate than those with negative DCP expression both in cancerous and non-cancerous tissues. These findings indicate that DCP production in the non-cancerous cirrhotic liver as well as in HCC might play an important role in HCC progression.
While the serum DCP level has been routinely used for the screening and postoperative follow-up of patients with HCC, many studies have shown that an elevated serum DCP level is associated with a poor clinical outcome in patients with HCC.28–31 Therefore, DCP is clinically effective as a serological tumor marker for the prediction of patient prognosis.32–35 The results of our study suggest that the serum DCP level can predict worse tumor behavior in patients with HCC and liver cirrhosis. This result was in accordance with a previous study on serum DCP levels in 92 patients with HCC, 57 of whom (62.0%) were found to have underlying cirrhosis.17 In addition, patients who had a good outcome despite a high serum DCP level were discerned by considering the tissue DCP expression profile. Although the serum DCP level alone was not a significant prognostic factor, the combined factor of positive tissue DCP expression and a high serum DCP level might represent a useful predictor for worse prognosis in patients with HCC and liver cirrhosis and help to suggest an optimal treatment plan.23 Since hepatic resections for HCC in the cirrhotic liver are characterized by early recurrence and poor survival,13,14 earlier detection of recurrent HCC in an increasing number of cirrhotic patients is critical to improve survival for this group of patients. Although measurement of serum DCP level is convenient and essential for diagnosing patients with HCC, tissue DCP expression may be effective at predicting the prognosis of patients with both HCC and liver cirrhosis.
In conclusion, both tissue DCP expression and the serum DCP level were significantly related to postoperative survival for patients with HCC and liver cirrhosis. Additionally, the combination of tissue DCP expression and serum DCP level might allow the prediction of the prognosis of patients with HCC and liver cirrhosis. DCP produced in non-cancerous hepatocytes and HCC cells and then secreted into the blood is considered to be significantly related to the malignant transformation of HCC. DCP can be used for clinical diagnosis and for other purposes although additional molecular medical innovations and further clinical validation are needed.
ACKNOWLEDGMENT
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science.




