Stability of Flavonoids in the Presence of Riboflavin-photogenerated Reactive Oxygen Species: A Kinetic and Mechanistic Study on Quercetin, Morin and Rutin
Abstract
Kinetic and mechanistic aspects on the stability of the flavones (FL) quercetin (Que), morin (Mor) and rutin (Rut), in methanolic solution and in the presence of reactive oxygen species (ROS) generated by visible light-promoted riboflavin (Rf, vitamin B2) photoirradiation were studied. The system was chosen as a model for the evaluation of the in vivo protective effect of biological targets by the flavones. The overall picture includes the vitamin as an endogenous natural photosensitizer. A systematic study on the effect of ROS on FL photostability shows that under work conditions Que is oxidized by singlet molecular oxygen (O2(1Δg)), superoxide radical anion (O2˙−) and hydrogen peroxide; Mor is degraded by O2(1Δg) and O2˙− whereas Rut only reacts with O2(1Δg). Que and Rut, with an extremely poor overall rate constant, are mainly physical quenchers of O2(1Δg). Mor, with O2(1Δg)-interception ability slightly lower than the recognized synthetic antioxidant trolox (Tx), behaves as a typical sacrificial scavenger provided that ca 80% of the collisions with O2(1Δg) cause its own degradation, whereas this parameter reaches around 50% in the case of Tx.
Introduction
It is well recognized that the oxidative damage of biologically relevant molecules in living tissues, induced by reactive oxygen species (ROS), plays an important role in a considerable number of pathological problems (1,2). Flavonoids are a family of polyphenol derivatives found in vegetables as well as in popular beverages such as red wine and tea (3). The main physiological benefits of these compounds have been largely attributed to their antioxidant properties, being particularly active against ROS (4,5). Following this line, it is a common temptation to straightforwardly correlate antioxidant protection with flavonoid content. Nevertheless, an essential requirement to ensure the effectiveness of flavonoids as antioxidants is their own stability in the presence of ROS. In this context, the main aim of this work was the evaluation of the reactivity and potential degradation of three flavones (FLs), taken as representative antioxidant protectors, in the presence of photogenerated ROS. The chosen FLs were quercetin (Que), morin (Mor) and rutin (Rut). Their respective chemical structures are shown in Scheme 1. The oxidative species were produced by photoirradiation of riboflavin (Rf, vitamin B2) with light of wavelength higher than 450 nm, a spectral region where the FLs are transparent. This combination reasonably mimics a natural picture in a living/biological system, in which the sensitizer, the oxidizable targets and the photoprotectors are simultaneously present in a given environment illuminated by daylight. Rf is one of the endogenous visible light absorbers which has been postulated as a possible sensitizer for the in vivo photo-oxidative degradation of several biologically relevant substrates (6,7). Upon photoirradiation in MeOH the pigment generates the oxidative species singlet molecular oxygen (O2(1Δg)), process [11] in Scheme 2) with a quantum yield of 0.49 (8) and superoxide radical anion (O2˙−) (process [3] in Scheme 2) with a quantum yield of 0.009 (9). Rf is synthesized by green plants and participates in a variety of enzyme-catalyzed oxidation–reduction reactions (10), being widely distributed also in human tissues, both in the free and in the conjugated form (11).
Chemical structure of the flavonoids quercetin, morin and rutin.

Major kinetic processes in the visible light irradiation of an air-equilibrated solution of an electron donor molecule (FL) in the presence of riboflavin (Rf).
In the present work we discuss the results of a systematic kinetic study on the interaction of Rf-photogenerated ROS with some FLs, toward the elucidation of the oxidative mechanisms that could account for the reactive interactions ROS–FLs. In this way we evaluate the stability of the chosen natural FLs in comparison with Trolox C (Tx), a well-recognized water-soluble analog of the natural antioxidant α-tocopherol (12,13).
Materials and methods
Materials. The flavones Que, Mor and Rut, Rf, sodium azide (NaN3), superoxide dismutase (SOD, from bovine erythrocytes, 2500–7000 U mg−1 protein), methionine (Met), eosin and monodeuterated methanol (MeOD) were purchased from Sigma. Water was triply distilled and methanol (HPLC quality) was provided by Sintorgan (Argentina).
Methods. Open or teflon-stopped 1 cm quartz cells were employed working under air- or nitrogen-saturation conditions, respectively. Ground-state absorption spectra were registered in an Agilent 8453 diodo-array spectrophotometer.
Stationary aerobic photolysis of solutions containing any FL (0.5 mm) and Rf (0.02 mm) were carried out in a PTI unit provided with a high-pass monochromator and a 150-W Xe lamp, irradiating with 450 ± 5 nm, or in a home-made photolyzer for nonmonochromatic irradiation (150 W quartz-halogen lamp). In the latter case, cut-off filters (450 nm) ensured that the light was only absorbed by the sensitizer.
The Rf-sensitized photo-oxygenation rates of the FLs and Met were evaluated from the initial slopes of oxygen consumption vs irradiation time, employing the specific oxygen electrode Orion 97-08. Anaerobic photodecomposition rates of Rf were determined by evaluation of the initial slopes of Rf consumption (decrease of absorbance at 446 nm) vs irradiation time.
The reactive rate constant, kr, for the reaction of O2(1Δg) with each FL (process [14]) was determined as described previously (14) using the expression slope/slopeR = kr [FL]/krR [R], for which the knowledge of the reactive rate constant for the photo-oxidation of a reference compound, R, at similar concentration, is required, and where slope and slopeR are the respective slopes of the first-order plots of FL and R consumption, or oxygen consumption by the same compounds, under sensitized irradiation. Oxygen uptake in water was monitored with a 97-08 Orion electrode. Employing Eo as a sensitizer, it was assumed that the reaction of O2(1Δg) with each FL is the only way of oxygen consumption. The reference R was FFA, with a reported pH-independent krR value in water of 1.2 × 108 m−1 s−1 (15).
The overall quenching rate constant of deactivation of O2(1Δg) by each FL (kt, the sum of kq plus kr, processes [13] and [14], respectively, Scheme 2) was determined using a previously reported system (16). Briefly, a Nd:YAG laser (Spectron) was used for the excitation (532 nm) of the sensitizer Eo (A532 = 0.3), and the emitted radiation (O2(1Δg) phosphorescence at 1270 nm) was detected at right angles using an amplified Judson J16/8Sp germanium detector, after passing through two Wratten filters. The output of the detector was coupled to a digital oscilloscope and to a personal computer for the signal processing. Usually, 16 shots were needed for averaging, so as to achieve a good signal-to-noise ratio, from which the decay curve was obtained. Air-saturated solutions were employed in all the cases. In the dynamic determinations, MeOD, instead of MeOH, was used as a solvent in order to enlarge the lifetime of O2(1Δg) (15). The O2(1Δg) lifetimes were evaluated in the presence (τ) and in the absence (τ0) of the quencher, and the data were plotted as a function of concentration, according to a simple Stern–Volmer treatment, 1/τ = 1/τ0 + kt [FL].
Results
Decrease in the photoprotective effect by a flavone
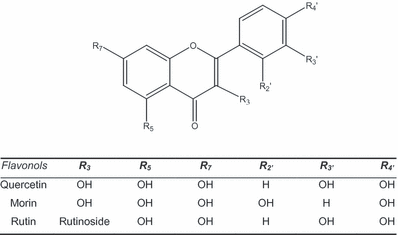
It is well known that several amino acids, including Met, are photo-oxidized when exposed to visible light in the presence of ROS generators in general and Rf in particular (17–20). We employed this fact to test the photoprotection of the FL Mor toward the oxidative damage of Met, taking the amino acid as a relevant oxidizable biological target.
The experiment was performed as follows: the visible light irradiation of a pH 7 aqueous solution containing 0.5 mm Met, and Rf (A445 = 0.5) as a photosensitizer, produced oxygen consumption (Fig. 1, trace “a”), due to the mentioned oxidation processes operated by the reaction of ROS with the amino acid. When the same experiment was carried out in the presence of 0.5 mm Mor the rate of oxygen uptake clearly decreases, in a process that could be interpreted as a sort of photoprotection exerted by the FL (trace “b”). Finally, a pH 7 aqueous solution of Rf (A445 = 0.4) plus 0.5 mm Mor, in the absence of Met, was photoirradiated with visible light for 5 min. Thereafter, Met was dissolved at a concentration of 0.5 mm and the solution was again photolyzed. The rate of oxygen consumption increased as shown in trace “c.” The rate is now indistinguishable, within the experimental error, from the initial trace “a” of Met, in the absence of Mor. The experimental conditions in the photolysis of solutions employed for traces “a,”“b” and “c” were identical.
Oxygen consumption as a function of the photoirradiation time of the following pH 7 aqueous solutions: methionine 0.5 mm + riboflavin 0.04 mm (a); methionine 0.5 mm + riboflavin 0.04 mm + morin 0.5 mm (b); the same as run (b) but the mixture riboflavin 0.04 mm + morin 0.5 mm was previously photoirradiated for 5 min.
A comparison of the respective rates of oxygen uptake observed in the solutions containing Met and Met + Mor strongly suggests that the photoprotection exerted by the flavonoid on the biological target varies with the degree of exposition of the FL to sensitized photoirradiation. This fact, in the context of the stability of FLs as biological photoprotectors, could indicate a loss of the antioxidative properties of Mor due to photoirradiation, and deserves to be carefully investigated. It was done through a comparative evaluation of the degradability of three flavones Mor, Que and Rut in the presence of ROS photogenerated by vitamin B2.
Possible reaction steps of Rf upon visible light photoirradiation
When Rf absorbs visible light, and in the presence of electron donating molecules, several ROS can be formed in solution, as depicted in Scheme 2 (21,22). The meaning of the different steps is self-defined within the context of photochemical reactions.
Phototransformations of the FLs due to Rf-sensitized irradiation
The visible light irradiation of methanolic or aqueous solutions of the individual 0.06 mm FL (Mor, Rut and Que) in the presence of Rf (A445 = 0.4) produces chemical transformations in the FL, as depicted by the absorbance evolution at different irradiation times. Figure 2 shows the case of Que in pH 7 water. Qualitatively similar changes were observed for Mor and Rut. From parallel experiments on similar photoirradiated solutions, oxygen uptake was observed (Fig. 3), and the respective rates for the three FLs are shown in Table 1.
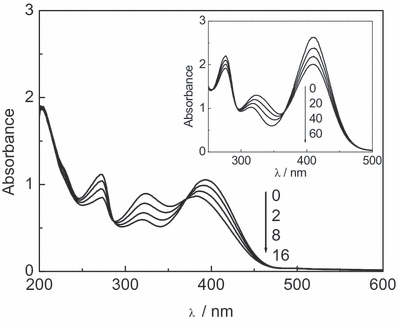
Spectral changes in quercetin 0.06 mm + riboflavin 0.04 mmvs riboflavin 0.04 mm in air-equilibrated aqueous solution (pH 7) after photoirradiation. Numbers on the spectra represent photoirradiation time in minutes. Inset: spectral changes in morin 0.12 mm + eosin 0.04 mmvs eosin 0.04 mm in air-equilibrated methanolic solution, in the presence of 10 mm KOH. Numbers on the spectra represent photoirradiation time in seconds.
Oxygen consumption as a function of photoirradiation for the following solutions: quercetin 0.5 mm + riboflavin 0.04 mm (a); morin 0.5 mm + riboflavin 0.04 mm (b); rutin 0.5 mm + riboflavin 0.04 mm (c).
| Compound | k t (MeOD) × 108 m−1 s−1 | k r (MeOH) × 108 m−1 s−1 | k r /k t (MeOH) | k r (MeOH/OH−) × 108 m−1 s−1 | ΔO2/Δt (relative), pH 7 water | |
|---|---|---|---|---|---|---|
| Eos | Rf | |||||
| Que pKa1 = 6.7* | 0.031 | 0.011 | 0.35 | Spontaneous decomposition | 0.06 | 1 |
| Mor pKa1 = 3.5* | 0.39 | 0.3 | 0.77 | 4.4 | 1 | 0.5 |
| Rut pKa1 = 7.1* | 0.009 | 0.001 | 0.11 | 1.1 | 0.01 | 0.25 |
| Trolox C pKa = 11.9* | 1.25† | 0.64† | 0.52† | 5.5† | ||
The results herein shown clearly indicate that either Rf electronic excited states or ROS produced through these states, or even both processes operating simultaneously, are responsible for the photodegradation of the FLs. On this basis, we carried out a systematic kinetic study in order to evaluate and characterize the nature, mechanism and extent of the possible processes involved in the Rf-sensitized degradation of FL.
The interaction of Rf electronically excited states with FL
The photoirradiation of nitrogen-saturated Rf aqueous-methanolic solutions of Rf in the presence of Que and Mor in the sub-mm concentration range, typically 0.5 mm, produces spectral changes that may be attributed to the reactive interactions between Rf electronically excited states and FL ground state (data not shown). These spectral perturbations were practically undetectable in the system Rut-Rf.
The reported lifetime of 1Rf* in water and methanol is 5 ns (7). A FL concentration in the sub-mm range, similar to those employed in all experiments performed in this work, is not enough to intercept 1Rf*, even assuming a diffusion-controlled value for the rate constant 1kq of process [2] in Scheme 1. Hence, the interaction 1Rf*-FL must be disregarded under work conditions.
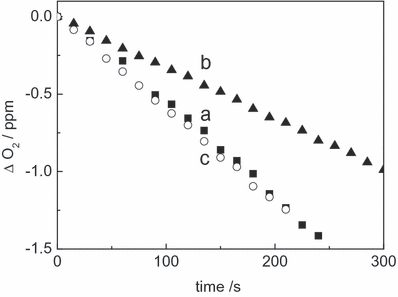
It is known that the photodegradation of Rf in solution, in the absence of oxygen and under visible light irradiation, predominantly proceeds through 3Rf* (6) for which a lifetime of 15 μs has been reported (16) and the rate of the process can be evaluated by the absorbance decrease in the Rf absorption spectrum at 445 nm (Fig. 4, inset). In the individual presence of 0.5 mm Mor and Que, the rate of Rf decomposition increased compared with the rate in the absence of FL, whereas in the presence of 0.5 mm Rut this rate suffers a delay (Fig. 4). These results strongly suggest the occurrence of some sort of interaction between 3Rf* and FL. In principle, and according to Fig. 4, this interaction could be different for Rut than for Mor and Que.
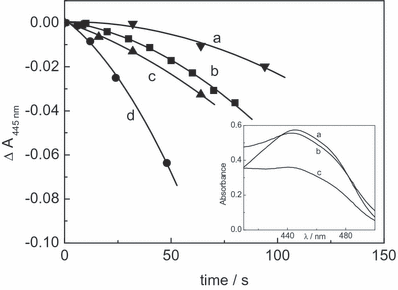
Absorbance decrease of 0.04 mm riboflavin at 445 nm in nitrogen-saturated methanolic solutions in the absence (b) and in the presence of: 0.6 mm rutin (a); 0.6 mm morin (c) and 0.6 mm quercetin (d). Inset: absorption spectra of Rf (ca 0.04 mm) in nitrogen-saturated solutions: (a) nonphotolyzed; (b) photolyzed 200 s in the presence of 0.5 mm quercetin; (c) photolyzed 200 s (for details, see Materials and Methods section).
The FL–ROS interaction
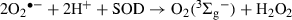 ([15])
([15])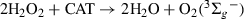 ([16])
([16])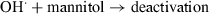 ([17])
([17])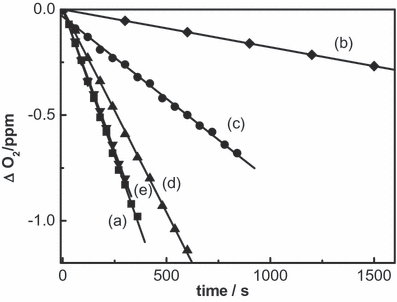
Oxygen uptake as a function of photoirradiation time of methanolic solutions containing 0.04 mm Rf plus: 0.5 mm quercetin (a); 0.5 mm quercetin + 1 μg mL−1 SOD (b); 0.5 mm quercetin + 2 mm NaN3 (c); 0.5 mm quercetin + 1 μg mL−1 CAT (d); 0.5 mm quercetin + 10 mm mannitol (e).
All experiments involving possible O2(1Δg)-mediated processes were made in methanolic solutions in order to facilitate the adequate dissolution of FL up to required concentrations. The exclusive O2(1Δg)-generator dye Eos (processes [1] + [11], with Eos (A515 = 0.5) instead of Rf) was employed as a dye sensitizer. Rf was not used in these kinetic determinations in order to avoid interferences by other oxidative species. Eos is one of the sensitizers most frequently employed in O2(1Δg) reactions, producing the oxidative species with a quantum yield of 0.30 in MeOH (26). In order to search for possible interactions of the FLs with Eos electronically excited states, argon-saturated methanolic solutions of Eos (A515 = 0.5) + 0.5 mm of the individual FLs were photoirradiated. No spectral changes could be observed after relatively prolonged irradiation time, strongly suggesting the absence of interactions by processes [2] and [4], with Eos instead of Rf.
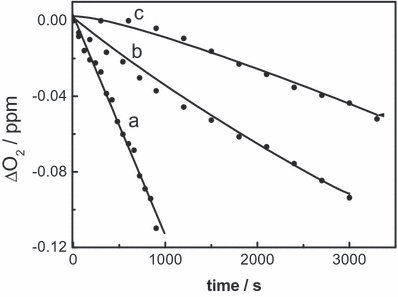
Photoirradiation with visible light of aerated mixtures of Eos (A515 = 0.6) plus a FL 0.12 mm, in MeOH and in MeOH in the presence of 10 mm KOH (alkaline MeOH), produces changes in the absorption spectrum of the particular FL (Fig. 2, inset), as well as oxygen consumption. These changes were not observed in Ar-saturated solutions. The presence of a FL in the sub-mm concentration range quenches the IR phosphorescence emission of O2(1Δg), as detected by TRPD experiments (see below). It is well known that phenols and polyphenols interact with O2(1Δg) through processes that are highly dependent on the degree of ionization of the OH groups (27). The rate constant kt for the overall interaction of O2(1Δg)–FL was independently determined in MeOD and in MeOD in the presence of 10 mm KOH, through a Stern–Volmer treatment (Fig. 6). The phosphorescence quenching experiments unambiguously demonstrate the existence of an O2(1Δg)–FL interaction, which may be physical in nature (process ([13]) and/or reactive (process [14]). The kt values, as determined by TRPD (Table 1), do not depend on the type of sensitizer or on potential interactions of the substrate with excited states of the sensitizer involved in O2(1Δg) generation.
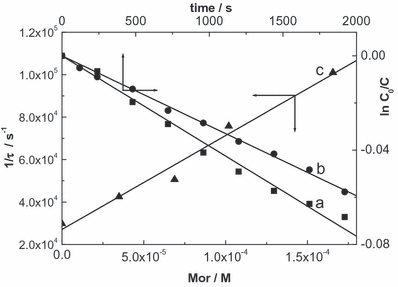
First-order plot of oxygen uptake in the eosin-sensitized photo-oxidation of furfuryl alcohol 0.5 mm (a) and morin 0.5 mm (b) in methanolic solutions, and Stern–Volmer plot for the quenching of O2(1Δg) phosphorescence by morin in MeOD solution, with eosin as a photosensitizer (c).
The overall rate constant values (kt) for the FLs were determined in MeOH and in alkaline MeOH, and are shown in Table 1. According to the respective absorption spectra, the OH groups are ionized in alkaline MeOH. Que was not employed in alkaline medium due to its spontaneous decomposition.
The reactive rate constant values (kr) (process [14]) were obtained by monitoring oxygen consumption upon visible light irradiation of mixtures Eos (A515 = 0.6) plus 0.5 mm FL, following a previously described method (14) (Fig. 6, Table 1). The calculated kr/kt ratios represent a measure of the efficiency of the degradation pathway via O2(1Δg) reaction.
Simple relative rates of oxygen consumption upon Eos-sensitized photoirradiation of the FLs, in pH 7 water are also included in Table 1. The comparison of these rates with the relative rates of oxygen consumption upon Rf-sensitized photoirradiation in the same medium strongly suggests that the oxidative mechanisms involved in both processes must be different from each other. The xanthene dye only generates O2(1Δg) (15) whereas in the Rf-sensitized process the oxygen uptake could be due to a combined action of several ROS.
Discussion
Although the importance of Rf as a natural endogenous photosensitizer and the abundance of different FLs in living organisms is well known (7,28), practically there are no studies involving the conjunctive visible light-induced photochemistry of these compounds. In this sense, we think that the employment of Rf as a dye sensitizer represents an adequate choice, given that the vitamin is a source for the in vivo generation of several important ROS, constituting an interesting model for the evaluation of the stability of FLs under natural conditions.
The Rf-sensitized photo-oxidation of Met is known to occur through the interaction with O2˙− and O2(1Δg) (17,19). It is also known that the quenching of O2(1Δg) is an entirely chemical process, with a rate constant kt = kr = 2.1 × 107 m−1s−1 in water. The delay in the rate of oxygen uptake by the system Rf + Met in the presence of 0.5 mm Mor must be ascribed to a physical deactivation of ROS by the FL (Fig. 1) and to the deactivation of 3Rf* by Mor. Nevertheless, as it can be appreciated from Fig. 4, the presence of Mor 0.6 mm minimally affects the anaerobic degradation rate of Rf, a fact that can be associated with a slight interaction of the electronically excited vitamin with the FL under aerobic conditions. Around 20% of the Mor–O2(1Δg) collisions (Table 1) deactivate the oxidative species without destruction of the FL. On the other hand, ca 80% of such collisions are reactive in nature, and the photoprotective effect exerted by Mor on Met photo-oxidation is practically cancelled upon enough exposure of the FL to the action of Rf-generated ROS. The loss of the photoprotective effect by the FL is just a question of photoirradiation time.
From the stationary photolysis experiments, the photodecomposition of the three FLs, Que, Mor and Rut, was detected in aerobic medium. The same was true in Ar atmosphere only for Que and Mor. As already stated in the Results section, the degradation of FL must be attributed, in anaerobic conditions, to reactive interactions between 3Rf* and FLs. It was confirmed by the results shown in Fig. 4 for the effect of the presence of FL on the photodecomposition of Rf. Que and Mor accelerate the Rf degradation through reactive interaction with 3Rf*, whereas Rut exerts a protection against Rf degradation presumably through a physical quenching of 3Rf*. On this basis, different yields for the generation of the diverse ROS should be expected from 3Rf* for each FL, in the presence of dissolved O2(3Σg−). Following this line, Rut appears as an inefficient producer of RfH˙, the O2˙− precursory species. Therefore, the negative result observed for Rut in the oxygen uptake experiments employing SOD as a selective quencher could be simply due to the absence of O2˙− in the medium.
We had previously reported that the quenching of 3Rf* by electron-donating compounds such as phenols and polyphenols, including flavonoids, occurs through an electron transfer reaction, represented by step [4] in Scheme 2, with relatively high values of rate constants 3kq, in the range of 108–109 m−1s−1 (16,22). The thermodynamic feasibility of the electron transfer process can be evaluated by means of the Gibbs free energy for electron transfer, ΔETG0 = E0(FL/FL+) − E0(Rf/Rf−) − ERf* + C, where E0(Rf/Rf-) is the standard electrode potential of the acceptor Rf (−0.80 V), ERf* is the 3Rf* energy (2.17 eV), C is the coulombic energy term (−0.06 V) (22) and E0(FL/FL+), is the standard electrode potential of the FLs, less than 1 V for the three FLs herein studied (29). The so-calculated ΔETG0 value, lower than −0.4 ev in the three cases, indicates that process [4] may be thermodynamically operative and consequently the species Rf˙− could be spontaneously formed by electron transfer from 3Rf* (process [4]). We should take into account that the quantum yield for direct O2˙− generation, through reaction [3], is practically negligible (9). Under aerobic conditions and after an effective electron transfer process, a cascade of reactions can occur (Scheme 2), most of them generating ROS. At neutral pH values the species RfH˙ [step 6] is produced. The bimolecular decay of RfH˙ is known to proceed through a disproportionation reaction yielding Rf and fully reduced Rf (RfH2) (process [6]). In the presence of O2(3Σg−), RfH2 is reoxidized, giving rise to RfH2˙+ and O2˙− (process [7]), and eventually Rf and H2O2 (process [8]).
In aerobic medium, results of oxygen uptake in the presence of specific ROS inhibitors indicate that, under work conditions, Que is oxidized by O2(1Δg), O2˙− and H2O2; Mor reacts with O2(1Δg) and O2˙− whereas for Rut only the reaction with O2(1Δg) was detected. Somewhat controversial are the reports on the ability of flavonoids to inactivate O2˙− (28). For example, various natural and synthetic flavonols were found to be efficient superoxide scavengers, whereas other authors reported opposite results for Que and kaempferol (30). A rapid decomposition of Que by enzymatically generated O2˙− has been reported by Ueno et al. (31) and more recently by us (29), whereas Rut proved to be an inefficient scavenger of the oxidative species under similar experimental conditions.
The quenching of O2(1Δg) by hydroxyaromatic compounds in general and flavonoids in particular has been extensively reviewed (28). Hydroxy-substituents promote the electron donor ability of the aromatic structure toward the electrophilic species O2(1Δg). The rate constants kt and kr of the studied FLs increase with the ionization of the OH groups, a property well described for hydroxy-aromatic compounds (27,32). This behavior has been explained on the basis of a mechanism involving an intermediate complex possessing charge-transfer character (27,33,34).
The O2(1Δg)-mediated photo-oxidation quantum efficiency ϕr (ϕr = kr [FL]/(kd + kt [FL])) (27) is not easy to evaluate, particularly in natural environments, because its determination includes the knowledge of the concentration of the photo-oxidizable substrates represented by the FLs in this case. A simpler and useful approach is the evaluation of the kr/kt ratio (Table 1), which indicates the fraction of overall quenching of O2(1Δg) by the substrate that effectively leads to a chemical transformation. In the present case, kr/kt values range from 0.11 to 0.77 for the three FLs. In the context of FL stability, low kr/kt values indicate a sort of self-protection of the substrates against O2(1Δg)-mediated oxidation. The prevalence of the physical process is a desirable possibility as the final result is the elimination of the oxidative species without considerable loss of the scavenger.
Mor is the most labile of the studied FLs. Around 80% of the O2(1Δg)–Mor collisions effectively oxidize FL. These results are in line with our previous reports on the O2(1Δg)-quenching ability of dihydroxybenzenes (35). We found that cathechol—a structure present in ring B of Que and Rut—only quenches O2(1Δg) in a physical fashion. On the other hand, the quenching of O2(1Δg) by the resorcinol structure—similar to that of Mor in ring B—is mostly reactive, producing the effective oxidation of the quencher. These facts are clearly reflected by the respective kr/kt values in Table 1. Finally, an additional reason for the high O2(1Δg) rate constant values found for Mor is the existence of an ionized phenolate structure for this FL under work conditions. Mor is the only among the studied FLs possessing a very low pK value for the first phenolic group ionization (30) (Table 1).
The FL molecules herein studied present two centers (Scheme 1) susceptible to attack by O2(1Δg): the already described ring B and the phenylchromen-4-one moiety, containing ring A (Scheme 1). Que and Mor possess OH substitution in position 3 at the chromen moiety, whereas Mor has a rutinosid residue in that position. All three FLs are OH-substituted in position 7. The substitution pattern in ring B, especially the presence of a OH group in that position, has been recognized as a determinant factor promoting O2(1Δg)–flavonoid interaction (36). This fact is neatly reflected by the low rate kt and kr values of Rut (Table 1).
Considering the structure–reactivity relationship reports of FL as quenchers of ROS in general, three main points are well established, through several studies (for a review, see Ref. [28]): (1) ortho-dihydroxy (cathechol) structure in the ring B is important for high O2˙− scavenging activity; (2) there is no unanimity about the role of the presence of an OH group in position 3; (3) a C2–C3 double bond enhances the antioxidant activity by stabilization of the flavonoid radical through electron delocalization across the molecule. In synthesis, the maximal overall antioxidant activity is shown by flavonoids with a free OH group in position 3, a cathechol moiety in the B-ring and a double in C2–C3, such as Que. Our results are coincident with these observations in the sense that Que, with the higher relative rate for oxygen consumption under Rf photosensitization (Table 1), is the most degradable FL.
In an attempt to standardize the persistence of the antioxidant capacity of the FLs Que, Mor and Rut in the presence of photogenerated ROS, their respective stabilities were compared with that of the recognized synthetic antioxidant Tx. It is known that Tx interacts with different radicals, including Rf-generated O2˙− with relatively high rate constants, to form the neutral trolox radical (12,30,37). Furthermore, the rate constants for the reaction of Tx with O2˙− is very similar to that reported for several flavonoids, including Que, Mor and Rut, determined by pulse conductivity in aqueous solution (30). In this sense Que and Mor behave in a fashion similar to Tx with respect to O2˙− under Rf-sensitized photoirradiation.
Regarding the O2(1Δg) interaction, the data in Table 1 indicate that the rate constants for both physical and chemical quenching of the oxidative species are much higher for Tx than for either of the studied FLs. It is necessary an increase in concentrations of ca 2 orders of magnitude for Rut, ca 40 times for Que and approximately three times for Mor, with respect to Tx concentration, to quench O2(1Δg) with a similar rate than the synthetic antioxidant. Considering the stability towards O2(1Δg)-mediated degradation, the balance between rate constants for physical and chemical deactivation of the oxidative species indicates that Que and Rut, the poorest O2(1Δg) quenchers, behave mainly as physical deactivators, with Rut being the most longstanding photoprotector in view that only around 10% of the collisions produce its effective photodegradation. In this context, Mor, the more promising of the FLs for the overall deactivation of O2(1Δg), is paradoxically a typical sacrificial scavenger, provided that ca 80% of the collisions with O2(1Δg) cause its own degradation, whereas this parameter reaches around 50% in the case of Tx.
As a conclusion we can say that the visible light photoirradiation of Rf in the individual presence of Que, Mor and Rut, in aqueous and methanolic solutions, generates ROS that degrade each FL by particular mechanisms and to different extents. This fact clearly reduces the characteristic photoprotective effect attributed to FLs. According to oxygen uptake experiments the photodegradation rate of the FLs is Que > Mor > Rut.
Acknowledgments
Acknowledgements— Financial support from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Secretarías de Ciencia y Técnica of the Universidad Nacional de Río Cuarto (SECyT UNRC) and Universidad Nacional de San Luis (SECyT UNSL), all from Argentina, is gratefully acknowledged.




