Photosensitized Oxidation of Tetrabromobisphenol A by Humic Acid in Aqueous Solution†
This article is dedicated to the memory of Dr. Colin Chignell.
Abstract
The brominated flame retardant 3,3′,5,5′-tetrabromobisphenol A (TBBPA) may accumulate in the environment, including surface waters, and degrade there to potentially toxic products. We have previously shown that singlet oxygen (1O2), produced by irradiation of rose bengal with visible light, oxidizes Triton X-100-solubilized TBBPA to yield the 2,6-dibromo-p-benzosemiquinone anion radical while consuming oxygen (Environ. Sci. Technol.42, 166, 2008). Here, we report that a similar 1O2-induced oxidation can be initiated in aqueous solutions by the irradiation of TBBPA dissolved in a humic acid (HA) solution. HA is a known weak 1O2 photosensitizer and we indeed detected the infrared 1O2 phosphorescence from HA preparations in D2O. When an aqueous preparation of HA was irradiated (λ > 400 nm) in the presence of TBBPA, oxygen was consumed, and the 2,6-dibromo-p-benzosemiquinone anion radical was generated and detected using electron paramagnetic resonance. Radical formation and oxygen consumption were inhibited by sodium azide, a singlet oxygen quencher. Our results suggest that solar radiation, in the presence of HA, may play an important role in the photodegradation of TBBPA in the aquatic environment.
Introduction
Brominated flame retardants (BFRs) are widely used in many consumer products ranging from fabrics and plastics to electronics (1). Because 3,3′,5,5′-tetrabromobisphenol A (TBBPA; Scheme 1 [1]) accounts for approximately half of all the BFRs used in manufacturing (2), there is concern about its bioaccumulation and interest in its biodegradation. Several studies have reported that TBBPA may bioaccumulate in the environment (3–5). Photolytic decomposition of TBBPA in the environment may occur both in the atmosphere and in surface waters.
The rate of photodecomposition of TBBPA in water is highly dependent on the pH, as the absorption spectra of the un-ionized and dissociated forms are very different (6). Eriksson et al. (7) reported that at pH values below its pKa of ∼7.4, the quantum yield of TBBPA photodecomposition decreases with decreasing pH, while at pH values above its pKa photodecomposition is independent of pH. In addition to direct photochemical reactions, TBBPA may also be degraded by reactive oxygen species present in the environment such as hydrogen peroxide, the superoxide anion and singlet oxygen (1O2). We have previously reported that at pH values at or above its pKa TBBPA is rapidly oxidized by 1O2 generated by rose bengal (RB) or methylene blue (6). In aqueous alkaline solutions, TBBPA was a very efficient 1O2 quencher (kq = 1.44 × 109 m−1 s−1) and even at neutral pH, where TBBPA is only partially dissociated, the quenching rate constant was quite high (kq = 3.9 × 108 m−1 s−1). Our EPR studies showed that the main oxidation photoproduct was the 2,6-dibromo-p-benzosemiquinone anion radical (6).
TBBPA in surface waters is also exposed to humic acid (HA). Humic substances are omnipresent in the natural environment and play a number of important roles including controlling the pH balance, governing the mobility of contaminants through absorption, aggregation and sedimentation (8), and chelating metals (8–10). The photochemistry of HA in the environment has been investigated previously (11–15); numerous articles have suggested that HA acts as a sensitizer or precursor for the production of reactive intermediates including 1O2, superoxide anion and/or hydrogen peroxide in oxygenated natural water. HA encompasses very diverse structures (16–19) containing chromophores that act as photosensitizers, either directly from the excited states (12,20), or indirectly via1O2 (21–23). A contribution from the non-1O2 mechanisms to the TBBPA degradation, if any, may depend on the identity of the photoactive chromophores specific to a given HA. In contrast, the contribution from the 1O2-initiated reactions will be the same regardless of the type of HA, as long as such HA is able to photosensitize 1O2.
The aqueous solubility of TBBPA increases in surfactant solutions (6); thus other agents that can produce surfactant-like microenvironments may have a similar effect. Osako et al. proposed that BFRs such as TBBPA leach from landfills in association with HA (24). The aim of this study was to investigate the possibility of photosensitized degradation of TBBPA in the presence of HA in aqueous environment with the focus on the reactions initiated by 1O2. To the best of our knowledge, this is the first study to examine experimentally the potential oxidation of TBBPA in irradiated HA solution.
Materials and methods
Materials. HA was purchased from Fluka (Switzerland). TBBPA, sodium azide, furfuryl alcohol and Fremy’s salt were purchased from Aldrich Chemical Co. (Milwaukee, WI). Dissolved organic matter (DOM), previously characterized by Bilski et al. (21), was obtained from a pond in Alburg Dune State Park, VT. Deuterium oxide was purchased from Cambridge Isotope Laboratories (Andover, MA). 5,5-Dimethyl-1-pyrroline N-oxide (DMPO), obtained from Alexis Biochemicals (San Diego, CA), was vacuum distilled and stored at −70°C until use. Catalase and superoxide dismutase (SOD) were from Boehringer Mannheim (NJ). All other chemicals were reagent grade or better.
All measurements were performed at room temperature. Buffers were prepared from reagent grade or better components, and pH was measured using a glass electrode.
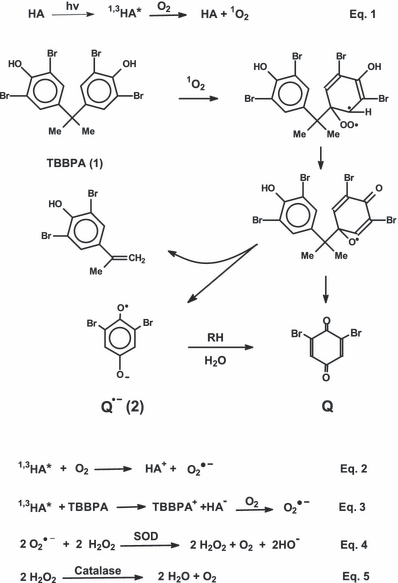
Singlet oxygen generation. Generation of 1O2 by HA was confirmed experimentally by measuring the 1O2 phosphorescence spectrum as described previously (25,26). Briefly, the 1O2 phosphorescence measurements were performed in D2O using a broadband interference filter for HA irradiation; the light source was a 450 W Hg lamp. A liquid nitrogen-cooled germanium diode was used for signal detection. RB was used as a 1O2 standard for spectral comparison as shown in Fig. 1.
Photosensitization of 1O2 by humic acid in D2O. HA, 1O2 phosphorescence spectrum photogenerated by HA (0.25 mg mL−1); RB, 1O2 spectrum generated by rose bengal for spectral reference. HA was irradiated using a broadband interference filter (270–360 nm), while RB was irradiated using a 546 nm interference filter. The spectra are scaled differently, thus they do not reflect the relative observed 1O2 phosphorescence intensities.
Absorption and irradiance spectra. Absorption spectra were recorded on Hewlett-Packard (Palo Alto, CA) model 8451A diode array spectrophotometer. The spectral irradiance of the sun was measured with a SPR-01 Spectroradiometer (Luzchem Research, Inc., Gloucester, ON, Canada).
EPR study. EPR spectra were recorded using a Bruker EMX spectrometer operating at 10 mW microwave power (9.78 GHz) with 1 G modulation amplitude and 100 kHz modulation frequency. Samples were placed in a quartz flat cell and irradiated directly inside the microwave cavity of the spectrometer using a 1 kW Xe arc lamp. The radiation from the lamp was passed through a 30 mm pathlength liquid filter (λ > 400 nm; aqueous solution containing in g L−1: NaNO2 48.4, Na2CO3 1, K2CrO4 0.2). Hyperfine coupling constants were obtained by accumulating, simulating and optimizing spectra on an IBM PC computer using software described elsewhere (27). The accuracy of the coupling constant measurements was ±0.02 G. Fremy’s salt was used as a standard for the determination of the EPR spectral g-values.
Oxygen consumption. Oxygen consumption was measured with the aid of a Clark electrode. Samples (2.2 mL) were placed in a jacketed cell and irradiated directly with a 150 W Xe arc lamp filtered to remove wavelengths below 300 nm. Water at 25°C was circulated through the jacket to prevent sample heating.
Results
Singlet oxygen
For most of our experiments, we used commercially available HA as a model for the naturally occurring HAs. We confirmed that this sample did indeed produce 1O2 upon broad band excitation as shown in Fig. 1. This signal is considerably weaker than the standard RB spectrum shown in Fig. 1 for comparison. Note that the HA signal intensity has been considerably expanded relative to the RB spectrum so that the intensities cannot be compared directly. While the HA singlet oxygen signal obtained here was too weak for a reliable quantum yield measurement, we have previously reported quantum yields from naturally occurring DOM that ranged from 0.5% to 6% (21,23). For example the singlet oxygen quantum yield of the Alburg Dune State Park DOM sample shown in Fig. 5 was 0.5%.
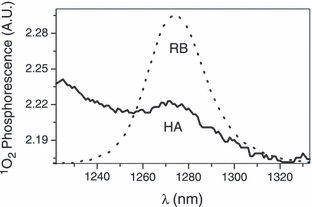
EPR spectrum of TBBPA (1 mm) and HA (1 mg mL−1) or DOM (1 mg mL−1) in 100 mm borate buffer (pH 9.0). (A) HA irradiated (λ > 400 nm). (B) Same as (A), but in the presence of sodium azide (20 mm). (C) DOM irradiated (λ > 400 nm).
EPR studies
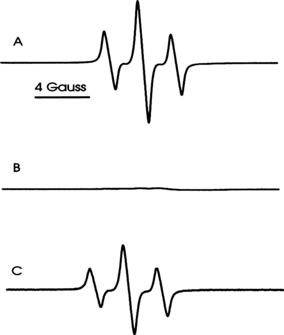
EPR spectra of aqueous HA. In a pH 9 buffer solution, irradiation (λ > 400 nm) of HA produced a singlet EPR signal (Fig. 2B), which was also present at lower intensity before irradiation (Fig. 2A). In the irradiated sample, the signal intensity increased by a factor of about 2. The peak to peak signal width was 2.3 G with g = 2.0045. These spectral parameters are similar to those previously reported for other HAs (14,28,29) and are typical of semiquinone radicals formed by one-electron reduction of quinone moieties in HA. The EPR signal intensity of HA was pH dependent, increasing as the pH was raised from 6 to 10 (Fig. 2C). Similar findings have been previously reported by Senesi and Schnitzer (28).
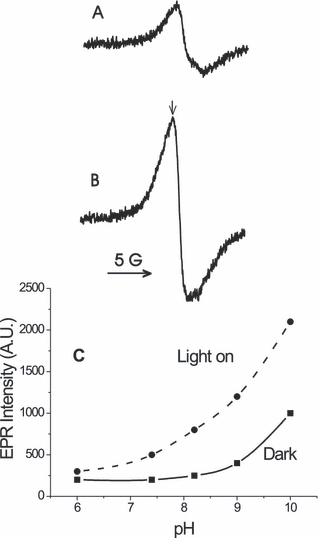
The EPR spectrum of HA (1 mg mL−1) in 100 mm borate buffer pH 9.0. (A) Dark. (B) After irradiation (λ > 400 nm). (C) Effect of pH on HA EPR signal intensity (measured at the arrow in B) before and after irradiation (λ > 400 nm).
We have investigated the temporal changes in the EPR signal to elucidate the possible mechanisms of the interaction between irradiation and HA (Fig. 3). When irradiated (λ > 400 nm), the signal intensity exhibited an initial sharp increase and then continued to increase more slowly. When the light was turned off, the signal showed a rapid decrease followed by a much slower decay toward the initial preirradiation level. A further on–off cycle of the light repeated the cycle. These results are very similar to those reported by Polewski et al. (14).
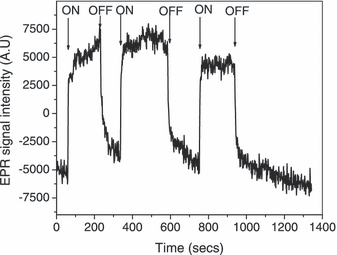
EPR signal intensity (monitored at arrow in Fig. 2) of HA (1 mg mL−1) in 100 mm borate buffer pH 9.0 during irradiation (λ > 400 nm). ON = light on; OFF = light off.
EPR spectra of aqueous HA containing TBBPA. At alkaline pH, TBBPA has an absorption maximum at 320 nm and no absorption above ∼375 nm (Fig. 4). When a solution containing TBBPA and HA was irradiated (λ > 400 nm) in pH 9 buffer, a strong triplet EPR signal (a2H = 2.36 G) was generated (Fig. 5A). The same EPR spectrum was observed when an unbuffered solution of TBBPA and HA adjusted to pH 9 was irradiated (data not shown). We attribute this spectrum to the 2,6-dibromo-p-benzosemiquinone anion radical, which we had previously observed during the oxidation of TBBPA in Triton X-100 by singlet oxygen generated from RB (6). Generation of the 2,6-dibromo-p-benzosemiquinone anion radical was completely inhibited by the addition of sodium azide (Fig. 5B), confirming that its formation was essentially 1O2 dependent. Irradiation (λ > 400 nm) of TBBPA and DOM generated the same EPR spectrum seen when TBBPA and HA were irradiated (cf. Fig. 5A,C).
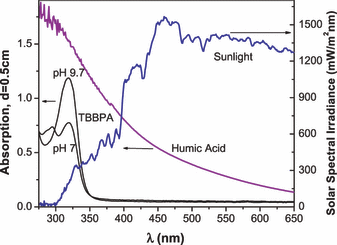
Absorbance of humic acid (0.17 mg mL−1) in 100 mm borate buffer, TBBPA (200 μm) in sulfobetaine-12 (25 mm) adapted from Han et al. (6) and the spectral irradiance of the sun measured at 36°00′N, 78°55′W (clear sky, noon, March 2008).
Spin trapping with DMPO of an aqueous solution of HA and TBBPA. The EPR spectrum of an aqueous solution containing DMPO and HA (Fig. 6A) contained the characteristic four-line signal of the DMPO/•OH adduct (aN = 15.0 G, aH = 14.8 G) and a single broad central line similar to that seen in Fig. 2A. Upon irradiation (λ > 400 nm) the intensity of both signals increased slightly (Fig. 6B). When TBBPA was also present the dark spectrum (Fig. 6C) was similar to that observed in the absence of TBBPA (Fig. 6A). However, irradiation (λ > 400 nm) of this solution generated a more intense DMPO/•OH signal (Fig. 6D). In addition, the single broad center line seen in Fig. 6B was replaced by an intense three-line spectrum attributable to the 2,6-dibromo-p-benzosemiquinone anion radical (cf. Fig. 5A). To determine whether the DMPO/•OH adduct resulted from the trapping of the •OH radical we repeated the experiment in the presence of 300 mm formate (Fig. 6E), which reacts with the •OH radical at the same rate as DMPO (30). As may be seen from Fig. 6E the DMPO/•OH adduct was partially replaced by the DMPO/CO2•− adduct (aN = 15.7 G, aH = 18.8 G). However, the DMPO/CO2•−:DMPO/•OH EPR intensity ratio (calculated from Fig. 6E by simulation [27]) was 1.5, whereas the calculated ratio based on the relative concentrations of DMPO and formate (their rate constants for reaction with the •OH radical are the same) would be 3.4 (i.e. 300/88) if all of the DMPO/•OH adduct was derived from reaction of DMPO with the •OH radical.
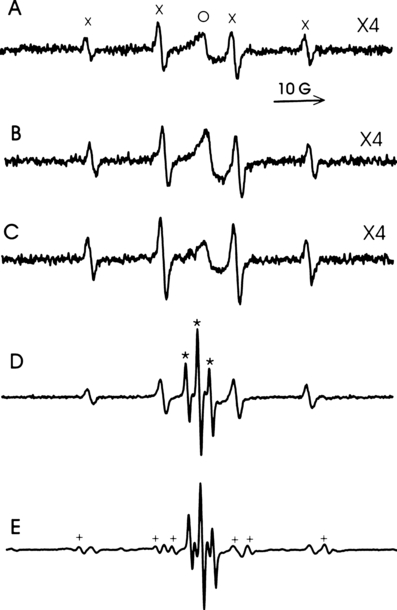
EPR spectra of TBBPA (1 mm), HA (1.0 mg mL−1) and DMPO (88 mm) in 100 mm borate buffer pH 9.0. (A) HA, DMPO in the dark; (B) HA, DMPO irradiated (λ > 400 nm); (C) TBBPA, HA, DMPO, in the dark; (D) TBBPA, HA, DMPO, irradiated (λ > 400 nm); (E) Same as (D) with 300 mm formate. X = DMPO/•OH (aN = 15.0 G, aH = 14.8 G); O = HA•−; * = 2,6-dibromo-p-benzosemiquinone anion radical (a2H = 2.37G); + = DMPO/CO2•− (aN = 15.7 G, aH = 18.8 G).
Oxygen consumption
When TBBPA was irradiated (λ > 300 nm) in the presence of HA, oxygen was consumed (Fig. 7B–E). In the absence of HA, irradiated TBBPA also consumed some oxygen, but at approximately one-third of the rate in the presence of HA (Fig. 7A). Turning off the light completely stopped oxygen consumption in both cases. When NaN3 (20 mm) was added to a solution of TBBPA and HA undergoing irradiation, oxygen consumption slowed markedly (Fig. 7C) because NaN3 is a physical quencher of 1O2. Increasing the NaN3 concentration to 40 mm had no further effect. Adding furfuryl alcohol, which reacts with singlet oxygen to generate peroxide(s) (31), increased the rate of oxygen consumption (Fig. 7E). More importantly, the addition of catalase to TBBPA/HA after irradiation resulted in the recovery of ∼50% of the oxygen consumed (Fig. 7B), indicating that most of the oxygen had been converted to hydrogen peroxide. Subsequent addition of SOD did not alter the oxygen concentration (Fig. 7B). Reversing the order of SOD and catalase addition gave the same result (data not shown). Furthermore, the addition of SOD during irradiation did not alter the rate of oxygen consumption (Fig. 7D).
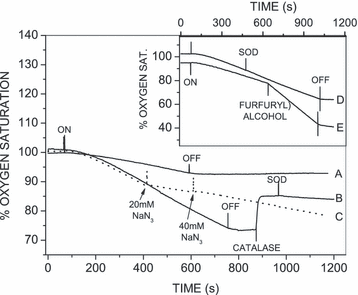
Oxygen consumption during the irradiation (λ > 300 nm) of TBBPA (1 mm) and humic acid (1 mg mL−1) in 100 mm borate buffer pH 9.0. Line A, TBBPA alone; lines B–E, TBBPA and humic acid. Where indicated, NaN3 (20 or 40 mm), furfuryl alcohol (100 mm), SOD (50 μg mL−1) or catalase (320 U mL−1) were added. ON = light on; OFF = light off.
Discussion
The HA that we mainly used in this study is a natural organic matter derived from coal, and it has been used extensively as a model for DOM in surface waters (32–39). Nonetheless, we confirmed that natural DOM which we had previously isolated from an aquatic source (21) gave results identical to HA. Irradiation of TBBPA in the presence of HA resulted in the generation of radicals (5, 6) and oxygen consumption (Fig. 7) both of which were inhibited by sodium azide, a physical quencher of 1O2. Figure 5A,C shows that the same 1O2-induced radical species were produced from DOM, which, based on our previous study (21), was a relatively weak 1O2 producer. Thus it would appear that 1O2 yield of our HA preparation (Fig. 1) was sufficient to initiate the rapid reaction of TBBPA with 1O2 at pH values above its pKa of 7.4 (6), confirming HA as a valid DOM model.
A possible mechanism that would explain our current results is shown in Scheme 1.
The primary radical transients produced from TBBPA (1) by HA were detected using EPR and their identification is based on the similarities to our previous work (6). Reaction of 1O2 initially generates a hydroperoxide (31) that is oxidized to a peroxyl radical followed by conversion to an alkoxyl radical, which decomposes to give the 2,6-dibromo-p-benzosemiquinone anion radical (Scheme 1 [2]) detected by EPR (Fig. 5A). There are, however, noticeable differences regarding oxygen consumption and recovery between the previous results and our current observations. First, in our previous findings with RB as a 1O2 photosensitizer, none of the consumed oxygen could be recovered by catalase (6), which is in contrast to the photosensitization by HA. Second, in contrast to the previous studies with RB, the present photoconsumption of oxygen was not completely inhibited by sodium azide, suggesting some contributions other than those initiated by 1O2. Our present results can be rationalized if HA is not only needed for 1O2 generation (Scheme 1, Eq. 1) but also for other oxidation processes (Scheme 1, Eqs. 2 and 3).
The addition of catalase (Scheme 1, Eq. 5) resulted in the recovery of ∼50% of the consumed oxygen from hydrogen peroxide (Fig. 7), unlike the experiments in which HA was lacking (6). This suggests other oxidation mechanisms that must be HA dependent. SOD (Scheme 1, Eq. 4) had no effect on oxygen consumption, although superoxide radical is a known precursor of hydrogen peroxide. The dismutation reaction may be less sensitive to SOD because the substrates are adsorbed on HA (24) and diffusively free superoxide does not seem to be generated during TBBPA oxidation. The oxidation of a variety of hydroquinones by oxygen may be either increased or decreased by SOD (40), which further complicates data interpretation. In addition, based on the published reports, other ways of hydrogen peroxide production are theoretically possible.
Skurlatov et al. (41,42) proposed that 1O2 may react with polychlorinated semiquinone anion radicals to generate hydrogen peroxide without superoxide as an intermediary. The mechanism involves the formation of a partial charge transfer intermediate between 1O2 and the polychlorinated phenol. If a similar intermediate is formed between 1O2 and the 2,6-dibromo-p-benzosemiquinone anion, then hydrolysis would generate hydrogen peroxide and 2,6-dibromo-p-benzoquinone. The 2,6-dibromo-p-benzosemiquinone anion radical (2) might also be directly reduced by oxygen or 1O2. However, the presence of two electron-withdrawing bromine atoms in the 2,6-dibromo-p-benzosemiquinone anion radical may inhibit such a process. Interestingly, even a highly oxidative hydroxyl radical can be generated in the TBBPA-HA system. The hydroxyl radical was trapped by DMPO (Fig. 6D) and could be generated by the reaction of brominated phenolic TBBPA photoproducts with hydrogen peroxide as previously reported by Zhu et al. (43) or by the Fenton reaction.
We presume that a substantial part of the oxygen consumption and all its recovery must be due to the reactions involving HA. Note that HA slowly photoconsumed oxygen (Scheme 1, Eq. 2) even without the presence of TBBPA (Fig. 7). These new reactions must involve a direct (redox) interaction between the excited HA chromophores and the phenolic moiety in TBBPA (Scheme 1, Eq. 3). The oxidation initiated by such means is not quenchable by sodium azide, yet the superoxide radical anion should be an intermediate. Its production may be less sensitive to SOD due to the heterogeneous nature of the TBBPA-HA system (24). The concomitant oxidation of TBBPA and HA explains the production of hydrogen peroxide and subsequent oxygen recovery by catalase. While the direct oxidation of TBBPA by excited HA may deserve more consideration, it was not the main objective of our present investigation.
The photochemical degradation of TBBPA in surface waters may also occur directly as a result of absorption of sunlight. However, at pH values above its pKa TBBPA has an absorption maximum at 320 nm and no absorption at wavelengths longer than ∼360 nm (Fig. 4). Thus while direct photolysis of TBBPA can occur (6,7), its absorption maximum is at a wavelength where the UV emission of the sun is weak (Fig. 4). Because TBBPA has poor aqueous solubility (∼10−3 mg L−1), when present in surface waters it is likely to be associated with dissolved organic materials such as the HAs (24). In contrast to TBBPA, the optical absorption of HA extends well into the visible range where solar radiation is strong (Fig. 4). Thus the degradation of TBBPA in the environment may result from reaction with photochemically generated reactive species such as hydrogen peroxide, the superoxide anion and 1O2. While the 1O2 yield of our HA preparation was quite low (Fig. 1), we have previously characterized several HA samples from natural sources, such as Mississippi and Atchafalaya River plumes (23) and some inland sources such as Minnesota and Vermont ponds (21), and found that they were all capable of generating 1O2 upon irradiation with quantum yields that ranged from 0.5% to 6%.
Our results demonstrate that TBBPA is directly oxidized by 1O2 photogenerated by HA, and that this mass-produced BFR is degraded via subsequent radical reactions. It seems reasonable to assume that similar processes may contribute to the environmental degradation of TBBPA in natural aquatic systems. Similar transient radical products from TBBPA were found to be toxic in vivo (44). In addition, halogenated phenols and quinones are potential end products likely to accumulate during TBBP oxidation (Scheme 1). Halogenated phenols are known to be potential endocrine disruptors (45), while brominated quinones may be potent photosensitizers. The photodegradation of TBBPA may result in the generation of a wide variety of brominated phenols (7). Our data also show that HA is not only needed for 1O2 generation but also for other oxidation processes involving TBBPA. All these suggest that photoproducts from the photoinduced degradation of TBBPA may pose a hazard for natural aquatic bio-systems and humans.
Acknowledgments
Acknowledgements— This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and the Mokpo National Maritime University Fund.




