Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy
Abstract
Photodynamic therapy (PDT) is now a well-recognized modality for the treatment of cancer. While PDT has developed progressively over the last century, great advances have been observed in the field in recent years. The concept of dual selectivity of PDT agents is now widely accepted due to the relative specificity and selectivity of PDT along with the absence of harmful side effects often encountered with chemotherapy or radiotherapy. Traditionally, porphyrin-based photosensitizers have dominated the PDT field but these first generation photosensitizers have several disadvantages, with poor light absorption and cutaneous photosensitivity being the predominant side effects. As a result, the requirement for new photosensitizers, including second generation porphyrins and porphyrin derivatives as well as third generation photosensitizers has arisen, with the aim of alleviating the problems encountered with first generation porphyrins and improving the efficacy of PDT. The investigation of nonporphyrin photosensitizers for the development of novel PDT agents has been considerably less extensive than porphyrin-based compounds; however, structural modification of nonporphyrin photosensitizers has allowed for manipulation of the photochemotherapeutic properties. The aim of this review is to provide an insight into PDT photosensitizers clinically approved for application in oncology, as well as those which show significant potential in ongoing preclinical studies.
Introduction
Heliotherapy, the therapeutic exposure to sunlight, was known to the ancient Greeks and Indians as a treatment for several skin disorders (1,2). However, the earliest endeavor to manipulate the phenomenon of heliotherapy (photosensitization) was made by von Tappeiner and Jesionek in 1903 (3) utilizing topical eosin and visible light for the treatment of a skin tumor. Later, photodynamic therapy (PDT) was described as a dynamic interaction involving light, a photosensitive agent and oxygen which results in tissue damage (4).
The principle of PDT is based on the selective uptake of a photosensitizer that localizes to a specific tumor cell/tissue type, followed by irradiation with low-energy tissue-penetrating light of the appropriate wavelength to activate the sensitizer (5). Upon activation, the photosensitizer is promoted from its ground state (S0) to the first excited singlet state (S1), from which point a chain of further electronic transitions can occur (Fig. 1). This pathway involves an intersystem crossing from the sensitizers S1 state to its longer-lived triplet state (T1). In the absence of triplet state population, the excited photosensitizer returns to its ground state with the emission of light, known as fluorescence, and/or by means of radiationless transitions whereby energy is given to or taken up by another particle or system. Population of the triplet state is necessary in order to produce the reactive oxygen species (ROS) necessary to initiate cell death. In most cases, the key ROS of PDT is singlet oxygen (1O2), which is the lowest excited electronic state of oxygen. There is much controversy regarding the half-life and consequently, diffusion distance of the transient oxygen species. However, recent studies have shown that the intracellular lifetime of singlet oxygen is ∼3 μs in a viable, metabolically active, H2O-containing cell (6). This lifetime is significantly longer than that reported previously by investigators (7–9). The resulting intracellular diffusion distance of singlet oxygen has been estimated to be 2–4 × 10−6 cm2 s−1 (10). This short singlet oxygen lifetime and resulting diffusion distance makes PDT a highly selective form of cancer treatment due to the localized effect it produces.
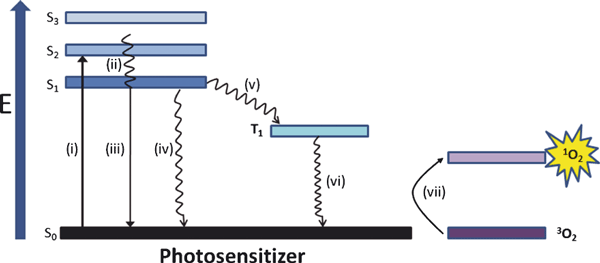
Jablonski diagram showing the photoprocesses involved in excitation of photosensitizers. Absorption (i), internal conversion (ii), fluorescence (iii), radiationless transitions (iv), intersystem crossing (v), phosphorescence (vi) and energy transfer (vii).
The mechanism of action of PDT is dependent on various factors including cell genotype (11,12), PDT dose (13), intracellular adenosine triphosphate (ATP) levels (14) and photosensitizer localization. Most PDT sensitizers tend not to accumulate in nuclei; therefore, PDT is unlikely to induce DNA damage, mutations and carcinogenesis (8). Mitochondrial-localizing photosensitizers are likely to induce apoptosis, while plasma membrane-localizing sensitizers are more likely to cause necrosis when exposed to light (15,16). In general, the mode of cell death switches from apoptotic to necrotic cell death when the intensity of the insult is excessive, producing rapid cell lysis rather than an organized programmed cell death effect (17). In recent years numerous publications have also reported activation of an autophagic mode of cell death following irradiation of certain photosensitizers (18,19). Moreover, the immune response to PDT is now well-documented and has been shown to enhance efficacy of PDT in vivo (20,21). The complex cellular mechanism of action and activation of immune response to PDT is beyond the scope of this review; nevertheless, several manuscripts focusing on this area have recently been published (11,22–26).
The clinical application of PDT in the treatment of cancer has now begun to change. Most recently, treatment regimens have been applied which seek to elicit a vascular-targeting effect. The advantage of targeting tumor vasculature over individual tumor cells is the resultant irreversible destruction to tumor blood supply resulting in long-term tumor cure rates (27,28). As seen with the palladium-bacteriopheophorbide, Tookad, a short photosensitizer exposure-light interval (even simultaneous drug and light administration), confines the photosensitizer to the tumor vasculature and prohibits accumulation within cellular compartments of neoplatic tissue. Immediate irradiation induces vessel occlusion and stasis, leading to ischemia and necrosis of tissue, and eventually tumor ablation (29). Combined therapy utilizing PDT and anti-angiogenic drugs such as Avastin (30) or appropriately targeted inhibitors of angiogenesis such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP) is also emerging as a major focus of PDT research (31).
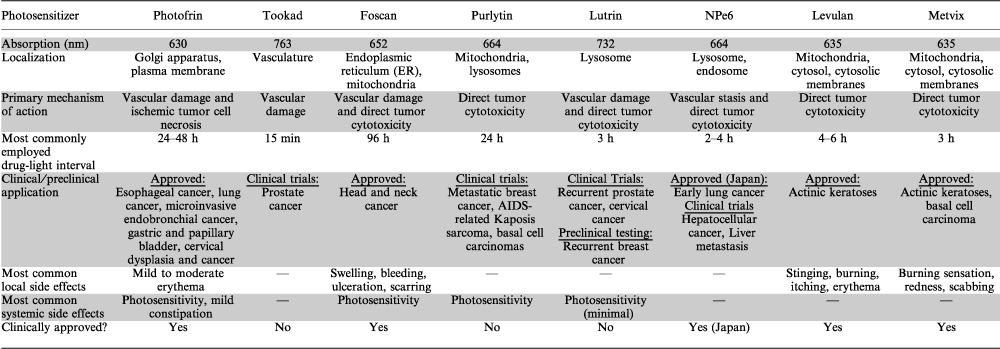
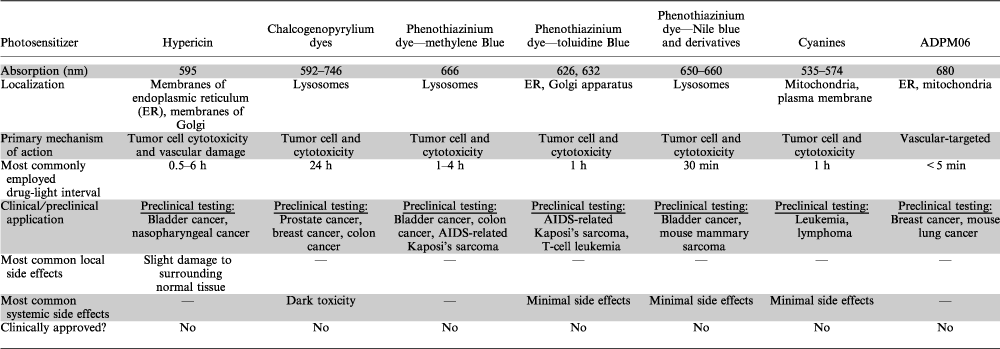
The aim of this review is to discuss the leading porphyrin and nonporphyrin PDT photosensitizers currently in development for the treatment of cancer. Each photosensitizer will be discussed in terms of its photophysical, photochemical, pharmacological and photobiological properties. Tables 1 and 2 summarize these properties. A number of porphyrin photosensitizers have entered clinical trials and indeed many have now received approval for application in oncology (Table 3). The side effects associated with some of these agents, as well as poor light absorption properties and suboptimal tissue penetration have stimulated interest in the development of new photosensitizers. Herein, we will discuss how many of these newer PDT agents are nonporphyrin based, and how they may demonstrate improved activity and reduced toxicity compared with certain porphyrin-based PDT agents.
| Photosensitizer | Approved indications |
|---|---|
| Photofrin | Obstructing esophageal cancer, obstructing lung cancer, microinvasive endobronchial cancer, gastric and papillary bladder cancer, cervical dysplasia and cancer |
| Levulan | Actinic keratoses |
| Metvix | Actinic keratoses basal cell carcinoma |
| Foscan | Head and neck cancer |
| NPe6 | Early lung cancer |
- PDT, photodynamic therapy.
Ideal photosensitizers
In order to compare established as well as emerging photosensitizers, specific characteristics of an ideal photosensitizer should be considered (Table 4). Although certain features of a photosensitizer can be easily modified (such as wavelength absorption), other aspects such as manipulation of the pharmacokinetic (PK) profile of the photosensitizer are not as easily controlled (32). Therefore the pursuit of a novel photosensitizer with the ability to fulfill the necessary chemical, physical and biological requirements of an ideal photosensitizer is still ongoing.
| Purity | Should be a single pure substance of known composition that is stable at room temperature |
| Toxicity | Minimal toxicity in the absence of light and only cytotoxic in the presence of light of defined wavelengths. Photosensitizer should not yield toxic metabolites. |
| ADME | Optimal absorption, distribution, metabolism and excretion (ADME) properties for relevant indication |
| Activation | Absorb light wavelengths between ∼700 and 850 nm for maximum light penetration through tissue with minimum light scattering |
| Quantum yield | High single oxygen quantum yield (Φ) for photochemical event—usually production of singlet oxygen and other ROS |
| Cost and availability | Inexpensive and commercially available in order to promote extensive utilization of treatment |
| Selectivity | Accumulation within tumor tissue is favorable. Subcellular localization within organelles which induce an apoptotic rather than necrotic mode of cell death such as mitochondria is also beneficial |
| Mutagenicity/carcinogenicity | Neither mutagenic nor carcinogenic effects should result from the photosensitizer |
- ROS, reactive oxygen species.
Classification of PDT photosensitizers
Photosensitizers are generally classified as porphyrins or nonporphyrins (Fig. 2a,b, respectively). Porphyrin-derived photosensitizers are further classified as first, second or third generation photosensitizers. First generation photosensitizers include hematoporphyrin derivative (HpD) and Photofrin. A number of second generation photosensitizers have been developed to alleviate certain problems associated with first generation molecules such as prolonged skin photosensitization and suboptimal tissue penetration (1). These second generation photosensitizers are chemically pure compared with first generation compounds, absorb light at a longer wavelength and cause significantly less skin photosensitization post-treatment. In addition, second generation compounds must be at least as efficient in eradicating tumors as Photofrin, the current gold standard for PDT (33). Second generation photosensitizers bound to carriers such as antibodies and liposomes for selective accumulation within tumor tissue are referred to as third generation photosensitizers and currently represent an active research area in the field (24). Although the majority of photosensitizers at the preclinical stage are porphyrin derivatives, a diverse number of nonporphyrin photosensitizers also exist. Significant effort is now being employed in the synthesis of pure chemical derivatives with improved activity and minimal side effects.
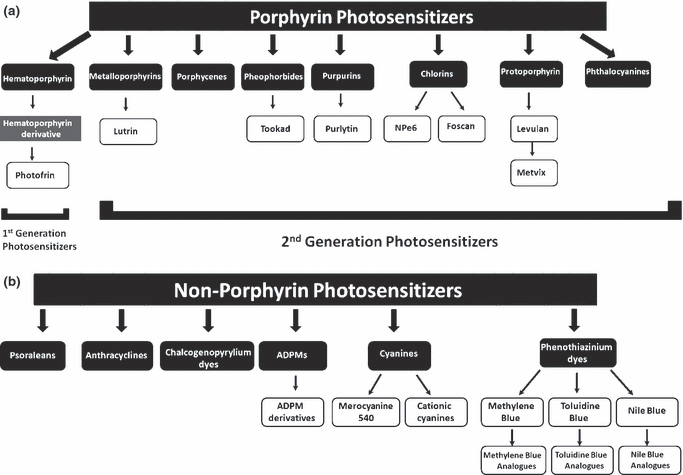
Classification of photosensitizers as (a) porphyrin-based or (b) nonporphyrin-based molecules.
Porphyrin-based PDT agents
Porphyrins are a class of tetrapyrroles which comprise a major component of hemoglobin and myoglobin, two O2-binding proteins found in blood. Porphyrins are essential for the biological activity of all living organisms. These molecules possess a highly conjugated, heterocyclic macrocycle and may also contain a central metallic atom such as ferrous iron and magnesium. The presence of a 22 π electron system gives rise to their long wavelength absorption. As a result, porphyrins have attracted the attention of researchers globally for application as photosensitizing agents in medicine (34). Various classes of porphyrins exist, which are responsible for controlling oxidation and reduction processes in nature (35). HpD is one porphyrin which has been successfully used as a PDT photosensitizer in the area of oncology and is discussed in some detail below.
First generation photosensitizers. Hematoporphyrin (Hp) was first produced in 1841 by Scherer during investigations into the nature of blood. Scherer found that heat treatment of dried blood with concentrated sulfuric acid with subsequent removal of iron and treatment with alcohol produced Hp. It was not until 1867 that the fluorescent properties of Hp were described (36) with the name Hp being given to the compound in 1871 (37). Early studies (38–40) demonstrated the potential of Hp as a diagnostic tool for the detection of cancers; however, one of the major drawbacks was the significant dose required to achieve consistent photosensitizer uptake in tumors, which resulted in inappropriate photosensitivity. In 1955, Schwartz et al. (41) demonstrated Hp to be impure, consisting of a mixture of porphyrins with different properties. Following partial purification, the partly purer Hp localized very poorly to tumors, whereas the residue left behind from purification was shown to possess a great affinity for malignant tissue. Subsequent studies demonstrated that treatment of crude Hp with acetic and sulfuric acids, followed by filtering and neutralizing with sodium acetate and dissolution in saline produced a substance which became labeled as HpD. The tumor localizing property of HpD was found to be superior to Hp. A number of studies carried out in the 1960s by Lipson et al. (42–44) revealed that HpD has the ability to accumulate selectively in neoplastic tissue using much lower doses than Hp.
Following the early work of von Tappeiner and Jesionek (3) into photosensitization, modest research on the clinical therapeutic applications of PDT was performed until 1972 when Diamond et al. (45) demonstrated that a crude hematoporphyrin preparation resulted in regression of an experimental glioma in rats when irradiated with white light. Six years later, Dougherty et al. (46) reported the complete response of various malignant tumors, including carcinomas of the breast, colon, prostate and malignant melanomas using HpD-mediated PDT. Subsequently, considerable attention was focused on refining this seminal PDT therapeutic and determining the active component involved in HpD-based treatment of cancers. Partial purification of HpD was successfully completed, with many of the less active monomers removed, to produce Photofrin, a lyophilized concentrated form of monomeric and oligomeric hematoporphyrin derivatives which continues to represent the “gold standard” in PDT applied to various nonskin-derived cancers (47). Hp, HpD and Photofrin are all referred to as first generation photosensitizers of PDT.
Photofrin. Photofrin, also known as Porfimer sodium and dihematoporphyrin ether, is a purified HpD with an absorption maximum of 630 nm and a low molar extinction coefficient of 1170 m−1 cm−1 (5). This low molar extinction coefficient means that high concentrations of Photofrin and light are required for adequate tumor eradication. Photofrin has also been shown to have an exceptionally long half-life of 452 h causing long-lasting photosensitivity (48). Photofrin-mediated PDT involves intravenous (I.V.) administration of photosensitizer followed by irradiation 24–48 h later. During this period Photofrin is cleared from a number of tissues. However, clearance from tumor tissue, skin and organs of the reticuloendothelial system is significantly slower (49). Upon irradiation, tumor cell damage is caused by generation of ROS. In addition, tumor ablation is also caused by ischemic necrosis resulting from vascular damage of diseased tissue (50).
Photofrin has been approved for clinical use in the treatment of early- and late-stage lung cancers, esophageal cancer, bladder cancer, malignant and nonmalignant skin diseases and early-stage cervical cancer (33). It is also being considered as a potential therapy against Kaposi’s sarcoma, Barrett’s esophagus with high-grade dysplasia, psoriasis and cancers of the head, brain, neck and breast (51).
For early-stage lung cancer, Photofrin-mediated PDT is a relatively recent addition to the already well-established treatment methods of surgical resection, radiotherapy and chemotherapy (52). Successful application of Photofrin-based PDT has been achieved in patients with nonsmall cell lung cancer (NSCLC), each of which were considered suitable surgical candidates prior to PDT treatment. Of one group of 13 patients, 12 (92%) attained a complete response, with 10 patients demonstrating such response following the initial PDT treatment. The remaining two patients required a second treatment for a complete response to be obtained (33).
In a pilot study using Photofrin-mediated PDT in patients with inoperable NSCLC, 10 of 11 patients achieved complete remission at Stage 1 NSCLC. The remaining patient and 11 out of 15 stage III NSCLC patients showed only a partial response to treatment (52).
Photofrin-mediated PDT of superficial bladder cancer has also been successfully implemented. Seventy-five to eighty percent of bladder cancer patients initially present with superficial tumors (53), making PDT an ideal alternative treatment option for this tumor type. Photofrin-mediated PDT has been evaluated in the treatment of resistant superficial transitional cell carcinoma (TCC) and refractory carcinoma in situ (CIS) of the urinary bladder in 58 patients (54). A single PDT treatment of Photofrin (1.5 or 2 mg kg−1 and 10–60 J cm−2 light [630 nm]) resulted in a therapeutic response in 84% and 75% of patients with residual resistant papillary TCC and refractory CIS, respectively. At a median follow-up of 50 months, 59% (34/58) of the responders were still alive, of which 31 remained disease-free.
The mechanisms of action of Photofrin-mediated PDT include vascular endothelial cell damage with hypoxia and thrombosis, ischemic tumor cell necrosis, as well as intense local inflammation associated with immune response (55). Consequently, treatment of bladder cancer patients with Photofrin PDT sometimes triggers the onset of numerous side effects including an increase in urinary frequency, urgency, nocturia, suprapubic pain, bladder spasm and occasionally permanent bladder contraction (56). These side effects have mainly occurred in patients where the integrating sphere effect of light in the bladder is not taken into account (57). As a result, the light dose required for activation of Photofrin will vary largely from patient to patient.
Although Photofrin remains one of the most frequently used PDT agents and is still viewed as the “gold standard” for this form of therapy in oncology, it is far from being an ideal photosensitizer. Photofrin is a complex and undefined mixture of dimeric and oligomeric compounds having poor tissue penetration due to its relatively weak absorbance in the red region of the spectrum. In fact, the precise composition of Photofrin remains unresolved. Capillary electrophoresis of the mixture has revealed that it contains in excess of 60 components. Therefore, it is doubtful that a single active compound exists. In addition, the drug is not selective at 2 mg kg−1, the normal dosing concentration, thus substantial prolonged photosensitivity occurs forcing patients to stay out of sunlight for at least 4 weeks post-treatment (58). For these reasons, alternatives to Photofrin have now been developed.
Second generation photosensitizers. While no single photosensitizer has yet been found which can be regarded as an ideal candidate for all conceivable applications in oncology, a wide variety of second generation photosensitizers have been developed to surmount the inadequacies of Photofrin and other first generation photosensitizers (51). One of the most promising of these second generation photosensitizers is palladium-bacteriopheophorbide (Tookad) (Fig. 3b), a novel compound which is derived from the photosynthetic pigment BChl a.
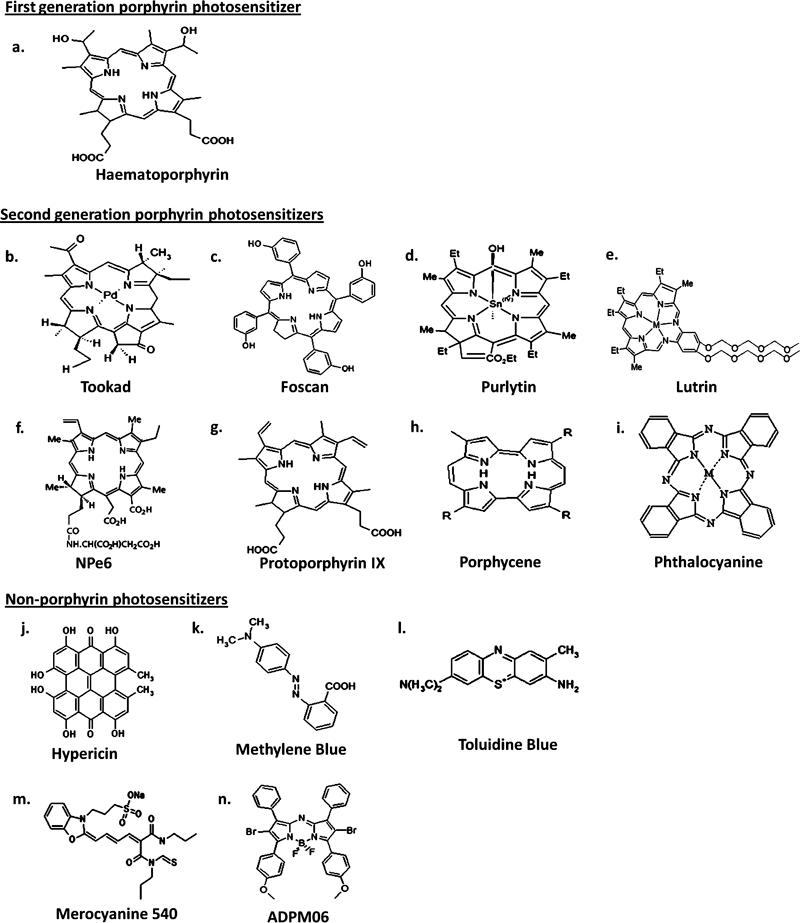
Chemical structures of PDT photosensitizers used in oncology indications.
Tookad. Tookad (palladium bacteriopherophorbide photosensitizer, WST09) (Fig. 3b) is a palladium-metalated bacteriopherophorbide derived from BChl, the bacterial equivalent of plant chlorophyll. Tookad possesses a high molar extinction coefficient (ε0 = 10.86 × 104 m−1 cm−1 in chloroform), maximum absorption wavelength in the near-infrared region (763 nm), light penetration depth of 4 mm using a 763 nm light source (compared to 1.6 mm light penetration with Photofrin using a 630 nm light source [59]) and fast PKs—95% of compound is cleared from circulation of mice in 15 min preventing accumulation in tissues, a characteristic necessary for localized tumor and isolated metastases treatment (60). In addition, it has been shown to have a plasma half-life of ∼20 min in mice (61). Tookad-based PDT possesses an advantage over several well-established PDT regimens in terms of its short drug exposure–light interval. This facilitates minimal delay between administration of drug and irradiation with light. When activated, Tookad targets the vasculature of the tumor, initiating inflammation, hypoxia, necrosis and tumor eradication (62).
Studies have been performed using Tookad-mediated PDT on a localized human prostate cancer xenograft model and normal canine prostate. The results suggest that Tookad PDT may offer a feasible treatment option for primary prostate cancer.
Studies examining Tookad efficacy in vivo have also been carried out using human prostatic small cell carcinoma xenografts, as well as established bone metastasis and orthotopic prostatic models (62). A single treatment of Tookad-based PDT produced a 69% cure rate in subcutaneous tumors, 90 days post-treatment. Metastatic tumors responded with complete tumor remission in 50% of cases. Orthotopic models of disease also yielded similar results.
Preclinical studies have also revealed that treatment of normal canine prostate using Tookad-mediated PDT causes minimal structural or functional damage (60). No urethral obstruction or sensitivity of the colon, rectum and bladder was observed in the canine prostate model post-PDT. This represents a further safety margin, thus allowing for a more forceful therapy against prostate cancer using Tookad-based PDT.
Recently, an assessment of the safety and treatment response of Tookad in 24 prostate cancer patients was completed (63). Early follow-up of patients revealed no serious complications associated with the treatment. PK measurements of plasma drug concentration revealed negligible quantities of drug remaining in patients 2 h postinfusion and no cutaneous photosensitivity was evident 3 h after infusion.
Further studies examining the effect of escalating light doses for Tookad-mediated PDT for the treatment of recurrent prostate cancer after failure of external beam therapy have recently been published (64). Patients received a fixed photosensitizer dose of 2 mg kg−1 and treatment response was determined through prostate-specific antigen (PSA) levels, measurement of avascular areas of tissue 7 days post-treatment through magnetic resonance imaging (MRI) and a 6-month biopsy. Eight out of 13 patients who received a light dose greater than the calculated threshold 23 J cm−2 had a complete response to treatment and side effects were minimal. However, some patients who received similar light doses only responded partially to treatment which may possibly be due to patient variation in drug PKs. Further clinical trials assessing Tookad-mediated PDT have commenced.
Tookad appears to be photochemically and pharmacologically superior to other clinically used photosensitizers to date. There is little or no skin-associated photosensitivity due to the extremely short half-life of the drug. A further advantage is its ability to be activated at a relatively long wavelength resulting in greater tissue penetration. Tookad overcomes several of the disadvantages associated with first generation photosensitizers and also appears to be superior to various second generation photosensitizers developed. Although it has yet to be clinically approved, preclinical studies to date would indicate that Tookad represents a promising photosensitizer in the PDT-based treatment of nonsuperficial cancers such as those of the prostate.
Foscan. Meso-tetra-hydroxyphenyl-chlorine (Foscan, Temoporfin) (Fig. 3c) is a single pure chlorine derivative which is photoactivated at 652 nm and has a molar extinction coefficient of 3 × 104 m−1 cm−1. It is an extremely potent second generation photosensitizer which is hydrophobic in nature and has been shown to have a plasma half-life in humans of ∼45–65 h (65). Foscan was approved in Europe for the palliative treatment of neck and head cancers in 2001. Due to the potency of Foscan, smaller drug dose (as low as 0.1 mg kg−1) and light intensity (as low as 10 J cm−2) are required for obtaining a very robust response (5). Moreover, drug doses and light intensity required to obtain a similar response to Photofrin are up to 100 times lower due to the superior photophysical properties and higher singlet oxygen yield of Foscan (66). The mechanism of action of Foscan is via direct tumor cell toxicity as well as vascular damage. PK studies have revealed that neither lipoprotein nor cholesterol metabolism has an effect on the PK of Foscan in humans. The prolonged photosensitizer concentrations observed in plasma over time following I.V. injection may therefore be attributable to the formation of a drug depot in the vasculature (67). Importantly, early clinical studies in patients suffering from malignant mesothelioma have shown that a 10 mm deep tumor necrosis occurs following Foscan treatment (68). In addition, Foscan has been shown to preferentially accumulate in the tumor cells of orthotopic brain tumor implants, with a tumor to normal brain tissue ratio of 100:1, thus making Foscan-mediated PDT a highly selective form of cancer therapy (69–71).
A nonrandomized Phase II study examining the effect of Foscan-PDT in patients with primary cancer of the lip resulted in complete tumor response rates equivalent to those obtained for surgery or radiotherapy (72). Of 25 patients treated, 96% showed a complete tumor response 12 weeks post-treatment, as confirmed by biopsy. Tumor recurrence occurred in two patients, one of whom was effectively retreated with Foscan-induced PDT. In addition to the high cure rate achieved with Foscan-PDT in lip cancer, treatment also provided excellent preservation of function and minimal side effects.
Recently, Foscan-mediated PDT for early oral squamous cell carcinoma (SCC) was investigated in 114 patients (73,74). A response was observed in 85% of patients with tumor remission maintained in 85% and 77% of responders, 1 and 2 years post-treatment, respectively. Importantly, Foscan-based PDT resulted in only mild to moderate skin photosensitivity, mostly only mild erythema, in a small proportion of patients and caused neither functional nor cosmetic impairments that would often result from standard surgery or radiotherapy of this type of cancer.
Studies examining the effects of Foscan in 128 patients with advanced incurable SCC of the head and neck have also been carried out (75). No further standard treatment options were available for these patients and the majority had previously received surgery, radiotherapy and/or chemotherapy as part of their treatment regimes. Foscan-PDT produced a tumor response of 54%, with a complete tumor response observed in 30% of patients, as well as a substantial clinical quality of life benefit demonstrated in 61% of patients. This study clearly proves that Foscan-mediated PDT is of significant clinical benefit and can improve the quality of life of patients who have exhausted standard treatment regimes.
Although Foscan appears to be one of the most active photosensitizers, a major drawback of the drug is prolonged skin photosensitivity which can last for several weeks following treatment. The optimal activity of Foscan requires light treatment up to a week following I.V. administration of the drug which also represents a disadvantage of the treatment regime (76). Aside from the drawbacks, Foscan is an effective photosensitizer and may offer clinical benefit in patients who have exhausted standard treatment regimes.
Purlytin. Tin ethyl etiopurpurin (SnET2, Purlytin) (Fig. 3d) is a second generation photosensitizer which has shown significant potential in Phase I/II studies in the treatment of metastatic breast adenocarcinoma, basal cell carcinoma (BCC) and Kaposi’s sarcoma in patients with acquired immunodeficiency syndrome (77). Purlytin is a synthetic purpurin that is structurally analogous to chlorophyll and is activated by 664 nm light. Structurally, Purlytin has a tin atom chelated into its center which results in a redshift absorbance of ∼20–30 nm (with respect to Photofrin) (78). It has a molar extinction coefficient of ∼2.8 × 104 m−1 cm−1 (51). The mechanism of action of Purlytin is via direct tumor cell cytotoxicity. Upon I.V. injection of Purlytin in a Cremophor-EL emulsion, a preferential binding by low-density lipoproteins (LDL) and high-density lipoproteins (HDL) has been observed (79). It has also been reported that Purlytin causes photosensitivity up to 14 days postadministration due to accumulation in the skin which represents a significant drawback of this photosensitizer (80,81).
The application of SnET2 in the treatment of prostate cancer in a canine model has been assessed (82). Significant amounts of SnET2 were found to be retained within the prostate up to 168 h postadministration. The greatest volume reduction of prostate tumor (60%) was observed using multiple diffusers for transperineal interstitial light delivery compared with transurethral and transperineal illumination (52%).
Studies have assessed the efficacy of tin ethyl etiopurpurin in a single patient suffering from BCC with 13 lesions in total (83). The patient had previously undergone several surgical excisions, as well as initially successful PDT with benzaporphyrin derivative-monoacid ring. A complete response was observed in the patient treated with Purlytin-PDT, with the effect maintained up to 6 months post-treatment.
Further studies examining the effect of Purlytin-mediated PDT on metastatic cutaneous adenocarcinoma of the skin have now also been completed (84). Three patients with biopsy-proven metastatic adenocarcinoma of the skin, all of which had failed previous conventional therapies, were treated and assessed up to 6 months post-treatment to determine efficacy, safety, recurrence, etc. All patients showed a complete response after a single dose of Purlytin-PDT, demonstrated minimal side effects and exhibited exceptional local control of the metastatic lesions, which was maintained up to 6 months post-treatment.
Purlytin possesses some advantage over Photofrin due to its deeper light penetration into tissue. However, the insolubility of this photosensitizer in water requires formulation in lipid-based emulsions which may result in dark toxicity. The photosensitivity resulting from Purlytin is also a concern (77). However, it has been shown to be efficacious in numerous clinical studies to date with complete response rates achieved in various studies.
Lutrin. Lutrin (motexafin lutetium, Lu-Tex) (Fig. 3e) is a member of the family of texaphyrin molecules acting through tumor and vascular-targeting mechanisms. It is a second generation tripyrrolic pentaaza-expanded porphyrin which is mono-anionic in nature (85). Lutrin has an absorption band at 732 nm which allows for better interstitial light delivery due to greater depth of penetration and reduced absorption of light by hemoglobin (86). It has a molar extinction coefficient of 4.2 × 104 m−1 cm−1 (51). Importantly, Lutrin is water soluble and produces good singlet oxygen yield upon irradiation. Moreover, it has been shown to have a plasma half-life of 7 h following I.V. injection in humans resulting in reduced cutaneous photosensitivity (87). Using 14C radiolabelling studies it has been shown that Lutrin selectively accumulates in tumor tissue with a tumor-to-muscle ratio of 10.55:5 h following photosensitizer administration (88). Lutrin accumulation has also demonstrated selectivity for tumor microvasculature, leaving the surrounding vasculature of normal tissue unharmed (89,90).
Studies in a murine EMT-6 sarcoma model have shown that Lutrin localizes primarily to the lysosomal compartment of cells inducing lysosomal degradation, cytoplasmic blebbing and eventually cell death upon irradiation (91). Cell death of EMT-6 neoplasms is initially apoptotic post-PDT, advancing with time towards mass necrosis of neoplastic tissue.
Further studies have assessed Lutrin-mediated PDT in a B16F10 murine melanoma model using both normal and ApoE-deficient mice, the latter displaying a lipoprotein profile which resembles humans (92). Results demonstrate a longer survival and greater efficacy in ApoE-treated mice which is attributable to a greater photosensitizer accumulation in B16F10 tumor as well as a more rapid removal of the photosensitizer from the blood. The improved efficacy and PKs are due to the greater association of Lutrin with LDL and very low-density lipoprotein particles relative to HDL particles in ApoE mice.
The response of mouse mammary adenocarcinoma to Lutrin-PDT has been assessed and compared with the response observed with Photofrin-mediated therapy (93). Treatment of subcutaneously implanted SMT-F mammary carcinoma tumors with 40 mg kg−1 Lutrin and 150 J cm−2 740 nm light resulted in a 100% tumor response and an overall 55% cure rate. Photofrin-PDT yielded a 67% response rate and an overall 16% cure rate. This difference may be due to some extent to the greater tissue penetration of the 740 nm light used with Lutrin-PDT versus the 630 nm used with Photofrin-PDT.
Lutrin has been assessed in a Phase I study of recurrent prostate adenocarcinoma in 16 patients (94). Overall, results were promising with minimal photosensitizer and light-associated toxicities. An immediate but transient increase in PSA was observed directly following PDT. This would suggest optimal concentrations of Lutrin and light fluence required to induce cell death within the prostate were achieved. Nevertheless, intra-patient as well as interpatient heterogeneity of light, oxygen, photosensitizer and tissue optical properties was a significant problem which arose during this study, emphasizing the need for further detailed studies.
A more recent study examining the short-term and long-term effects of Lutrin-mediated PDT on PSA levels has revealed that patients who received a high PDT dose demonstrated a short-term increase in PSA levels as well as a more durable PSA response, compared with patients who received low doses of PDT (95). This data clearly underlines the need for an individualized PDT dosing regimen for the treatment of prostate cancer.
Lutrin has undergone clinical trials for the treatment of locally recurrent breast and prostate cancers and appears to embody many of the characteristics of an ideal photosensitizer such as minimal residual photosensitivity following PDT, good absorbance at 732 nm and solubility in water. One concern associated with Lutrin-mediated PDT is the severe pain experienced by some patients during phototherapy (76). Nevertheless, this problem may be controlled with local anesthesia. Thus, Lutrin may now represent a good alternative to Photofrin and other second generation photosensitizers given its photophysical and pharmacological superiority.
NPe6. NPe6 (mono-l-aspartyl chlorine 6, talaporfin sodium and laserphyrin) (Fig. 3f) is a chemically pure second generation photosensitizer which has been approved in Japan for treatment of early centrally located lung cancer. It is a hydrophilic chlorine, mainly due to the presence of an aspartyl residue, which is derived from Chl a. It exhibits significant absorption at 664 nm and has a molar extinction coefficient of 4 × 104 m−1 cm−1 (96). Optimal irradiation for tumor areas was 2–4 h post-NPe6 administration. It has been shown that a drug–light interval of 6 h decreases the effectiveness of PDT by 50% and efficacy is almost abolished with a drug–light interval of 12 h (97). Morever, a high concentration of NPe6 in plasma rather than in tumor tissue facilitates a more effective tumor treatment (97). Vascular stasis resulting from platelet aggregation and thrombus formation, and direct tumor cytotoxicity are the mechanisms involved in NPe6-mediated PDT (98). Studies examining biodistribution of NPe6 in cancer patients revealed that 100% of I.V. injected NPe6 was bound to plasma proteins, regardless of the dose used. Further analysis showed that ∼67% of NPe6 was bound to albumin and other heavy proteins, ∼31% bound to HDL and 1–2% bound to LDL, with little change in these values over a 21-day period (99). PK profiles of NPe6 in these patients have also been elucidated and have been shown to be consistent with a two compartment model. Half lives of ∼9 and 143 h were determined for NPe6 (99). In vitro studies have revealed a propensity for NPe6 to localize within the lysosome at a subcellular level. NPe6-PDT has also been shown to induce the expression of VEGF, as well as proto-oncogenes c-jun and c-fos in a human oral carcinoma cell line (100).
Preliminary studies assessing the photosensitizing potential of NPe6 in vivo were performed utilizing the EMT-6 sarcoma model (101). A 100% cure rate was observed in animals that received 8 mg kg−1 NPe6 while animals that obtained 0.5–1 mg kg−1 of the drug demonstrated no response to treatment indicating the dose dependency of this drug. In addition, NPe6 and Photofrin were examined in terms of photosensitivity. While Photofrin-mediated PDT resulted in numerous undesirable skin reactions such as blistering and skin sloughing, an absence of skin photosensitization was observed following NPe6-PDT treatment (101).
Phase I clinical trials assessing the safety and tolerability of NPe6 in recurrent subcutaneous tumors have also been completed (102). In all cases, tumor necrosis was observed followed by regression of tumor 24–48 h post-treatment, eventually leading to formation of an eschar over the site of the tumor. At NPe6 doses of 1.65 mg kg−1 and lower, tumor recurrence occurred quite soon after treatment in two out of three patients. However, at NPe6 doses of 2.5 mg kg−1 and higher, a 66% cure rate was observed during the 12-week observation period, yet no perceptible tumor selectivity occurred at these higher concentrations compared with lower concentrations. Importantly, the rapid clearance of NPe6 allowed one patient to be given four sequential doses of the drug over the 12-week period, with no notable toxicity observed indicating the safety of this PDT agent.
A Phase II trial investigating the effect of NPe6-PDT in patients with early superficial SCC of the lung has also been performed (103). Patients possessed CIS or early invasive carcinomas which ranged in size from >1 cm to 2 cm. A complete response rate of 84.6% was attained with the 39 lesions treated and, on a per patient basis, a complete response rate of 82.9% was achieved. Minimal skin photosensitivity and pulmonary toxicity was also observed.
NPe6 is also undergoing Phase III clinical trials for the treatment of hepatocellular carcinoma. Reports have recently been published, outlining the safety and effect on time to tumor progression of an intratumoral light infusion technology in combination with NPe6 in a Phase II trial of patients suffering from refractory liver metastasis resulting from colorectal cancer (104).
NPe6 represents a promising photosensitizer for the treatment of various cancers. It is an effective and safe compound with minimal resultant skin photosensitivity due to its rapid clearance from the circulation. The hydrophilicity of NPe6 also allows for ease of formulation which is of significant benefit (76).
Levulan. Levulan (protoporphyrin IX, 5-aminolevulinic acid) is a second generation photosensitizer which produces a direct tumor cytotoxic effect. The Food and Drug Administration (FDA) approved Levulan for the topical treatment of actinic keratoses of the face and scalp in 1999. Levulan treatment employs the use of the naturally occurring hydrophobic 5-aminolevulinic acid (ALA) which is a porphyrin precursor involved in the heme biosynthetic pathway. ALA is metabolized to photoactive protoporphyrin IX (PpIX) (Fig. 3g) in mitochondria and accumulation of PpIX then occurs when the rate of conversion of ALA to PpIX is greater than the conversion of PpIX to heme (105). When Levulan is applied topically, the resulting PpIX is not only selective for malignant tissue but also the rate of PpIX production is 2–10-fold greater in neoplastic lesions compared with normal epidermis. Several factors contribute to this selective accumulation including significant variation in levels of heme synthesis enzymes, lower concentrations of iron within carcinoma cells reducing the rate of PpIX conversion to heme (106), presence of damaged stratum corneum in abnormal tissue (33) and vascular permability of tumor tissue (107). Following accumulation of PpIX, malignant lesions are subsequently irradiated with light of the appropriate wavelength. Similar to other porphyrins, PpIX has an absorption maximum of 410 nm, along with four smaller peaks near 510, 540, 580 and 635 nm, allowing for irradiation from multiple light sources with diverse spectral yields (108). The molar extinction coefficient of PpIX at 635 nm is <5 × 103 m−1 cm−1 (51). A short time interval of 1–8 h is required between administration of Levulan and maximal accumulation of PpIX in lesions, depending on the route of administration (24,109), with endogenously produced PpIX being cleared from the body within 24–48 h (24). However, the FDA has outlined that an interval of 14–18 h is required between Levulan administration and PpIX accumulation for the treatment of actinic keratoses.
The first study to successfully treat BCCs using Levulan-PDT was performed 18 years ago, with a 90% complete response rate obtained following a single treatment (110). Since then, Levulan-PDT has received considerable interest in the treatment of superficial carcinomas. Topical Levulan-mediated PDT has been approved for the treatment of actinic keratoses of the head and scalp, and it is also effective in treating various other nonmelanoma skin cancers (NMSC) (111).
Following on from these early successes, almost all reports of Levulan-PDT for treatment of BCC and SCC, Bowen’s disease and actinic keratoses in the early 1990s showed a 100% response rate. A 100% response in actinic keratoses, superficial BCC, Bowen’s disease and keratoacanthomas patients, and >80% response in nodular BCC and superficial SCC 30 days post-treatment was shown (112). Following histopathological examination and long-term follow-up of 24–36 months, 100% response rates were maintained in Bowen’s disease and keratocanthomas patients and final response rates of 84% and 86.9% were demonstrated for actinic keratoses and superficial BCC, respectively (112).
Studies investigating the synergistic effects resulting from addition of dimethylsulfoxide (DMSO) and edetic acid disodium (EDTA) salt, two possible enhancers of PpIX production to Levulan have also been carried out. Results demonstrate a substantial increase in porphyrin production in tumors treated with Levulan–DMSO–EDTA combination cream compared with Levulan cream alone, with the most significant difference observed in the upper 2 mm section of the tumor. Although the increase in porphyrin production did not correlate with an improved complete response rate of superficial BCCs in one study, encouraging results were obtained in Levulan–DMSO–EDTA-treated nodular BCCs indicating a possible method to improve therapeutic effectiveness of topical Levulan-PDT (113,114).
More recently, 209 patients with a variety of dermatological disorders including actinic keratoses, Bowen’s disease and superficial BCC, were successfully treated using Levulan-PDT. Response rates of 91–97% were achieved 48 weeks post-treatment with recurrence rates of 5–10%. Within this study, the use of broadband and laser light sources was compared. Both light sources were equivalent in terms of efficacy; however, a significant increase in pain was observed in patients irradiated with a broadband light (115).
Provided that sufficient pain relief medication is provided, Levulan-mediated PDT offers a noninvasive alternative to traditional treatments such as cryotherapy and topical 5-fluorouracil (5-FU) having excellent cosmetic benefits and allowing for repeated doses without patients experiencing cumulative toxicity (115).
A randomized paired comparison of Levulan-PDT and topical 5-FU (a well-established, selective treatment for actinic keratoses) was conducted for the treatment of actinic keratoses of the hands (116). A 6-month follow-up of the 17 patients treated revealed a 73% reduction in lesional area in PDT-treated lesions compared with a 70% reduction in 5-FU-treated patients. This study also revealed that a single treatment of topical Levulan-mediated PDT appears to be as successful as 3 weeks of twice-daily treatments of topical 5-FU. Additionally, Levulan-PDT appeared to be just as well-tolerated as 5-FU, with no considerable disparity in pain resulting from both treatments over a 4-week period.
Levulan has proven to be very successful in the treatment of actinic keratoses over the past several years. The more obvious advantages of Levulan include the use of the naturally occurring ALA, which is easily synthesized from succinyl CoA and glycine, ease of formulation due to its hydrophilicity, simple treatment protocol, minimal photosensitivity following treatment and excellent cosmetic results. Moreover, treatment can be repeated without cumulative effects. Nevertheless, there is some pain associated during treatment. Clearly, a significant disadvantage of the treatment is the limited depth of tumor necrosis (1 mm). This problem has, however, been addressed through the development of various ALA esters, such as Metvix, Benzvix and Hexvix, which exhibit improved skin penetration (117).
Metvix. Metvix (methyl aminolevulinic acid) is a methyl ester precursor of Levulan which has a greater lipophilicity allowing for greater penetration through the stratum corneum of skin. Its photophysical, photochemical, pharmacological and photobiological properties are similar to Levulan. Metvix is demethylated to ALA through the action of intracellular esterases once inside the target cell. The standard process of biochemical events associated with ALA then occurs (118). The depth of penetration of Metvix fluorescence in BCC lesions following a 3 h uptake has been shown to be up to 2 mm (119) compared to a 1 mm penetration depth of Levulan.
Metvix, which has been approved for treatment of BCC and actinic keratoses in Europe, was recently compared with Levulan-PDT in treatment of scalp actinic keratoses in a randomized, double-blind prospective study (120). Both Metix-PDT and Levulan-PDT elicited substantial reduction in actinic keratoses; however, no significant difference in efficacy was observed between the two. Nevertheless, the intensity of pain experienced by patients during and after Levulan-PDT was considerably greater than that experienced with Metvix-PDT.
Recently, Metvix-PDT was assessed in 97 patients suffering from primary nodular BCC (121). Fifty patients were treated topically with Metvix-PDT while the remaining 47 patients received excision surgery. Five years post-treatment, 14% of Metvix-treated tumors recurred compared to 4% with surgical excision. However, cosmetic results were improved with Metvix-PDT, with a good to excellent outcome in 87% of patients compared to 54% of surgery patients.
Studies assessing safety, tolerability, efficacy and cosmetic outcome of 2–8 individual sessions of Metvix-PDT have been completed in patients with nodular and superficial BCC (122). Complete regression of 89.4% of superficial BCC and 52.2% of nodular BCC was achieved 1 month following two Metvix-PDT sessions. Recurrence of superficial BCC occurred in 2.4% of patients 12 months post-treatment. In addition, cosmetic results were rated as excellent to good.
Metvix possesses a significant advantage over Levulan in that it allows for deeper penetration into malignant lesions and has a greater tumor selectivity, both of which may improve efficacy in cutaneous cancers. As noted previously, Metvix also results in reduced pain during treatment; Levulan and Metvix are now used routinely in dermatological oncology which represents a medical speciality where PDT is currently widely used.
Porphycenes. Porphycenes (Fig. 3h) are a group of second generation photosensitizers which are modifiable isomers of porphyrin. Porphycenes were discovered two decades ago following reorganization of the methine and pyrrole moieties of porphyrin (123). They are characterized by an absorption band above 600 nm, with a molar extinction coefficient of greater than 5 × 104 m−1 cm−1, efficient ROS generation following light irradiation and tumor selectivity (124). The major advantage of porphycenes is the capacity to structurally and chemically modify the side chain in order to enhance therapeutic efficiency through accelerated cellular uptake, enhanced ROS production, improved half-life, etc. (125). PK studies using a hydrophobic tetra-n-propyl-porphycene (TPP) have shown that selective transport of the porphycene by serum lipoproteins occurs upon I.V. injection (126). Moreover, delivery of TPP to malignant tissue is very efficient and selective with a measured ratio of TPP concentration in tumor versus peritumoral tissue of 16.7 at 24 h. Hydrophilic porphycenes on the other hand exhibit lower tumor uptake and selectivity as well as a rapid biodistribution. The plasma half-life of porphycenes is in the range of hours for hydrophobic porphycenes and minutes for more hydrophilic porphycenes (127).
Due to the significant number of porphycene derivatives that currently exist, it is not surprising that all do not follow the same trend in terms of subcellular localization. For example, a porphycene monomer has been shown to localize to mitochondria in P388 cells (128) whereas a 9-acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene localizes to the lysosomal compartment of L1210 cells (129).
Various studies investigating side chain–functionalized porphycenes have demonstrated dramatically enhanced therapeutic characteristics. One such study showed that porphycenes-containing nonionic polar side chain functionalities had significantly superior cellular uptake in fibrosarcoma cells (5–22-fold) compared with alkylporphycenes which resulted in LD50 values reaching nanomolar concentrations. The functionalized porphycenes were also shown to be between 17 and 220 times more photodynamically active than the first generation sensitizer Photofrin (125).
Examination of tumor microcirculation damage in vivo following functionalized porphycene-PDT has been performed (130). In vivo fluorescence microscopy revealed a maximum fluorescence in tumor tissue 30 s following I.V. administration of the porphycene. In addition, irradiation 1-min post-I.V. injection, at which time porphycenes have been shown to be mainly localized in the vascular wall of normal and tumor vessels, resulted in complete remission of all six tumors treated.
Photodynamic therapy, using a range of functionalized porphycenes, has also been performed (131). Fifty percent constriction in blood vessel diameter, substantial reduction in blood perfusion and 90% reduction in tumor size were observed as a result of functionalized porphycene-PDT. A direct correlation was observed between PDT efficacy and the number of polar constituents present on the porphycene side chain.
Porphycenes represent a unique class of second generation photosensitizers due to their modifiable structure. A vast range of porphycene derivatives have been developed; however, the photophysical properties of many of these have yet to be fully characterized. There is little or no photosensitivity associated with this class of molecule due to their rapid clearance from the body, especially in the case of the more hydrophilic porphycenes. In addition, the efficient ROS generation by porphycene-mediated PDT triggers a robust apoptotic and necrotic response in tumor tissue making the porphycenes a potentially valuable and promising class of compounds for clinical application.
Phthalocyanines. Phthalocyanines (Pc) (Fig. 3i) are a group of second generation photosensitizers which are structurally related to porphyrins. The first known appearance of the blue-colored pigment was observed by Braun and Tcherniac (132) in 1907; however, it was not until 1933 that the structure of Pc was elucidated (133). There are numerous commercial uses associated with Pc in the textile, photography and electrical industries, which have been broadly published over the years; however, Pc has attracted attention over the last number of decades in the area of PDT due to their optimal photophysical and photochemical properties. Pc exhibits strong absorption bands at 670–770 nm in the red region of the spectrum and the presence of an appropriate central atom, such as zinc, aluminum or silicon, yields high singlet oxygen production with long-lived triplet states (134,135). Pc also has a molar extinction coefficient of 2.5 × 105 m−1 cm−1. Over the past two decades considerable work has been focused on investigation of the phthalocyanine family of photosensitizers in medicine, of which the extensive work of various Russian authors should not go unnoticed (136).
Thus far, studies using the silicon phthalocyanine photosensitizer Pc 4 both in vitro and in vivo are promising and certainly warrant further assessment. This hydrophobic photosensitizer localizes exclusively to the mitochondria, and upon irradiation, localized damage to the organelle occurs through the formation of ROS. Pc 4 has also been shown to bind lipoproteins and serum albumin, with the proportion of binding to each being dependent on the delivery vehicle used for formulation (137). The choice of delivery vehicle used for photosensitizer formulation may therefore affect outcome in patients.
In vivo studies examining Pc 4-PDT in a U87 human glioma immunocompromised rat model have shown a specific accumulation of Pc 4 in tumor tissue compared with normal brain tissue with an average ratio of 3.89:1, respectively (138). Immunohistochemical and terminal deoxynucleotidyl transferase dUTP nick-end labeling staining of treated specimens demonstrated tumor-specific apoptosis. However, significant levels of necrosis were observed in high light fluence–treated animals.
Clinical evaluation of Pc 4 has been assessed in cutaneous and subcutaneous lesions from diverse solid tumor origins (5). A Phase I clinical trial of topically applied Pc 4 for treatment of mycosis fungoides, the most common form of cutaneous T-cell lymphoma is currently ongoing (139). Preliminary findings from this Phase I trial have been promising with no local or systemic toxicities observed.
One of the main advantages associated with Pc is the architectural flexibility of structure, allowing for the derivitization of molecules possessing differing properties. Pc 4 may potentially be used clinically to treat nonskin cancers, provided the appropriate delivery vehicle is employed (139). When applied topically a short drug–light interval of only 1 h is required. Pc 4 has shown also good efficacy both in preclinical and clinical studies. However, noninvasive spectroscopy has shown variability of Pc 4 within and between lesions which may lead to a heterogenous response in patients (140).
Third generation photosensitizers. A further generation of photosensitizers represents an emerging class worth addressing. Certain second generation photosensitizers have been conjugated to or packaged within carrier molecules that specifically deliver photosensitizers to tumor tissue. Such delivery biomolecules include monoclonal antibodies (mABs), liposomes and polymers (141).
Tumor cells possess cell surface antigens which differ greatly from those of normal cells, thus allowing for the exploitation of mABs as efficient delivery biomolecules of photosensitizers. The mAB-photosensitizer conjugate selectively binds to tumor cells, thus facilitating photosensitization of tumor tissue, while leaving normal tissue undamaged (142).
Recently, a third generation photosensitizer consisting of polyion complex micelles, containing polyethyleneglycol (PEG)-b-P(Asp) and a nano-scaled dendrimer porphyrin has been tested. The nanocarrier exhibited enhanced efficacy compared with dendrimer porphyrin alone in a Lewis lung carcinoma cell line. In addition, the nanocarrier demonstrated reduced dark toxicity which is thought to be due to the biocompatible PEG shell of the micelles (143).
Attempts have been made to improve the selectivity of hypericin for tumor cells using transferrin-conjugated PEG liposomes (144). Previous studies have shown that transferrin–PEG liposomes have the ability to extravasate to and accumulate in solid tumors in vivo (145). However, hypericin was found to leak from PEG liposomes and did not successfully target hypericin to tumor cells or increase photocytotoxicity compared with free hypericin and nontargeted PEG liposomes (144).
Phthalocyanine nanoparticles have also been developed and assessed in vitro in a cervical cancer cell line (146). Nanoparticle irradiation induced an apoptotic mode of cell death within the HeLa cell line. Moreover, PDT efficiency, as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (thiazolyl blue tetrazolium bromide) assay revealed a significant increase in efficacy compared with phthalocyanine-PDT alone.
Amine-functionalized polyacryamide nanoparticles containing Photofrin have also been assessed in a rat brain tumor model (147). Targeting of Photofrin within nanoparticles dramatically reduced the time between photosensitizer administration and irradiation. In addition, Photofrin-encapsulated nanoparticles were targeted directly to the tumor vasculature where they demonstrated a significant increase in treatment efficiency compared with Photofrin alone.
In summary, data thus far assessing third generation photosensitizers looks promising, with improved efficacy relative to the parental photosensitizer observed in the majority of cases. The enhanced biological specificity of these third generation photosensitizers over other generations allow for better photosensitizer delivery to target tissue, thereby improving the efficacy of treatment in many cases (141). Nevertheless, third generation photosensitizers are still largely in early stages of development. As such additional feasibility assessment is required.
Nonporphyrin-based PDT agents
The development of nonporphyrin photosensitizers for application in oncology has lagged considerably behind that of porphyrin-based PDT. To date, cationic photosensitizers, such as chalcogenopyrylium dyes, phenothiazinium and benzo[a]phenothiazinium derivatives, which include methylene blue and toluidine blue, have been the predominant focus. Many cationic dyes accumulate selectively in transformed cells with the mitochondria as their main cellular target (148). Nevertheless, novel photosensitizer families are now being developed (e.g. tetra-aryl-azadipyrromethenes [ADPM]) and provide an exciting new approach to the field of PDT.
Hypericin. Hypericin (Fig. 3j) is a naturally occurring extended quinone with a wavelength absorbance of 590 nm and a molar extinction coefficient of ∼4.5 × 104 m−1 cm−1. It is a naturally occurring compound found in Hypericum species, a genus of flowering plant which has historically been used in folk medicine (149). The discovery and characterization of hypericin occurred in the 1980s during investigations into the cause of hypericism, a skin photosensitivity seen in cattle after ingesting significant quantities of Hypericum plants (150). Hypericin-mediated PDT is strongly dependent on oxygen, with no photocytotoxicity observed in hypoxic environments (151,152). Under normal oxygen conditions, hypericin has been shown to have a high triplet quantum yield and is very efficient in the generation of singlet oxygen and superoxide anion (153–155). Direct tumor cytotoxicity combined with vascular damage is the mechanism of action of hypericin. The lipophilic nature of hypericin is responsible for its membrane localization. PK studies in humans following oral administration of hypericin have shown a half-life of 34–42 h, with maximal drug concentrations observed 4–5 h following administration (156–158). Attempts have also been made to elucidate the signaling pathways contributing to its photocytotoxicity. Numerous stress-activated protein kinases, as well as secondary messengers such as ceramides have now been shown to be involved (159,160).
Recent studies have demonstrated that hypericin-mediated PDT successfully compromises tumor growth in a RIF-1 mouse tumor model (161). Biodistribution and effects of PDT treatment were recorded over a 24 h time period following I.V. injection of hypericin. Efficacy was greatest when tumors were irradiated 0.5 h postadministration with 100% of mice cured. Although maximal uptake of hypericin was observed within the tumor 6 h postinjection, no cure was achieved when tumors were irradiated at this time period due to dispersion of hypericin from the tumor vasculature to surrounding tumor tissue.
Other studies examining the effects of hypericin-PDT on nasopharyngeal carcinoma revealed significant regression in tumors irradiated 6 h post-drug administration, at which time concentration of hypericin in tumor tissue was maximal (162). Comparable tumor remission was observed when irradiation was performed 1 h postinjection when plasma concentration of hypericin had peaked.
Further, the potential of hypericin as a PDT agent in the treatment of subcutaneous human SCC has also been demonstrated (163). Significant tumor regression was observed using 593 nm low power laser light for tumors less than 400 mm3 in volume. However, treatment of large SCC tumors showed a decreased PDT response and regrowth of tumor tissue, even when fiberoptics were placed in the center of the tumors, revealing that light penetration is a significant limiting factor in hypericin-mediated PDT.
Greater effect of treatment was observed in mouse P388 lymphoma tumor models following hypericin-PDT compared with Photofrin-mediated PDT which showed no considerable antitumor effect (164). In addition, PK measurements indicated a more rapid elimination of hypericin compared with Photofrin.
Small-scale clinical studies investigating the effect of intralesionally administered hypericin in patients suffering from BCC and SCC have shown a clinical remission of tumors 6–8 weeks post-treatment (165). Recently, further studies using topically applied hypericin in BCC, actinic keratoses and Bowen’s disease have produced slightly disappointing results. However, several parameters related to the treatment regime may be amended in an attempt to improve this inexpensive treatment modality (166).
Hypericin continues to demonstrate potential for further translational development. Although clinical trials to date have not been successful, optimization of the treatment regime through, for example, enhancement of hypericin delivery may significantly enhance efficacy of treatment (166). Hypericin has good tumor-localizing properties and minimal dark toxicity following treatment, although pain associated with current treatment regimens has been reported (166).
Chalcogenopyrylium dyes. The chalcogenopyrylium class of dyes has been considered as potential PDT agents due to their modifiable photochemical and photophysical properties and selectivity of these heavy atom-containing dyes for the mitochondria of cancer cells (167). Chalcogenopyrylium dyes are structurally related to the mitochondrial localizing xanthylium dye rhodamine (148). Chalcogenopyrylium sensitizers containing selenium and tellurium (the heavier of the chalcogen atoms) provide higher quantum yields for the production of singlet oxygen, as well as longer wavelengths of absorption compared with oxygen- and sulfur-containing sensitizers (168). Studies examining this “heavy atom” effect in PDT have been carried out using a series of heavy atom–substituted chalcogenopyrylium dyes, all of which are related in structure to the thiopyrylium dye, AA1, 2, 6-bis(4-aminophenyl)-4-[4-(dimethylamino)phenyl] thiopyrylium chloride, a mitochondria targeting dye (169). Dyes were found to be hydrolytically more stable having greater absorption maxima and singlet oxygen quantum yields. An inhibition of cytochrome c oxidase and increase in phototoxicity was also observed in rat mammary adenocarcinoma cells. Moreover, preliminary in vivo data displayed no obvious toxicity associated with substituted dyes.
In a related study using similarly substituted chalcogenopyrylium dyes, numerous molecules showed an increase in phototoxicity in murine colon carcinoma cell line compared with AA1 (168). In addition, several dyes were shown to localize to the mitochondria where they inhibited cytochrome c oxidase activity, an enzyme critical to the aerobic respiratory pathway, in dark and irradiated conditions. However, significant dark toxicity was observed with some dyes making them unsuitable as PDT agents.
Phenothiazinium dyes. The phenothiazinium dyes are a relatively old class of compound which include methylene blue (Fig. 3k) and toluidine blue (Fig. 3l) dyes. This class of dyes causes direct cell cytotoxicity, has absorption maxima greater than 600 nm and molar extinction coefficients greater than 104 m−1 cm−1.
Methylene blue, a thiazinium dye, is widely used in several disciplines of medicine and is well known to produce singlet oxygen and destroy nucleic acids in a nuclease-like manner (170). It displays good red light absorbance, having an absorption maximum at 666 nm and extinction coefficient of 82 000 m−1 cm−1 in aqueous solutions (171). I.V. injected methylene blue has a plasma half-life in humans of 5.25 h (172).
Methylene blue shows phototoxicity in vitro in an array of tumor cell lines including Ehrlich ascites, human, rat and mouse bladder carcinoma, human HeLa cervical adenocarcinoma and human T-cell and B-cell lymphoma (173). A significant reduction in tumor size, as well as complete tumor ablation was observed in mice with solid Ehrlich carcinomas treated intratumorally with methylene blue, followed by irradiation with a long wavelength red laser (174).
Intratumorally injected methylene blue accompanied by irradiation with red light resulted in 75% destruction of human colon tumor xenografts after a single treatment (175). In addition, methylene blue efficacy was assessed in patients suffering from recurrent inoperable esophageal cancer. The photosensitizer was administered intralesionally, thereby minimizing side effects. Of the three patients treated, tumor eradication was seen; however, another intraluminal tumor was seen in one patient 3 months following treatment and the other two patients showed liver metastasis 6 months following PDT (176).
However, there are numerous drawbacks associated with methylene blue as a PDT agent. These include lack of activity associated with I.V. or intravesically injected methylene blue resulting from poor tumor localization due to its high hydrophobicity (173). In addition, DNA single-stranded breaks resulting from methylene blue–induced oxidative damage to guanine bases is rapidly repaired within 4 h of treatment in certain cell lines, giving a likely explanation for the low phototoxicity of methylene blue (173). Lastly, methylene blue is reduced to a colorless photodynamically inactive form by ubiquitous cellular enzymes (177). Nevertheless, despite these disadvantages, methylene blue continues to be assessed as a PDT agent (178,179). Studies examining the effect of nanoparticle encapsulated methylene blue have shown an increase in efficacy over methylene blue alone (180,181). Nanoparticle encapsulated methylene blue may now offer new opportunities for the methylene blue–mediated PDT of cancer.
Toluidine blue, another member of the phenothiazine family of dyes, is a cationic thiazine dye with an absorption peak between 626 and 632 nm and a molar extinction coefficient of ∼3 × 104 m−1 cm−1. Toluidine blue is known to intercalate with DNA (177). Following light activation, toluidine blue has been shown to eliminate numerous microbial agents in vitro including Candida albicans (182) and methicillin resistant Staphylococcus aureus (183). Toluidine blue also appears to possess a selective affinity for neoplastic tissues in vivo, a characteristic which has been shown to be particularly valuable in the diagnosis of cancers of the oral cavity and upper gastrointestinal tract over the last few decades (184–186).
The photosensitizing potential of toluidine blue has been investigated in Jurkat cells in vitro. The dye has been shown to have the capacity to generate phototoxicity and apoptosis in malignant T cells (187).
Studies examining the efficacy of a combination of methylene blue – and toluidine blue–mediated PDT to treat acquired immunodeficiency syndrome–related Kaposi’s sarcoma have also resulted in a complete remission of lesions, as well as excellent cosmetic results in a single patient study (188). The patient had previously undergone five cycles of chemotherapy. Previous studies had reported significant cutaneous phototoxicity and unsatisfactory cosmetic results following Photofrin-mediated PDT.
The use of toluidine blue in the PDT of cancer has been somewhat limited. However, the low dark toxicity and photosensitivity of this photosensitizer represents a sound rationale for continuing the investigation on this photosensitizer towards application in oncology.
Another important class of phenothiazinium dye is the benzo[a]phenothiazinium dyes which consists of Nile Blue and its analogs. This class of photosensitizers has received much attention due to their perceived tumor selectivity and ability to obstruct tumor growth. However, these compounds were not shown to be photoactive (189,190). Through modification of the photochemotherapeutic properties of this class of dyes, there may be potential to develop them as PDT agents.
The photocytotoxicity of novel benzo[a]phenothiazinium analogs, 5-ethylamino-9-diethylaminobenzo[a]phenoxazinium, 5-ethylamino-9diethylaminobenzo[a] phenothiazinium (EtNBS) and 5-ethylamino-9 diethylaminobenzo[a] phenoselenazinium (EtNBSe) chlorides has also been examined (191). Sulfur- and selenium-containing derivatives increased singlet oxygen efficiency and absorption bands between 650 and 660 nm. EtNBSe was also found to be ∼1000 times more photocytotoxic than Photofrin II in an EMT-6 murine mammary sarcoma cell line. Importantly, it has been shown that these photosensitizers are capable of undergoing enzymatic reduction in the absence of oxygen yielding a colorless photoinactive (leuko) form. The ratio of oxidized to reduced photosensitizer may contribute to retention of dye, localization and efficacy of PDT treatment.
Further studies assessing the in vivo activity of EtNBS have also been completed (192). Complete response rates of 100% were achieved in an EMT-6 model and 70% complete response in a RIF-1 model with minimal or no damage to surrounding tissue following PDT. Efficacy may be attributed to the redox potential of EtNBS in the tumor compared with other tissue. Tumor tissue was found to contain mainly oxidized photoactive EtNBS, whereas the drug is present in its inactive leuko form in the skin preventing skin photosensitization following PDT treatment.
Subsequent studies using the EtNBS and EtNBSe derivatives have shown that a rapid redistribution of the photosensitizers from lysosomal to cytosolic compartments of EMT-6 cells occurs following irradiation (193). In addition, photodynamic oxygen consumption measurements have revealed that two distinct phases of oxygen consumption occur during treatment. Due to the higher singlet oxygen yield of EtNBSe, eight times fewer photons are required to commence its second oxygen consumption phase compared with EtNBS. The existence of a second oxygen consumption phase indicates that the lysosomally localized aggregates of the photosensitizer have suboptimal photodynamic oxygen consumption and it is not until the photosensitizer is released into the cytosol that oxygen consumption is optimized.
An additional series of phenoxazine Nile blue derivatives has also been developed which possesses substantially greater singlet oxygen yields as well as different photochemical properties (194). Greater than 90% of bladder carcinoma cells were destroyed following PDT treatment using nanomolar concentrations of these derivatives. Photosensitizer localization was confined to the lysosome by means of an ion-trapping mechanism, with lysosomes being a key intracellular target of PDT treatment.
While much work has been carried out to investigate the phenothiazinium dyes as potential photosensitizers, this class of dyes has yet to emerge as useful photosensitizers for clinical application in oncology indications despite being good singlet oxygen generators. A significant challenge towards the advancement of various members of the phenothiazinium class of photosensitizers lies in their rapid excretion, inherent dark toxicity and metabolic inactivation (195). Nevertheless, generation of photophysically and photochemically enhanced derivatives of many members of this class of dyes is now ongoing.
Cyanines. The cyanine dyes are a functionally diverse class of dyes which have been applied to dyeing textiles, silver halide photography, heat-mode optical recording and importantly, PDT (196). Generally, the wavelength absorbance of the cyanines increases further into the red region of spectrum with lengthening of the polymethine chain; however, this lengthening may cause unfavorable additional changes in the photosensitizers’ properties such as less chemical stability and decreased solubility (177). Most cyanine compounds are cationic and tend to accumulate in mitochondria of neoplastic cells with a much greater selectivity compared with normal cells (197). The simple structure of cyanines is such that significant numbers of derivatives, with differing photophysical properties, can be synthesized through straightforward structural modification around the core structure (177).
Merocyanine 540 (MC540) (Fig 3m) is a fluorescent impermeant dye which has been used for the selective purging of leukemic, as well as lymphoma and neuroblastoma cells in autologous bone marrow grafts (197). It is one of the few anionic members of the cyanine family and binds preferentially to fluid-like or cholesterol-free domains present in the outer leaflet of the lipid bilayer of intact cell membranes (198). Excitation of MC540 stained cells with light of a specific wavelength causes an increase in dye uptake, followed by collapse of osmotic properties of membrane eventually leading to cell death (199,200).
Evaluation of MC540 as a PDT agent has been performed in vitro and in preclinical models to assess efficacy against leukemic and neuroblastoma cells from human autologous bone marrow grafts (198,200,201). Data reveal that MC540-mediated PDT is highly effective against leukemia and lymphoma cells (202). Importantly, MC540-PDT is well tolerated by normal hematopoietic stem and progenitor cells with a significant percentage of granulocyte macrophage colony-forming units and erythroid burst-forming units remaining preserved (203). However, efficacy appears to be limited in the treatment of solid tumor cells (204).
Recent clinical applications of MC540 have employed the use of the alkyl-lysophospholipid, Edelfosine, in order to assess the potential benefits of combinational therapy in the purging of hematopoetic stem cells from patients with either breast cancer (124) or pediatric solid tumors (125). Data which have been obtained from these studies demonstrate a significant synergistic effect using MC540 and Edelfosine which may warrant further investigation.
Numerous studies investigating the photophysical and photosensitizing properties of various cationic cyanines have also been completed but have progressed little in recent years (197,205). Although Merocyanine 540 localizes to leukemia cells, the phototoxicity induced following irradiation is not selective. Also, comparative studies with Foscan have shown that Foscan is considerably more potent and selective in the destruction of leukemia cells following PDT (206).
Tetra-aryl-azadipyrromethenes. Due to the problems associated with the use of many porphyrin- and nonporphyrin-based photosensitizers, such as photosensitivity, dark toxicity and low wavelength absorbances, it is evident that completely new classes of compounds should be explored.
A promising group of compounds being developed by the current authors is the ADPMs (207). These compounds are generated from readily available precursors and may be sequentially modified, allowing for the refinement of essential therapeutic parameters to match the specific therapeutic target of choice by straightforward functional group manipulation of the core photosensitizer scaffold. In order to potentiate the singlet oxygen generating ability of this family of photosensitizers, bromine heavy atoms have been attached to the azadipyrromethene chromophore (207). Both brominated and nonbrominated ADPMs are converted to their corresponding BF2 chelates in order to introduce structural constraint to the chromophore (208). Each BF2 chelate demonstrates good absorption of light within the body’s therapeutic window between 650 and 700 nm with molar extinction coefficients ranging from 7 to 8.5 × 104 m−1 cm−1.
One member of this family, brominated ADPM06 (Fig. 3n), has been studied in detail and its distinguishing properties have now been elucidated. ADPM06 has been shown to possess inherent characteristics as a valuable therapeutic agent such as a limited reduction in light-induced activity in hypoxic when compared with normoxic conditions and an EC50 value in the nanomolar range for a range of tumor cell types. It potently inhibits the viability of a broad range of cell types, with no apparent bias towards tumor cells of any particular tissue origin. Moreover, isogenic drug-resistant and metastatic derivatives displayed sensitivities to ADPM06 (209). Preclinical in vivo studies with ADPM06 have recently been completed and demonstrate a 70% cure rate in a xenograft model of breast cancer. Clearance of the drug occurs 24 h following I.V. administration, with maximal uptake seen 15 min postinjection. The mechanism of action of ADPM06-mediated PDT in vivo when employing a short drug–light interval has been assessed by positron emission tomography and MRI and would indicate a vascular-targeted response (210).
Consideration of the photophysical properties of the dibrominated ADPMs reveal that this class of photosensitizer could be individually fine-tuned for precise therapeutic requirement. It has been observed that phenyl ring substituents modulate the wavelength of absorption maxima within the therapeutic window and the inclusion of bromine heavy atoms onto the periphery of the chromophore enhances the ability of the photosensitizers to generate singlet oxygen. Moreover, our group has previously reported a new approach to achieve selectivity of the BF2-chelated azadipyrromethene based upon the reversible off/on switching of the key therapeutic property (singlet oxygen generation) of a supramolecular photonic therapeutic agent in response to an external stimulus in the surrounding microenvironment (211).
There is minimal photosensitivity or dark toxicity associated with ADPM06. The easily modifiable structure of this photosensitizer, easy synthetic pathway, good efficacy and PKs as well as the vascular response to ADPM06-mediated PDT in vivo are all key advantages. This class of photosensitizers possesses significant potential for further translational development.
Other nonporphyrin photosensitizers
While a variety of “other” nonporphyrin photosensitizers exist (178), surprisingly few advances have been made in recent years to develop these compounds as photosensitizers. Further investigation of these largely unexplored classes of compounds which include psoralens, anthracyclines, triarylmethanes and acridines is certainly warranted.
Photodynamic molecular beacons
Molecular beacons are a recently introduced concept into the PDT arena. The idea of combining a photosensitizer molecule and a fluorescence resonance energy transfer (FRET)-based imaging probe has been investigated over the last few years (212). The aim of photodynamic molecular beacons (PMBs) is to activate the photosensitizer only in the presence of a cancer cell-specific biomarker that is recognized by a disease-associated linker. This linker, the majority of which are cleavable, holds the photosensitizer and the quencher in close proximity, so that the phototoxicity of the photosensitizer is silenced in normal cells and activated only in diseased cells/tissue where the biomarker is expressed. These PMBs allow for extra level of control within PDT, in addition to the preferential accumulation of a photosensitizer in tumor tissue and the fact that only diseased tissues are irradiated, make this a highly selective treatment modality for cancer (213).
The earliest publication outlined the production of PMBs consisting of a Pyropheophorbide, a photosensitizer and a carotenoid quencher molecule maintained in close proximity through a cleavable caspase 3 linker (212). This PMB was shown to efficiently quench singlet oxygen production in solution. On addition of caspase 3 to solution, a four-fold increase in 1O2 signal was detected as a result of linker cleavage and subsequent quencher removal from the vicinity of the photosensitizer.
Subsequent studies have described a PMB specifically activated by MMP7 (214), a protein of epithelial origin which plays a role in metastasis and has been shown to be highly expressed in various types of cancer including breast, pancreatic and colon cancers (215). The PMB consisted of a Pyropheophorbide a photosensitizer and black hole quencher 3 (BHQ3) held in close proximity by a MMP7-cleavable linker quenching 1O2 by means of FRET. Cleavage of the peptide linker mediated by MMP7 in human nasopharyngeal epidermoid carcinoma cells resulted in complete restoration of 1O2 production reducing the cell viability to <5% when compared with control in some cases. Moreover, in vivo studies have demonstrated an increase in tumor fluorescence up to 3 h post-I.V. administration of the PMBs indicating an activation of the beacon by MMP7 in vivo. In addition, a complete regression of MMP7 and tumors was observed 30 days post-PDT with no tumor recurrence reported.
Further studies have shown that PMBs can successfully minimize photodamage to normal nontargeted cells through the use of a carotenoid quencher molecule (216). Carotenoids are antioxidants which play a major role in quenching excited triplet state of chlorophyll in plants thereby preventing harmful 1O2 production. By employing the use of carotenoids as quencher molecules for PMBs a second level of quenching can be achieved (scavenging of 1O2 and deactivation of photosensitizer). In vitro assessment of the PMB in nontargeted hepatoblastoma-G2 cells confirmed a silencing of the photodynamic effect while the photosensitizer lacking a carotenoid quencher remained highly phototoxic.
Most recently, studies on the successful synthesis of fibroblast activation protein (FAP)-triggered PMBs has been reported, which has shown promising diagnostic and therapeutic potential for FAP-expressing epithelial cancers such as colorectal cancers (217). FAP is a cell surface glycoprotein which plays a significant role in tumor growth and metastasis. It is expressed in more than 90% of cancer-associated fibroblasts of various types of human epithelial cancers and therefore has emerged as an important biomarker (218,219). In vitro and in vivo examination of the FAP-specific PMB has shown activation and subsequent fluorescence detection in FAP-transfected human embryonic kidney (HEK) 293 cells, as detected by confocal microscopy, flow cytometry and in vivo imaging. Detection of two distinct peaks in HPLC analysis corresponding to the cleavage fragments following PMB activation has also been shown. No activation or fluorescence was detected with a FAP noncleavable control PMB or when the cleavable PMB was tested in HEK vector- only cells.
The new era of PMBs provides a wide range of potential cancer applications through the versatility and easy modification of the probes. mRNA-activatable beacons are also emerging as an additional attractive probe for future studies (220). Not only will these vast arrays of PMBs serve as valuable therapeutic agents in their own right, but also may offer significant potential in image-guided therapy as well as imaging response to therapy (221), for example, through built-in apoptosis sensors (222,223).
Conclusions
The various photophysical and photochemical properties of photosensitizers can dictate specific photosensitizer application in oncology indications, for example, photosensitizers with low wavelength absorbances may be more suitable for the treatment of superficial cancers while photosensitizers with wavelength absorbances above 700 nm can be used in the treatment of more deeply embedded lesions. This continues to extend the utility of PDT in oncology and its application in the treatment of a variety of intractable cancers. However, despite the myriad of achievements in the application of PDT in oncology, the field continues to require the development of new photosensitizers with improved efficacy and side-effect profiles. The majority of photosensitizers currently involved in clinical trials and utilized in the treatment of cancer are porphyrins and their derivatives. Although many significant advantages exist with porphyrin-based PDT treatment such as long absorbance wavelengths resulting in deep tissue penetration (e.g. Lutrin, Tookad), easy formulation of photosensitizer (e.g. Levulan, Foscan, NPe6) and overall encouraging efficacy profiles resulting from the application of a variety of treatment protocols, the disadvantages associated with the use of porphyrins and porphyrin-like compounds in PDT is still considerable. Side effects are repeatedly associated with the majority of first generation porphyrins, including Photofrin, the current “gold standard” for PDT, as well as some second generation porphyrins and porphyrin derivatives (Tables 3 and 4). Some major disadvantages associated with porphyrin-based photosensitizers include relative insolubility in water, dark toxicity (e.g. Purlytin), skin photosensitivity which may last for several weeks (e.g. Photofrin, Foscan, Purlytin), severe pain to patients during irradiation (e.g. Lutrin, Levulan), limited depth of tumor necrosis (e.g. Levulan, Metvix) and multistep synthesis required for formulation of some photosensitizers (e.g. Lutrin). Additionally, the nonmodifiable structures of many porphyin photosensitizers does not allow for the generation of analogs. The heterogenous nature of Photofrin, one of the most commonly used photosensitizers in cancer, is also a significant drawback.
Therefore, significant potential exists to develop various new classes of nonporphyrin photosensitizers having short half-lives and reduced toxicities. Due to the limited potency and various side effects associated with many nonporphyrin photosensitizers, the majority of these compounds have now been abandoned. Nevertheless, there may be value in synthesizing analogs of certain photosensitizers, which possess the major advantage of having easily modifiable structures. Synthesis and purification of these classes of compounds is nevertheless challenging. A significant advantage of nonporphyrin photosensitizers is that they are more defined compounds which may result in reduced toxicity. Even though many of the nonporphyrin PDT agents investigated pre-date the development of second generation photosensitizers, none have yet been approved clinically.
Future work on the development of photosensitizers is likely to focus on increasing therapeutic efficacy and selectivity for malignant tissue, while minimizing side effects, in order to increase chances of clinical approval. Attention to the development of novel targeted delivery systems for photosensitizers such as nanoparticles and mABs, which may significantly decrease the time required for accumulation within tumor tissue, should also prove invaluable. In addition, the new era of PMBs may facilitate development of a more specific PDT treatment regime with minimal effects on normal tissue. Several photosensitizers may also be examined as potential agents for intra-operative applications such as photodynamic diagnosis where boundaries of tumor tissue may easily be visualized by fluorescence to assist in surgical resections (224). Finally, significant emphasis is now likely to be placed on a vascular targeting approach of PDT and the combination of photosensitizers in order to significantly reduce drug–light intervals and prevent tumor recurrences.
Acknowledgments
Acknowledgements— Sincere thanks to Prof. Johan Van Lier for providing advice on chemistry aspects of this article. Funding is acknowledged from Science Foundation Ireland, the Health Research Board of Ireland and University College Dublin. The UCD Conway Institute is funded by the Programme for Third Level Institutions (PRTLI), as administered by the Higher Education Authority (HEA) of Ireland.