Effect of UV-C on Algal Evolution and Differences in Growth Rate, Pigmentation and Photosynthesis Between Prokaryotic and Eukaryotic Algae
Abstract
Insight into the influence of UV-C radiation on the evolutionary relationship between prokaryotic and eukaryotic algae was studied in seven species of algae exposed to different UV-C irradiances. The order of their acclimation (from most tolerant to sensitive) is Synechococcus sp. PCC7942 (Cyanophyta), Synechocystis sp. PCC6803 (Cyanophyta), Chlorella protothecoides (Chlorophyta), Chlamydomonas reinhardtii (Chlorophyta), Phaeodactylum tricornutum (Bacillariophyta), Alexandrium tamarense (Pyrrhophyta) and Dicrateria zhanjiangensis (Chrysophyta). These results are in accordance with the algal evolution process that is generally accepted and proved by fossil record. It shows that UV-C radiation is an important environmental factor that cannot be ignored in the evolutionary process from prokaryotic algae to eukaryotic algae. The threshold of UV-C radiation at which prokaryotic algae can survive but eukaryotic algae cannot was found to be approximately 0.09 W m−2. This was the first time to determine with precision the irradiance level at which UV-C contributed as a selection pressure of evolution. Furthermore, the effects of UV-C radiation on photosynthetic performance, growth rate and pigment content were investigated in two species of prokaryotic algae: Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803, and two species of eukaryotic algae: C. reinhardtii and C. protothecoides. After 6 days of exposure, the contents of chlorophyll a and carotenoids decreased in all species, moreover reduction in C. reinhardtii and C. protothecoides was more obvious than in Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803. The ability to photosynthesize followed the same trend as the pigments.
Introduction
Solar UV radiation (UVR) has always been regarded as a ubiquitous factor in biological evolution in terrestrial organisms since the early Archean. The most damaging wavelengths of UVR are the short-wavelength UV-C (200–280 nm) and UV-B (280–315 nm) regions. As far as we know, there are only a few reports of UV-C influence on photosynthesis (1), because within the modern atmosphere, UV-C radiation is absorbed and scattered by the stratospheric ozone layer (2). The research on UVR effects is mainly focused on the impact of UV-B radiation on the physiology of photosynthetic cells of both higher and lower organisms, in particular cyanobacteria and other algae (3–6). Damage to motility, DNA molecule, protein biosynthesis, photosynthesis, nitrogen fixation and survival of cells may occur either by direct photochemical reaction or by indirect oxidation, due to reactive oxygen products induced by UV-B (7–10).
At present UV-C does not reach the earth’s surface. However, during the early Precambrian era, the levels of UV-C radiation on the earth’s surface was much higher than today due to the lack of oxygen in the atmosphere and the absence of ozone in the stratosphere (11). It is believed that UV-C radiation, due to its high energy resulting from its short wavelength, acted as a major selection pressure during the earlier stages of evolution from prokaryotes to eukaryotes. As on Earth today, ancient microbes probably protected themselves from UV exposure by living under UV-absorbing materials (12–15) including water (16), by living under layers of dead cells (17), by producing UV-screening compounds or migrating in response to UV exposure in microbial mats and mud flats (18,19). While all of these strategies may have mitigated the damaging effects of UVR, the phenomenon that anaerobic bacteria are resistant to UVR (20) suggests that organisms that populated the anaerobic early Earth were exposed to UVR and such organisms developed mechanisms to cope with this exposure. Anaerobic bacteria are the most primitive organisms. There is a significant body of bona fide evidence for the presence of abundant anaerobic life forms on the surface of the earth in 3.5–3.3 Ga (21). At that time, the ecosystems were anaerobic (22).
It has been shown in previous research that anoxygenic photosynthesizers are not more sensitive to UV-C than oxygenic photosynthesizer (20), if considering the arising-order of anoxygenic and oxygenic photosynthesizers, and the intensity of UV-C at that time. We also can get this deduction.
The initial period of eukaryotic algae is one of the most influential periods concerning the evolution of life. So far, scientists suggest that the appearance of eukaryotes is the result of the evolution of the earth’s environmental chemistry during the Precambrian. These suggestions arose from investigations on the morphology of algal fossils from that age. During the Precambrian when prokaryotic algae were prosperous and produced O2 continually, the O2 in the air gradually formed the ozone, which ultimately resulted in the decrease of irradiance of UV-C. Intensive levels of UV-C radiation were one of the defining characteristics of the environment. Proceeding to the late Precambrian, the decrease of UV-C radiation was closely related to the great reproduction of eukaryotic algae.
As mentioned above, early living organisms must have been adapted to high UV irradiation and have some UV-acclimation characters. For hereditary conservation, some inheritable characters of these oldest organisms, e.g. cyanobacteria and some basal eukaryotes may be preserved (23), although mutation also occurs. Furthermore, it is a common method that uses present oldest organisms as materials to investigate the interaction between paleoenvironment and the organisms at that time (20,24,25). To provide an insight into the UV-C radiation influence on the evolution relationship between prokaryotic and eukaryotic algae, two species of prokaryotic algae, Synechococcus sp. PCC7942 (Cyanophyta) and Synechocystis sp. PCC6803 (Cyanophyta), and five species of eukaryotic algae Chlorella protothecoides (Chlorophyta), Chlamydomonas reinhardtii (Chlorophyta), Phaeodactylum tricornutum (Bacillariophyta), Alexandrium tamarense (Pyrrophyta) and Dicraeta zhanjiangensis (Chrysophyta) were chosen for study. These species are also recognized as ubiquitous organisms of oceanic or lacustrine microbial loops and as the most abundant primary producers in modern times. We used biochemical and biophysical techniques to find the irradiance level of UV-C that contributed as a selection pressure to evolution from prokaryotes to eukaryotes and to what degree UV-C influenced the physiology of photosynthetic cells.
Materials and Methods
Species and culture conditions. Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 were provided by Dr. Wim F. J. Vermaas (Arizona State University). Chlamydomonas reinhardtii was presented by Dr. Pan (Tsinghua University, China). Cells were cultured photoautotrophically at 25 ± 1°C in BG11 medium (26). Chlorella protothecoides was provided by the Culture Collection of Alga at the University of Texas (Austin, TX). The components of basal culture medium (27) are as follows: KH2PO4 0.7 g L−1, K2HPO4 0.3 g L−1, MgSO4·7H2O 0.3 g L−1, FeSO4·7H2O 3 mg L−1, glycine 0.1 g L−1, vitamin B1 0.01 mg L−1 and A5 trace mineral solution 1 mL L−1. The other strains, including P. tricornutum, A. tamarense and D. zhanjiangensis were from Dr. Gao (Xiamen University, China). The algae were cultivated in Guillard f/2 medium (28) at 25 ± 1°C, with silica added for the diatom. The medium was made from filtered seawater (Whatman GF/F glass microfiber filters; Whatman International Ltd., Maidstone, UK) diluted with deionized water to a salinity of 26 psu and then autoclaved.
Light conditions and UVR treatment. All algae were cultivated in a 14:10 h light:dark cycle, in 2 L borosilicate glass. The experiment started when the culture was in exponential growth, almost 1 × 105 cells per mL. Each culture was equally divided into six parts. Three of them were treated with UV-C, while the other three were treated without UV-C to be used as controls. All of the cultures were placed in 250 mL UV-transparent quartz Erlenmeyer flasks (the transmittance of 254 nm is larger than 93%) that were closed with sterile cotton stoppers allowing gas exchange. White light was provided by fluorescent tubes (Philips 40 W-1/29) at an irradiance of 30 μmol photon m−2 s−1. A germicidal lamp (254 nm) was used as a UV-C source. The UV-C radiation was applied from 0800 to 1800 h (local time) in the middle of the light period. Based on preliminary results (we tested the growth response of these algae under different UV-C irradiances, from 0.01 to 0.20 W m−2, data not shown), four UV-C irradiances, 0.03, 0.07, 0.09 and 0.18 W m−2 (±0.05), were used in the experiments because they are the transition point. The different irradiances were obtained by changing the distance between the lamp and the sample. The UV-C irradiance was measured by a UV radiometer equipped which was new and calibrated at the factory; the radiometer had specific UV sensors sensitive at 254 nm (Beijing Electro-Optical Equipment Factory, China). The depth of samples was just 1 cm, so the attenuation was very small. The irradiance of transmitted light was almost equal to the incident intensity. All samples were gently agitated by bubbles produced by aeration flow during irradiation to ensure uniform distribution. Aeration flow was kept an 1:1 vvm by bubbling air at the atmospheric pressure. Each treatment was replicated three times. All of the control samples grew in the same conditions except for UV-C irradiation. Additionally, all the cultures (treated and controls) were grown in the same radiation conditions in a thermo-constant chamber, 25 ± 1°C.
Growth measurements. Samples were taken daily from each treatment to determine cell concentration using a microscope. Immediately after sampling, cells were fixed with 0.2% of glutaraldehyde (Sigma-Aldrich, St. Louis, MO) for 30 min at 4°C. When using a blood cell counting board to count cell number under microscope, each sample was diluted 1000 times by fresh medium.
Pigment concentration. Chlorophyll a (Chl a) was determined from the absorbance of the methanol extracts at 666 nm (29). For the determination of carotenoids, samples were harvested by centrifugation, and the pellet was saponified by suspension in 30% (vol/vol) methanol containing 5% (wt/vol) KOH. The remaining pellet was neutralized by addition of 70% (vol/vol) acetic acid, and the carotenoids extracted by addition of pure dimethylsulfoxide at 70°C for 5 min. The absorbance of the supernatant was measured at 490 nm and the concentration of carotenoids was calculated using the specific absorption coefficient ε% = 2200 (30).
Measurement of oxygen evolution. Evolution of O2 was measured using a Clark-type electrode (Hansatech, Kings Lynn, UK). Cells were harvested by centrifugation at 1500 g for 15 min and resuspended in 25 mM HEPES/NaOH buffer, pH 7.0. Light–response curves of photosynthesis were obtained by measuring the rate of O2 evolution at 600 μmol m−2 s−1 flux densities.
Chlorophyll fluorescence measurements. The optimal quantum yield (Fv/Fm) and other fluorescence parameters were determined using a portable pulse amplitude modulated fluorometer (Pam-Water-Ed, Walz, Germany). The maximum photochemical efficiency of photosystem II (PSII) was determined from the ratio of variable Fv to maximum Fm fluorescence (Fv/Fm = [Fm − Fo]/Fm) (31).
Statistics. All statistical analyses were performed with SPSS software. Normal probability plots and residual plots were examined to ensure that the data met the assumption of ANOVA. Prior to ANOVA (32), mean values of three replications were calculated and data were tested for normality. The critical level of significance was P ≤ 0.05.
Results
Growth response under UV irradiation
Growth recorded at different radiation conditions indicated strong species-specific differences. A lower UV-C irradiance of 0.03 W m−2 had almost no influence on all species (data not shown). With an increase of UV-C irradiance, the growth rate of some species decreased. After 6 days of UV-C treatment at 0.07 W m−2, the cultures of A. tamarense, D. zhanjiangensis and P. tricornutum indicated zero growth. Chlamydomonas reinhardtii and C. protothecoides showed obvious decrease in growth through the whole period but still some positive increase. The two Cyanophyta species, Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 just showed little difference between controls and treatments. The order of acclimation of these species to UVR (from most tolerant to sensitive) was Synechococcus sp. PCC7942, Synechocystis sp. PCC6803, C. protothecoides, C. reinhardtii, P. tricornutum, A. tamarense and D. zhanjiangensis (Fig. 1). When the irradiance of UV-C increased to 0.09 W m−2, C. reinhardtii and C. protothecoides increased slightly in the first 3 days. After that, cell numbers stopped increasing. The growth rate of treatments of Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 was roughly 30% less than the rate of the controls. The order of acclimation of these species had the same ranking as those treated with 0.07 W m−2 (Fig. 2). Only when UV-C irradiance was increased to 0.18 W m−2 growth stopped (Fig. 3).
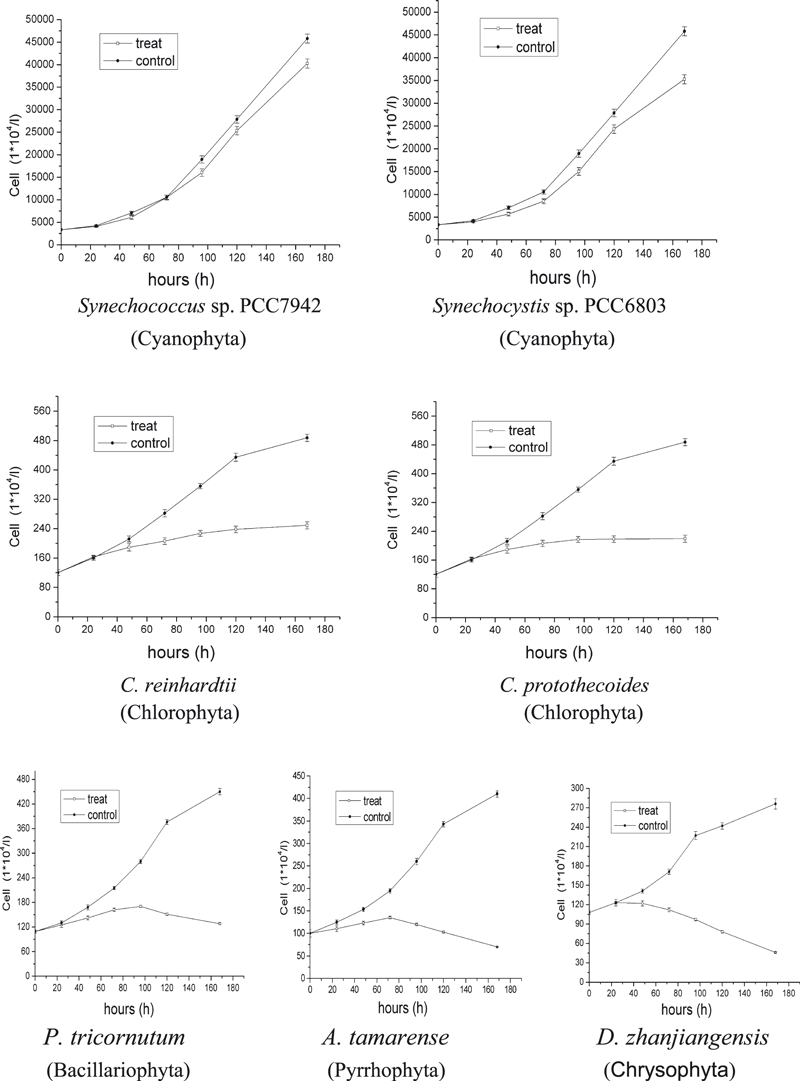
Growth of the seven algal species Synechococcus sp. PCC7942, Synechocystis sp. PCC6803, Chlamydomonas reinhardtii, Chlorella protothecoides, Phaeodactylum tricornutum, Alexandrium tamarense and Dicrateria zhanjiangensis under UV-C radiation conditions: 0.07 W m−2 at 25°C and 14:10 h light:dark cycle. Data are expressed as mean values ± SD (n = 3).
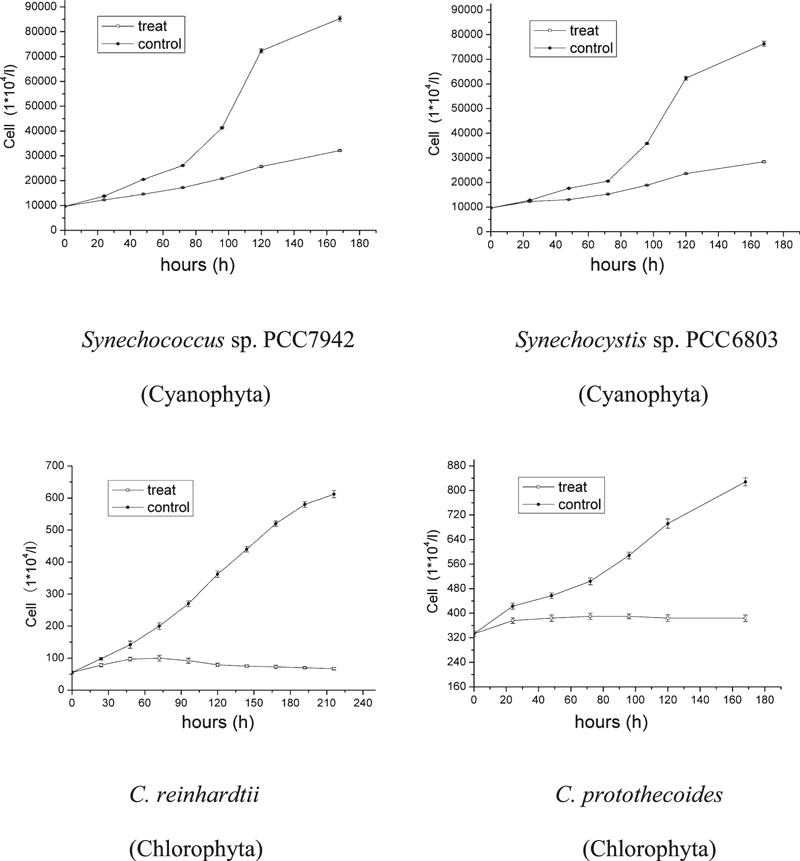
Growth of the four algal species Synechococcus sp. PCC7942, Synechocystis sp. PCC6803, Chlamydomonas reinhardtii and Chlorella protothecoides under UV-C radiation conditions: 0.09 W m−2 at 25°C and 14:10 h light:dark cycle. Data are expressed as mean values ± SD (n = 3).
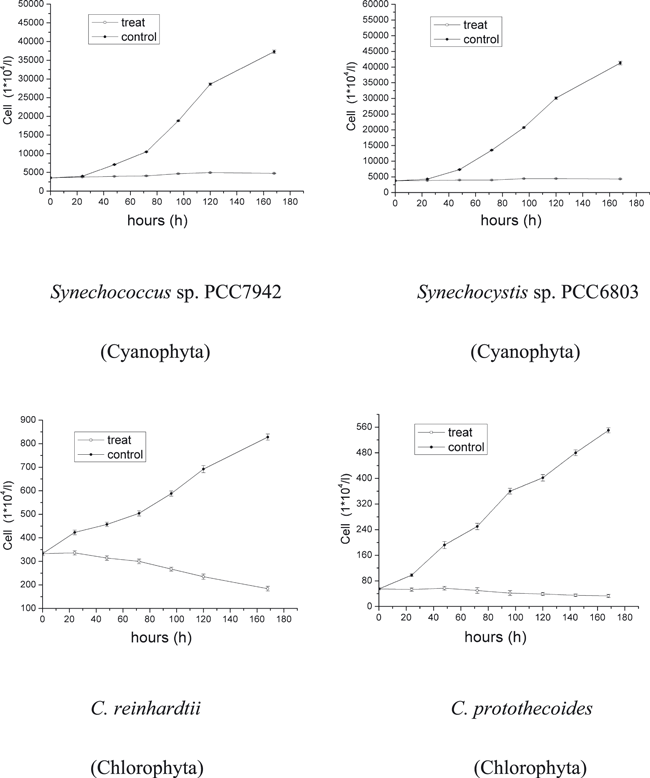
Growth of the four algal species Synechococcus sp. PCC7942, Synechocystis sp. PCC6803, Chlamydomonas reinhardtii and Chlorella protothecoides under UV-C radiation conditions: 0.18 W m−2 at 25°C and 14:10 h light:dark cycle. Data are expressed as mean values ± SD (n = 3).
Induction of pigments
After 6 days of UV-C treatment at 0.09 W m−2, the amount of Chl a of Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 was reduced by 48.5% and 60.4% compared with controls. The amount of Chl a of C. reinhardtii and C. protothecoides cells decreased so rapidly and became undetectable after 4 days (Table 1). The color of the culture of these two species turned from a hue of green to yellow, finally to translucence. Under UV-C, the carotenoid content of these four species did not decrease as much as the Chl a. The declining ratios compared to controls were: Synechococcus sp. PCC7942, 22.8%; Synechocystis sp. PCC6803, 23.7%; C. protothecoides, 40%; and C. reinhardtii, 54% (Table 2).
| Exposure time (days) | Chl a (μg per 107 cells) | |||||||
|---|---|---|---|---|---|---|---|---|
| PCC7942 | PCC6803 | C. protothecoides | C. reinhardtii | |||||
| Control | +UV-C | Control | +UV-C | Control | +UV-C | Control | +UV-C | |
| 0 | 0.365 ± 0.05 | 0.365 ± 0.05 | 0.308 ± 0.04 | 0.308 ± 0.04 | 1.33 ± 0.11 | 1.33 ± 0.11 | 1.58 ± 0.08 | 1.58 ± 0.08 |
| 2 | 0.354 ± 0.03 | 0.308 ± 0.02 | 0.313 ± 0.04 | 0.235 ± 0.03 | 1.25 ± 0.08 | 0.42 ± 0.05 | 1.49 ± 0.07 | 0.53 ± 0.03 |
| 4 | 0.366 ± 0.04 | 0.236 ± 0.02 | 0.299 ± 0.03 | 0.165 ± 0.01 | 1.36 ± 0.08 | 0.008 ± 0.001 | 1.35 ± 0.05 | 0.015 ± 0.004 |
| 6 | 0.358 ± 0.03 | 0.188 ± 0.03 | 0.315 ± 0.04 | 0.122 ± 0.02 | 1.28 ± 0.07 | ND | 1.43 ± 0.07 | ND |
- ND = no detection. Data are expressed as mean values ± SD (n = 3).
| Exposure time (days) | Carotenoids (μg per 107 cells) | |||||||
|---|---|---|---|---|---|---|---|---|
| PCC7942 | PCC6803 | C. protothecoides | C. reinhardtii | |||||
| Control | +UV-C | Control | +UV-C | Control | +UV-C | Control | +UV-C | |
| 0 | 0.075 ± 0.05 | 0.075 ± 0.05 | 0.068 ± 0.04 | 0.068 ± 0.04 | 0.25 ± 0.03 | 0.25 ± 0.03 | 0.28 ± 0.02 | 0.28 ± 0.02 |
| 2 | 0.074 ± 0.03 | 0.068 ± 0.02 | 0.068 ± 0.04 | 0.065 ± 0.03 | 0.25 ± 0.04 | 0.22 ± 0.03 | 0.29 ± 0.03 | 0.23 ± 0.03 |
| 4 | 0.068 ± 0.04 | 0.066 ± 0.02 | 0.065 ± 0.03 | 0.055 ± 0.01 | 0.26 ± 0.05 | 0.18 ± 0.001 | 0.25 ± 0.04 | 0.17 ± 0.004 |
| 6 | 0.072 ± 0.03 | 0.058 ± 0.03 | 0.067 ± 0.04 | 0.052 ± 0.02 | 0.23 ± 0.04 | 0.15 ± 0.012 | 0.23 ± 0.03 | 0.13 ± 0.01 |
- Data are expressed as mean values ± SD (n = 3).
Photosynthesis measurements
The rate of photosynthesis was monitored by O2 evolution from the cultures. After 6 days of UV-C treatment at 0.09 W m−2, there was a progressive reduction of O2 evolution in the treatments of C. reinhardtii, C. protothecoides, Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 compared to the control cultures. In Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803, the maximal decreasing ratio that appeared on the fourth day was almost 73%. On the sixth day, the O2 evolution increased 10% compared to the fourth day. The ratios of reduction of O2 evolution in the treatments of two species of Chlorophyta were significantly greater than that of Cyanophyta. The O2 evolution was too low to measure on the fourth and sixth days in C. reinhardtii and C. protothecoides (Table 3). The maximum quantum efficiency of PSII photochemistry (Fv/Fm) of these four species was measured prior to the UV-C exposure every 2 days for a 6-day period. There were also clear differences between the two species of Chlorophyta and the two species of Cyanophyta. Its yield correlated well with O2 evolution. After 6 days of exposure Synechococcus sp. PCC7942 exhibited some degree of recovery (Table 4).
| Exposure time (days) | O2 evolution (μmol O2 per 107 cells h−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| PCC7942 | PCC6803 | C. protothecoides | C. reinhardtii | |||||
| Control | +UV-C | Control | +UV-C | Control | +UV-C | Control | +UV-C | |
| 0 | 300 ± 8.3 | 300 ± 8.3 | 268 ± 7.8 | 268 ± 7.8 | 560 ± 19.2 | 560 ± 19.2 | 447 ± 15.9 | 447 ± 15.9 |
| 2 | 288 ± 9.5 | 197 ± 8.5 | 272 ± 10.4 | 178 ± 6.3 | 532 ± 13.5 | 150 ± 5.8 | 428 ± 10.5 | 132 ± 6.8 |
| 4 | 296 ± 6.7 | 86 ± 6.2 | 250 ± 9.3 | 72 ± 3.6 | 548 ± 23.5 | ND | 420 ± 21.8 | ND |
| 6 | 267 ± 7.2 | 95 ± 4.3 | 237 ± 12.4 | 81 ± 4.9 | 515 ± 18.8 | ND | 404 ± 17.9 | ND |
- ND = no detection. Data are expressed as mean values ± SD (n = 3).
| Exposure time (days) | Fv/Fm | |||||||
|---|---|---|---|---|---|---|---|---|
| PCC7942 | PCC6803 | C. protothecoides | C. reinhardtii | |||||
| Control | +UV-C | Control | +UV-C | Control | +UV-C | Control | +UV-C | |
| 0 | 0.706 ± 0.025 | 0.706 ± 0.045 | 0.718 ± 0.052 | 0.718 ± 0.052 | 0.758 ± 0.042 | 0.758 ± 0.042 | 0.733 ± 0.08 | 0.733 ± 0.08 |
| 2 | 0.688 ± 0.03 | 0.423 ± 0.026 | 0.694 ± 0.049 | 0.446 ± 0.033 | 0.732 ± 0.029 | 0.212 ± 0.011 | 0.719 ± 0.052 | 0.253 ± 0.024 |
| 4 | 0.7 ± 0.028 | 0.215 ± 0.013 | 0.679 ± 0.061 | 0.195 ± 0.016 | 0.738 ± 0.037 | ND | 0.72 ± 0.043 | ND |
| 6 | 0.693 ± 0.031 | 0.323 ± 0.028 | 0.655 ± 0.048 | 0.193 ± 0.02 | 0.699 ± 0.045 | ND | 0.708 ± 0.058 | ND |
- ND = no detection. Data are expressed as mean values ± SD (n = 3).
Discussion
In the modern age, there is an increased absorption of direct UV-C radiation by stratospheric ozone due to the increased path length at lower solar angles—a magnitude of six times greater path length at low solar angles than those at solar noon. Additionally, UV-C is scattered more than visible radiation through this longer path length. As mentioned above, before the formation of ozone in the stratosphere, UV-C could directly irradiate over the earth and have the same high level of irradiance early in the day as well as later in the day. It is expected that in the Archaean oceans the average weighted instantaneous UV exposure might therefore have been approximately 0.1 W m−2 at a depth of 50 m (33). Photosynthetic phytoplankton must have been irradiated by high UV-C. Being the most energetic wave band of UVR, UV-C is thought to have acted as a selection pressure during algal evolution.
In this experiment, we tested the acclimation of seven alga species belonging to five classes to different UV-C irradiance in order to determine whether we could get direct evidence to support the connection between UV-C radiation and algal evolution. By their growth rates at different UV-C irradiance we found their adaptability to UV-C ranked from most tolerant to sensitive as Synechococcus sp. PCC7942, Synechocystis sp. PCC6803, C. protothecoides, C. reinhardtii, P. tricornutum, A. tamarense and D. zhanjiangensis (Fig. 1). The finding was exciting because this order was in accordance with the algal evolutionary order shown by phytoplankton fossil record found thus far (Fig. 4). We also found that the threshold of UV-C radiation, beyond which the two species of prokaryotic algae could grow and the five species of eukaryotic algae could not live was near 0.09 W m−2. Though we need to explore more species of algae to confirm this evidence, this is the first direct measurement of how the UV-C could have influenced algal evolution. According to the UV-C curve of Fig. 4, the eukaryotic algae might have appeared after 1.4 Ga. The discovery of this UV-C threshold has also built a foundation needed to stimulate paleoenvironmental research in the evolutionary process from the prokaryotic to the eukaryotic algae. As growth is considered to be an important ecological parameter that integrates all positive and negative stress in several biochemical cellular processes it should receive much more attention. Although photosynthesis is an important target of UVR (34), growth studies on macroalgae indicate that more cellular processes are impaired besides photosynthesis (35). Laboratory studies have revealed that effects of UV-B on cell growth and division are often evident at lower dosages than effects on photosynthesis (34). In contrast, our UV-C experiment showed that low doses of UV-C had a larger influence on photosynthesis than on cell division in the two species of Cyanophyta (Fig. 1, Table 5). We thought that maintaining the cell division did not require full photosynthesis. In addition, due to UV-C-induced DNA damage, cell elongation was observed (data not shown). This might be an adaptation to decrease the radiation per unit area by reducing surface area to volume ratio. There are many studies indicating that the cell size is a critical factor for significant differences in UV-B sensitivity (36,37), i.e. the larger the cells, the higher the tolerance. But under UV-C radiation, the smaller prokaryotic algae showed more tolerance than the larger eukaryotic algae. DNA damage is probably the major effect of UV-C radiation on most microbes, and can result in heritable changes via mutagenesis, resulting in increased genetic variation (38). We expect mutation of prokaryotic algae to have been higher than that of eukaryotic algae, making prokaryotes DNA more resistant or that other internal mechanisms, such as a more efficient DNA-damage repair, made the prokaryotic algae better protected from UV-C (23).
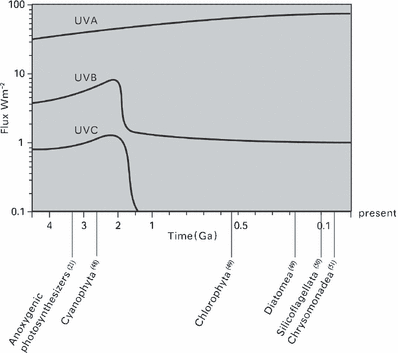
Photobiological history of earth. Changes in UVA, B and C radiation from 4.5 Ga to the present. With earliest reasonably confident fossil dates for the anoxygenic photosynthesizers and the major algal groups.
| Exposure time (days) | O2 evolution (μmol O2 per 107 cells h−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| PCC7942 | PCC6803 | C. protothecoides | C. reinhardtii | |||||
| Control | +UV-C | Control | +UV-C | Control | +UV-C | Control | +UV-C | |
| 0 | 310 ± 7.6 | 310 ± 7.6 | 287 ± 6.8 | 287 ± 6.8 | 553 ± 17.5 | 553 ± 17.5 | 459 ± 15.7 | 459 ± 15.7 |
| 2 | 294 ± 9.8 | 255 ± 7.3 | 278 ± 9.7 | 242 ± 6.2 | 542 ± 17.3 | 380 ± 8.9 | 441 ± 9.0 | 338 ± 10.7 |
| 4 | 298 ± 8.5 | 206 ± 6.9 | 261 ± 8.9 | 198 ± 7.7 | 551 ± 6.9 | 298 ± 6.7 | 427 ± 8.8 | 265 ± 8.5 |
| 6 | 275 ± 6.9 | 158 ± 5.9 | 249 ± 9.5 | 143 ± 6.2 | 523 ± 13.5 | 186 ± 5.2 | 417 ± 12.2 | 162 ± 7.7 |
- Data are expressed as mean values ± SD (n = 3).
The changes in pigments and photosynthesis in C. reinhardtii, C. protothecoides, Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 at the threshold of UV-C radiation of 0.09 W m−2 showed that the decrease of Chl a in the two species of Cyanophyta was slower than in the two species of Chlorophyta. This may be partly attributed to the phycobilisomes in Cyanophyta being able to absorb the damaging UV-C radiation prior to Chl a. As for the Chlorophytes, Chl a was directly influenced by UV-C radiation. The slower decrease of Chl a in Cyanophyta could potentially keep their ability to photosynthesize and thus increase their survival under UV-C stress. Carotenoids can act as UV-B screens in many species of algae. They may have both a screening and an antioxidant function. The latter has been established for many carotenoid structures (39). In cyanobacteria, soluble carotenoid proteins (40) or carotenoids in the cell wall (41) could act as a filter for UV-B radiation. In Synechococcus PCC7942 zeaxanthin provides the highest protective potential (42). It was also shown that zeaxanthin is the most effective protectant against UV-B radiation in Escherichia coli transformants (43) and that it prevents radical peroxidation processes in liposomes better than β-carotene (39). In our experiments, the content of carotenoids decreased day by day under the UV-C treatment in all four species. The ratio of decrease in Cyanophyta was half that of Chlorophyta. This perhaps indicates that carotenoids also could help algae tolerate UV-C, and they might play an important role in the differential tolerances to UV-C between Cyanophyta and Chlorophyta. In fact, their role in photoprotection against UV-C radiation still needs further research. We assume that the analysis of specific carotenoids could shed more light on this question.
Reduction of photosynthetic efficiency in order to avoid photodamage while exposed to high photosynthetically active radiation (PAR) is a well-known protective mechanism to dissipate energy absorbed by PSII as heat via the xanthophylls cycle (44). UV-C exhibited an additional effect in the reduction of O2 evolution and Fv/Fm in all species. The measurable effects of both PAR and UVR in the reduction of photosynthetic efficiency are similar, but the mechanisms behind PAR- and UVR-induced inhibition of photosynthesis are different (45). UVR exhibits adverse effects on photosynthesis causing direct molecular damage due to the absorption by biomolecules (46). Photosynthetic depression by UVR implicates the damage of the oxidizing site of the reaction center of PSII (47). In our experiment, the Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 displayed higher O2 evolution and Fv/Fm than C. reinhardtii and C. protothecoides after 6 days of treatment with the UV-C radiation of 0.09 W m−2. On the sixth day, the photosynthetic ability of treatments of Cyanophyta showed some recovery. We expect this recovery in cyanobacteria is mostly due to their efficient photosynthetic machinery, and the presence of a variety of UV-stress responses, including “UV stress proteins” (23).
During the early Precambrian era, due to the lack of oxygen in the atmosphere and the consequent absence of ozone in the stratosphere, UV-C irradiance was much higher than that of today. It may have acted as a major selection pressure during earlier stages of evolution from prokaryotes to eukaryotes. If so, there must exist a threshold of UV-C. If the UV-C radiation was higher than that level, the prokaryotic algae could live, but the eukaryotic algae would die. Since the photosynthesis of the prokaryotic algae produced O2 continually, and the O2 in the air gradually formed the ozone, this ultimately resulted in the decrease of the irradiance of UV-C. When the UV-C irradiance fell below the threshold, the eukaryotic algae would have been allowed to live and develop in the world. Through this experiment, our results indicate that the threshold of UV-C is near 0.09 W m−2. The five species of eukaryotic algae belonging to four classes could not grow and conduct photosynthesis if the UV-C irradiance was higher than this irradiance; however the two species of prokaryotic algae were still able to live and release O2.
Acknowledgements— This work was supported by the NSFC project 30670476 and u0633009. We thank Min Zhu and Jing Jin for their valuable assistance.




