Differential Expression Profiles of microRNAs in NIH3T3 Cells in Response to UVB Irradiation
Abstract
MicroRNAs (miRNAs) are known as a kind of small, noncoding RNA, which play an important role in mediating many biological processes such as development, cell proliferation and differentiation in plants and animals. Here we report the differential expression profiles of miRNAs and characterized putative target genes in NIH3T3 cells at a series of different time points after UVB irradiation (compared with no UVB irradiation). The relative expression of mature miRNA genes was determined by miRNA microarray technique and the results were confirmed by real-time reverse transcriptase polymerase chain reaction (qRT-PCR). Potential target genes of these miRNAs were classified into different function categories with the GOstat software (http://gostat.wehi.edu.au/cgi-bin/goStat.pl). Several miRNAs in this study expressed highly at different time points, especially mmu-miR-365 and mmu-miR-21. Three miRNAs were lowly expressed, of which mmu-miR-465 showed low levels of expression at all time points, whereas after 50 J m−2 UVB irradiation mmu-miR-296 and mmu-miR-376c showed low levels of expression at 6 and 12 h, respectively. Our study provided a basis for the global characterization of UV-regulated miRNA expression.
Introduction
MicroRNAs (miRNAs) are endogenous 18–25 nt RNAs that control many aspects of cellular functions, including cell growth, differentiation, death, metabolism and development through regulation of gene expression (1,2). In recent years, a few miRNAs involved in cell cycle and apoptosis have been studied. For example, the mir-17-92 cluster (3), miR-14 (4) and miR-372/373 (5) have been proved to be proproliferative and antiapoptotic. On the contrary, several recent studies have found a conserved miRNA family, the miR-34s, that can induce cell cycle arrest and promote apoptosis. Of considerable interest, miR-34s are direct transcriptional targets of p53 and loss of miR-34s can impair p53-mediated cell death (6–10). In addition, both let-7 (11) family and mir-15/16 (12) are antiproliferative and proapoptotic.
However, these investigations mostly focused on the cancers and the miRNA profiles induced by UVB remain poorly understood. In solar radiation, although only 0.5% UVB (280–320 nm) can reach the earth’s surface, the wavelength of UVB is in the vicinity of absorption peaks of DNA and protein, thereby rendering them susceptible to damage (13). Many studies have reported the biological effect of low-dose UVB in NIH3T3. Jeong et al. (14) and Ibuki et al. (15) reported that UVB induced DNA repair synthesis and apoptosis in NIH3T3 cells. Hu et al. also revealed that monolayer cultures receiving 100 J m−2 UVB could cause damage to the function and molecular structure of NIH3T3, ultimately resulting in apoptosis or necrosis (16). Thus, cell cycle arrest, apoptosis and necrosis are the major biological effects of UVB radiation. As a new class of sequence-specific regulators of gene expression, miRNAs may form a regulatory network with growth factors and transcription factors participating in UVB-induced cell cycle progress and apoptosis.
In this study, we explored the expression profile of miRNAs in the response of NIH3T3 cells to low-dose UVB at different time points using miRNA microarray analysis. Our results showed that UVB irradiation resulted in robust changes in the miRNA expression profile in a time-dependent manner, and aberrant miRNAs in the array expressed at different time points. These results demonstrated the complexity of the miRNA profile of the UVB response, and provided a basis for the global characterization of UVB-regulated miRNA expression. The identification of miRNAs induced by UVB should facilitate future studies of the cellular mechanisms underlying the UVB regulation of gene expression.
Materials and methods
Cell line culture and treatment. The cell lines NIH3T3 of mouse fibroblast origin were cultured in modified Eagle’s medium (MEM; Invitrogen) containing 10% calf serum, 100 μg mL−1 penicillin, 100 μg mL−1 streptomycin and incubated at 37°C in a humidified atmosphere with 5% carbon dioxide. UVB irradiation was carried out using a UVB light source (Shanghai Institute of Metrology and Measurement Detection Technology) and the dose rate was measured by the China Measurement Academy of Science (Shanghai). The wavelength peak of the UVB lamp used is 305 nm. The approximate irradiance of UVB at the sample level (at a distance of 40 cm from the lamp) is 16.5 μW cm−2. The duration of UVB irradiation for obtaining a dose of 50 J m−2 is 5 min. When the confluent cells were irradiated, the lid of the culture dish was removed and the culture medium was replaced by 5 mL PBS after the cells were washed with PBS twice and then exposed to 0, 50, 70, 100 J m−2 of UVB. The cells were randomly divided into several groups, including the control group (sham irradiation group) and the treated group (UVB-irradiated group). After irradiation, the cells were cultured in MEM again for 0, 2, 4, 6 and 12 h.
Cell viability assay. Cells were seeded onto 96-well plates (200 μL/well, 10 000 cells/well) and were treated in two ways: (1) cells were treated with 0, 10, 30, 50, 70, 100 and 150 J m−2 UVB. After irradiation, the cells were cultured in complete culture medium again for a further 12 h. (2) Cells were incubated for 0, 2, 4, 6, 12, 18, 24, 36 and 48 h after exposure to 50 J m−2 UVB radiation. Subsequently, MTT was added to the culture medium to yield a final MTT concentration of 0.5 mg mL−1. Cells were incubated with the MTT for 4 h in a CO2 incubator and were then collected and dissolved in dimethyl sulfoxide. Colorimetric analysis at 570 nm was measured. For trypan blue exclusion assay, the cell suspension was mixed with the same volume of 0.4% trypan blue solution. Light microscopy was used to count the cell viability by estimating the percentage of the number of unstained cells in comparison with the untreated control. All experiments were repeated in triplicate.
Apoptosis assay and FACS. Apoptosis morphological changes of the NIH3T3 cells treated with 0, 50, 70 and 100 J m−2 UVB were observed by acridine orange (AO) and ethidium bromide (EB) staining. One microliter AO/EB (AO: 100 μg mL−1; EB: 100 μg mL−1) was mixed with 25 μL treated or untreated cells of 1 × 106 mL−1, and then 10 μL was tested using a fluorescence microscope (Olympus, Japan) immediately. Cell cycle analysis was performed by flow cytometry for the treated group (6, 12 and 18 h after 50 J m−2 UVB) and the control group. The cells were fixed in chilled 70% ethanol for at least 24 h before staining with 100 μg mL−1 propidium iodide (PI) and 100 μg mL−1 RNase A (Sigma) and were analyzed using FACScan (Coulter) 30 min after staining. All experiments were repeated in triplicate.
RNA isolation and miRNA microarray. Total RNA was extracted by using Trizol reagent (Invitrogen) from NIH3T3 cells irradiated with 50 J m−2 UVB (2, 4, 6 and 12 h postirradiation) and the control group. RNA sample (5 μg) was labeled using the miRCURY Hy3 labeling kit (Cat. 208032; Exiqon) and the labeled sample was concentrated with RNeasy Mini Kit (Cat. 74104, Qiagen) following the manufacturer’s instruction. The concentrated samples were then hybridized on the miRCURY LNA (locked nucleic acid, LNA) Array (v. 8.0) using miRCURYTM Array microarray kit (Cat. 208000V8.0; Exiqon) and Hybridization Chamber II (Cat. 40080; Corning) as well as Bioarray LifterSlip coverslip (Ambion). The capture probes are LNA™-enhanced oligonucleotides. Labeling efficiency was evaluated by analyzing the signals from control spike-in capture probes. The resulting signal intensity values were normalized to per-chip median values and then used to obtain geometric means and standard deviations for each miRNA. Microarray scanning and normalization of the data were performed using GenePix 4000B scanner (635 nm) and GenePix Pro 6.0 software (Axon Instruments). Two independent hybridizations for each sample were performed on chips with each miRNA spotted in quadruplicate. However, we took the typical one with relatively good reproducibility.
Real-time qRT-PCR analysis for miRNA expression. Total RNA was isolated from the treated group (2, 4, 6 and 12 h after 50 J m−2 UVB) and the control group. The miRNA sequence-specific primers for mmu-let-7a (Cat. AM30000), mmu-miR-24 (Cat. AM30121), mmu-miR-376b (Cat. AM30269), mmu-miR-21 (Cat. AM30102) and endogenous control 5S rRNA (Cat. AM30302) were purchased from Ambion. Real-time reverse transcriptase polymerase chain reaction (qRT-PCR) analysis was carried out on a Stratagene MX3000P instrument with mirVana qRT-PCR miRNA detection kit (Cat. AM1558, Ambion) and amplified product levels were detected by real-time monitoring of SYBR Green I dye fluorescence under the following conditions: 37°C, 30 min; 95°C, 10 min of reverse transcription; 95°C, 3 min; 95°C, 15 s; 60°C, 30 s for the amplification. The PCR reaction was carried out up to 40 cycles (n = 3). The gene expression ΔCT values of miRNAs from each sample were calculated by normalizing with internal control 5S rRNA. The ΔΔCt method for relative quantitation of gene expression was used to determine miRNA expression levels. ΔCt was calculated by subtracting the Ct of 5S rRNA from the Ct of the miRNA of interest. ΔΔCt was calculated by subtracting the ΔCt of the reference sample (control) from the ΔCt of each sample. Fold change was generated using the equation 2ΔΔCt. All experiments were repeated in triplicate.
Target prediction and function analysis. PicTar (17) (http://pictar.bio.nyu.edu/) were employed for the prediction of miRNA targets. To evaluate the PicTar target predictions for all single miRNAs, we searched for significantly overrepresented GO terms (18) of all target genes for all differential miRNAs separately using the GOstat software (http://gostat.wehi.edu.au/cgi-bin/goStat.pl) (19). In brief, the program will determine all annotated GO terms and all GO terms that are associated (i.e. in the path) with these for all the genes analyzed. It will then count the number of appearances of each GO term for the genes inside the group and for the reference genes. First, we pasted mouse RefSeq ID of target genes into the text area, and then we chose “mgi” (Mus musculus) in available GO gene-association databases. For the remaining options we selected the default value. Frequently, the most significant GO terms all represent the same subset of genes, as the genes may have several GO annotations that are similar. Fisher’s exact test was performed to judge whether the observed difference was significant or not. This resulted in a P-value for each GO category that the observed counts could have been due to chance. In addition, a pathway analysis of the targets was performed using DAVID Bioinformatics Resources 2008 (http://david.abcc.ncifcrf.gov/).
Statistical analysis. All data were expressed as mean ± standard deviation (SD). Statistical Package for the Social Sciences (SPSS) 13.0 software package was used for the F-test and statistical significance was defined as P < 0.05.
Results
Cell viability assay and cell cycle analysis
UV irradiation decreased cell viability in a dose-dependent manner. The survival ratio of NIH3T3 cells decreased gradually after being irradiated by UVB at different dosages and then incubated at the same duration. Our data showed that UVB exposure, at every dose, resulted in decreased cell viability and increased apoptosis. When the dose was added to 50 J m−2, the mortality increased dramatically (Fig. 1A). According to the above data, together with our morphological data (data not shown), we have chosen 50 J m−2 UVB for the next series of experiments. When cells were exposed to UVB (50 J m−2) and then incubated with complete culture medium for 2, 4, 6, 12, 18, 24, 36 and 48 h, the results showed that there was a large change at 2, 4, 6 and 12 h (Fig. 1B). The effects of UVB irradiation on the progress of NIH3T3 cells were studied by flow cytometry using PI staining. It showed that UVB-induced DNA damage led to cell cycle arrest in the G1 phase (Fig. 2C).
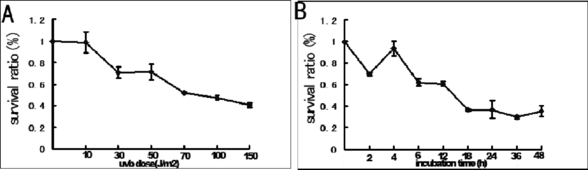
(A) Effect of various doses of UVB irradiation on cell viability. Cells were irradiated with different doses of UVB irradiation and then incubated for 12 h. (B) Cell viability after treatment with the 50 J m−2 UVB for different incubation times. Cell proliferation was determined using the MTT assay.

DNA contents of NIH3T3 cells treated with 50 J m−2 UVB for different incubated times. (A) Control; (B) 6 h; (C) 12 h; (D) 18 h. Typical sub-G1 peaks appeared in the treatment cells for 12 h. The sum of G1, G2 and S fractions was normalized to 100% to strengthen G1 block. AP = apoptosis.
Apoptosis of NIH3T3 cells induced by UVB irradiation
To assess whether apoptosis is induced by UVB irradiation, the following major characteristics of apoptosis were studied. The incidence of chromatin condensation and the production of apoptotic sub-G1 DNA content fractions (Fig. 2C) were observed at 12 h after the postincubation period following UVB light exposure. Chromatin condensation of NIH3T3 cells, irradiated with 50 J m−2 UVB and then stained by AO/EB, was detected by using the fluorescence microscope and showed the morphological features of apoptotic cells, such as cell surface protuberances and nuclear fragment in the early apoptotic cells by AO staining (green). The typical apoptotic bodies were found in the late apoptotic cells and the death cells were dyed high uniform red by EB staining (Fig. 3). These characteristics of apoptosis were more obvious in the cells irradiated with 70 and 100 J m−2 UVB, whereas almost no apoptotic nuclei were observed in the control cells.

AO/EB staining of NIH3T3 cells at 12 h after UVB radiation. (A) Control (arrow indicates normal cell). (B) After the NIH3T3 cells were treated with 50 J m−2 UVB, the apoptotic cells can be observed: some cells appear as typical apoptotic blebs (right arrow). In some cells stained with EB, the nuclei exhibit bright condensed chromatin or fragmented chromatin (left arrow). (C) After being treated with 70 J m−2 UVB, the same apoptotic cells can be observed (arrow represents apoptotic bleb phenomenon). (D) Dead cells can be observed in NIH3T3 cells with 100 J m−2 UVB (arrow shows dead cell). Cells in which nuclei are red or yellow-red indicate apoptotic cells (original magnification ×40).
Relative expression of miRNA genes responsive to UVB in NIH3T3 cells
Recently, a new microarray platform using LNA-modified capture probes shows increased sensitivity and specificity (20). The technology in question is based on the use of LNA-modified oligonucleotides that increase the binding affinities for complementary RNA and DNA sequences (21). In this microarray platform, the capture probes are so designed that they consist of either unmodified DNA oligonucleotides or of a defined combination of unmodified and LNA-modified nucleotide analogs (22). Using this miRNA microarray platform, differentially expressed miRNAs emerged in treated cells compared to the control (Table 1). Furthermore, clustering analysis was performed using CLUSTER 3.0/TreeView software (Fig. 4). It showed 4 h expression clustered to 6 h and 2 h clustered to the 12 h expression, suggesting that a peak of differential expression was between 4 and 6 h and that the expression levels returned to 2 h base level at 12 h.
| Name | Fold-change† |
|---|---|
| 2 h | |
| mmu-miR-24 | +3.3515 |
| mmu-let-7e | +2.5681 |
| mmu-miR-140* | +2.5854 |
| mmu-let-7c | +2.6624 |
| mmu-miR-193 | +2.1943 |
| mmu-miR-31 | +3.1023 |
| mmu-let-7a | +2.4927 |
| mmu-miR-465 | −2.3838 |
| 4 h | |
| mmu-miR-101b | +2.0208 |
| mmu-miR-292-3p | +2.1465 |
| mmu-miR-466 | +2.0331 |
| mmu-miR-542-3p | +3.7746 |
| mmu-miR-126-5p_MM2§ | +3.4450 |
| mmu-miR-200b | +2.1398 |
| mmu-miR-217_MM1‡ | +2.1330 |
| mmu-miR-338 | +2.1421 |
| mmu-miR-292-3p_MM2 | +2.0851 |
| mmu-miR-487b | +2.0192 |
| mmu-miR-369-5p | +2.8270 |
| mmu-miR-376b* | +2.6654 |
| mmu-miR-295 | +2.2503 |
| mmu-miR-302c | +2.0435 |
| mmu-miR-465 | −2.0576 |
| 6 h | |
| mmu-miR-365 | +6.7136 |
| mmu-miR-542-3p | +3.5698 |
| mmu-miR-126-5p_MM2 | +3.3272 |
| mmu-miR-376b* | +2.2297 |
| mmu-miR-296 | −2.0777 |
| mmu-miR-465 | −3.1385 |
| 12 h | |
| mmu-miR-24 | +2.0440 |
| mmu-miR-21 | +5.8962 |
| mmu-miR-143 | +3.6896 |
| mmu-let-7a | +2.0440 |
| mmu-miR-540 | +2.2097 |
| mmu-miR-376c | −2.0576 |
| mmu-miR-465 | −2.7428 |
- The data are presented as fold change increase (+) or decrease (−) compared with the control.
- *Some miRNA hairpin precursors give rise to two excised miRNAs, one from each arm. An asterisk has been used to denote the less predominant form. †Fold-change was generated using standard values at different time points divided by standard values of the control.‡MM1 represents one mis-match (MM) base. §MM2 represents two mis-match bases.

Hierarchical clustering analysis of miRNA expression. Red color indicates genes with a positive (highly expressed) two-fold change and green color denotes genes with a negative (lowly expressed) two-fold change.
As expected, UVB irradiation resulted in robust changes in the miRNA expression profiles in a time-dependent manner. These results show that the early UVB response (at 4 h) involved a relatively big change in miRNA expression with 14 upregulated miRNAs, whereas the change at 12 h was relatively small only with four upregulated miRNAs and two downregulated miRNAs.
Validation of microarray expression by real-time qRT-PCR
A real-time qRT-PCR assay was used to study the expression of four miRNA genes in order to confirm the array data. These miRNAs were selected for two reasons. One is that mmu-let7a and mmu-miR-24 were expressed at not only 2 h but also at 12 h, and mmu-miR-376b was expressed at 4 and 6 h after 50 J m−2 UVB radiation. The other is that their function in the progress of cell cycle and proliferation is relatively clear (11,23).Yanaihara et al. observed that the let-7 family regulated cellular growth and differentiation by RAS gene (11) whereas Lal et al. (24) reported that miR-24 mediates the cell cycle by suppressing p16 expression in human diploid fibroblasts. Real-time qRT-PCR analysis showed that Mmu-let7a and mmu-miR-24 were highly expressed at 2 and 12 h, although levels were variable, whereas their expression levels at 4 and 6 h were less than two times. The expression level of mmu-miR-24 was increased by more than three-fold in treated cells (2 and 12 h) in comparison with the control (Fig. 5A). And the expression level of mmu-let-7a was more than two-fold higher in the treated cells at 2 and 12 h than the control group (Fig. 5B). For mmu-miR-376b, we found that it was expressed at a lower level in the treated group, but was still upregulated at 4 and 6 h compared with the control (Fig. 5C). In addition, the level of mmu-miR-21 was upregulated at 12 h along with mmu-miR-24 (Fig. 5D). Taken together, these results suggest that the expression of these three miRNAs observed in the arrays was consistent with that observed in the real-time qRT-PCR.

Real-time qRT-PCR analysis of miRNA expression. Relative expression of mmu-mir-24 (A), mmu-let-7a (B), mmu-mir-21 (C) and mmu-mir-376b (D) at different time points after 50 J m−2 UVB irradiation. n = 3, Mean ± SD.
Predicted targets of miRNA genes in NIH3T3 cells
Prediction of miRNA-regulated gene targets is a necessary step to understand the functions of miRNA. However, due to the imperfect binding between the majority of human miRNAs and their targets, individual miRNAs may regulate hundreds of target genes (25). Recently a study indicated that PicTar had an excellent recovery rate in target gene prediction (26), so we utilized the database PicTar (17) to obtain predicted gene targets of all differentially expressed miRNAs in response to UVB radiation. These miRNA genes, as expected, could potentially regulate several hundred targets (data not shown).
Discussion
UVB radiation is the carcinogenic factor in sunlight; damage to skin cells from repeated exposure can lead to the development of cancer (27). Cellular mechanisms exist to repair the DNA damage, or to induce apoptosis to remove severely damaged cells. The molecular events in the induction of skin cancer are being actively investigated. However, most of the studies have focused on examining protein-coding genes. Recently, studies have found that miRNAs have been implicated in the regulation of proliferation, differentiation and apoptosis; it seemed possible that miRNAs might contribute to understanding the cellular mechanisms of cell cycle arrest and apoptosis induced by UVB. Our present study uncovered the miRNAs sensitive to UVB.
The LNA modification raises the thermostability of nucleic acid duplex by up to 4°C per nucleotide when the complementary strand is DNA, and up to 8°C when the complementary strand is RNA (22). Furthermore, capture probes with a uniformly normalized Tm of 72°C are designed to distinguish between closely related sequences. By achieving uniform hybridization conditions, LNA-modified probes offer higher sensitivity, specificity and equality for miRNA detection. We analyzed miRNA expression of NIH3T3 cells at a series of different time points (2, 4, 6 and 12 h) after UVB irradiation and the control using a miRNA microarray platform which was able to assess the expression level of 274 mouse mature miRNAs. To select differentially expressed miRNAs from the microarray data, we set the cutoff limit as a two-fold change: a miRNA was regarded as significant if its expression was changed by at least two-fold at one or more of the four time points.
Interestingly, mmu-miR-465 was downregulated throughout all the time points after UVB irradiation. Yu et al. found that mmu-miR-465, which was mapped to the genome in a 62 kb region on the X chromosome, was enriched in prepubertal stages of mouse testicular development and decreased in adult testis, suggesting that their presence was primarily in early stage germ cells and/or in the somatic cells of the testis (28). In our study, the results demonstrated that mmu-miR-465 could be expressed in NIH3T3 cells, and dramatically decreased after UVB irradiation. What is more important is that mmu-miR-465 showed low levels of expression at all time points. These findings suggest that mmu-miR-465 was sensitive to UVB and it may elicit some direct or indirect effects of UVB on fibroblasts. Moreover, we found that the level of mmu-miR-365 at 6 h was significantly higher than that of the control (nearly seven-fold), and the levels of several miRNAs at this time point, such as mmu-miR-542-3p, mmu-miR-126-5p and mmu-miR-376b*, increased more than two-fold in comparison with the control. Shen et al. reported that miR-126 may function in normal hematopoietic cells to modulate HOXA9 protein in F9 cells (29). Not very much is known about the function of miR-365, miR-376 and miR-542 currently.
As miRNA sequences were conserved in mice, chickens and humans with similar genomic organizations, several miRNAs differentially expressed in NIH3T3 cells after UVB irradiation were also discovered in human cancer genes (30–32). Strikingly, we observed that miR-21 and miR-24 appeared together at 12 h after exposure to 50 J m−2 UVB. MiR-21 is known to be involved in cancer progression and has been described as an oncogenic miRNA (33). However, when it appears together with miR-24, it shows a characteristic of inhibiting growth. The association of miR-21 and miR-24 with growth inhibition was also observed in A549 cells (34) and quiescent CHO cells (23). The results of cell cycle and apoptosis at this point had an apparent sub-G1 DNA content fraction (Fig. 1C) and apparent apoptotic cells (Fig. 2B), suggesting that the association of miR-21 and miR-24 inhibited growth.
MicroRNAs have the potential to regulate diverse target genes including cell cycle and apoptosis. It is important to remember that these are predicted targets, and experimental validation of these targets is necessary to determine a bona fide regulatory relationship. Nonetheless, it is tempting to speculate that these miRNAs may represent the first noncoding genes to be implicated in cell cycle induced by UVB irradiation. One of the strongest links to support the notion of a role for specific miRNA genes in this aspect is the regulation of PIK3R1 and BCL2 by miR-21. The available data suggest that overexpression of miR-21 leads to downregulation of PTEN and a more active survival signaling through the PI-3 kinase-Akt pathway rendering the cells less susceptible to apoptosis and cell cycle arrest (35). Moreover, Li and Si demonstrate that Bcl-2 is also involved in miR-21-mediated cellular effects (36,37). Although there is no evidence whether these genes were the direct targets for miR-21 or not, the prediction of these target genes provides clues for further study.
For further analyzing the relationship between the patterns of target gene expression and their functional implications using the results in this database, we classified the target genes of all differentially miRNAs into several function categories using the GOstat software (http://gostat.wehi.edu.au/cgi-bin/goStat.pl) (Supporting Information) (20). Among them, target genes of mmu-miR-369-5p, mmu-miR-540 and mmu-miR-487b were too few to get the results of function categories. The results of the five miRNAs genes with the largest changes about cell differentiation and cell cycle progress are listed in Table 2. Moreover, the targets of these five miRNAs were chosen to perform a pathway analysis on DAVID Bioinformatics Resources 2008 (http://david.abcc.ncifcrf.gov/) (Table 3; Table S2).
| MiRNA | GOs | Target genes | Count | Total* | P-value† |
|---|---|---|---|---|---|
| Mmu-mir-24 | GO:0048869 Cellular developmental process | cd28 myog sox6 prdm1 dyrk2 bcl10 vav1 madd qk nr6a1 bbc3 ybx2 sema6b sema7a flt1 chrd hnf1b cdk5 irak3 sema5a bcl2l11 mapk7 mapk8ip1 tsc22d3 srpk2 pim2 trp53inp1 calcr tcf7 bcl2l2 sema4g klhl1 neurod1 skp2 cdx2 dll1 bmpr1b neo1 pak7 | 39 | 1677 | 6.6e-06 |
| GO:0050794 regulation of cellular process | tcfap2b cnot6 rap1a htra3 men1 bcl10 ybx2 bbc3 chrd zfp697 hnf1b igfbp5 maml1 suv420h2 bcl2l11 per1 tsc22d3 ppm1g pim2 ell mxi1 esrrg rab5c nfib calcr pogz hira taf5 trim66 bcl2l2 ccni sp1 cdx2 rab5b sp6 tcerg1 cd28 klf6 rsc1a1 myog foxo1 sox6 nlk prdm1 vav1 madd nr6a1 qk cdk5 irak3 mapk8ip1 bzw1 axl trp53inp1 trim33 ankrd6 tcf7 cited4 nfat5 neurod1 cntfr cry2 skp2 rnf12 dll1 rnf2 neo1 pak7 | 68 | 3140 | 4.65e-09 | |
| GO:0048869 Cellular developmental process | cd28 myog sox6 prdm1 dyrk2 bcl10 vav1 madd qk nr6a1 bbc3 ybx2 sema6b sema7a flt1 chrd hnf1b cdk5 irak3 sema5a bcl2l11 mapk7 mapk8ip1 tsc22d3 srpk2 pim2 trp53inp1 calcr tcf7 bcl2l2 sema4g klhl1 neurod1 skp2 cdx2 dll1 bmpr1b neo1 pak7 | 39 | 1677 | 6.6e-06 | |
| GO:0030154 cell differentiation | cd28 myog sox6 prdm1 dyrk2 bcl10 vav1 madd qk nr6a1 bbc3 ybx2 sema6b sema7a flt1 chrd hnf1b cdk5 irak3 sema5a bcl2l11 mapk7 mapk8ip1 tsc22d3 srpk2 pim2 trp53inp1 calcr tcf7 bcl2l2 sema4g klhl1 neurod1 skp2 cdx2 dll1 bmpr1b neo1 pak7 | 39 | 1677 | 6.6e-06 | |
| Mmu-mir-21 | GO:0050794 regulation of cellular process | lcorl klf6 suz12 sox5 dazl sox6 reck ntf3 pitx2 yap1 cbx4 dlx2 bcl2 spry1 zfp654 msx1 trim33 tiam1 cadm1 taf5 jag1 pdcd4 il12a stat3 pclo sox2 cntfr caskin1 elf2 glis2 fasl alx1 fubp1 a930001n09rik foxp3 zfp367 sox7 | 37 | 3140 | 8.95e-06 |
| GO:0048522 Positive regulation of cellular process | il12a sox2 cntfr ntf3 glis2 fasl suz12 cadm1 yap1 dlx2 jag1 dazl bcl2 foxp3 | 14 | 719 | 8.67e-04 | |
| Mmu-mir-542-3p | GO:0030154 cell differentiation | bag4 ttn bcl11b whsc1l1 angpt2 pim1 ap3d1 cdx2 unc5d apbb2 atxn1 pten serpine2 rps6kb1 myh9 btg1 sfrs1 nrl kpna3 bcl11a narg1 bmp7 mtpn eif5 sh3kbp1 | 25 | 2389 | 9.23e-06 |
| GO:0048468 cell development | bag4 ttn bcl11b pim1 unc5d pten atxn1 apbb2 rps6kb1 btg1 myh9 sfrs1 nrl kpna3 bmp7 mtpn eif5 sh3kbp1 | 18 | 1865 | 1.48e-03 | |
| Mmu-mir-365 | GO:0050794 regulation of cellular process | api5 hhip eya3 kcnh2 creb5 sox6 bmi1 prdm1 5730403b10rik ing3 six1 zfp148 bcl2 pax6 socs5 4921505c17rik six4 nfib hif1an sertad2 epc1 ywhah cbfb maf pias1 asf1a pbx2 npas4 ncor1 ehf dlx3 csk adm nr1d2 trp63 etv1 tmod3 | 37 | 3140 | 5.29e-08 |
| Mmu-mir-465 | GO:0050794 regulation of cellular process | zfp516 cdc73 cdh5 rras2 tcfap2b lcorl hopx rbm39 foxg1 cdkn1c ash1l tsg101 zfx runx1t1 atxn3 sox6 tceb3 arhgef3 yaf2 eif2ak3 runx2 zxdc six1 zfp148 lrp1 fancl irx3 nfib tal1 hif1an kitl lrp6 actl6a cbx8 irf6 fgf10 nfat5 med14 boc hey1 ctnnd2 pax9 foxd3 strap etv1 tbx15 batf khdrbs3 | 48 | 3140 | 4.98e-06 |
- *Represents a total count of occurrences for each GO term. †P-value represents the probability that the observed numbers of counts could have resulted from randomly distributing this GO term between the tested group and the reference group. A chi-squared test is used in order to approximate this P-value.
| MiRNA | Database | Term | Count | % | P-value |
|---|---|---|---|---|---|
| Mmu-mir-24 | KEGG_PATHWAY | Axon guidance | 7 | 3.2 | 1.5E-2 |
| MAPK signaling pathway | 9 | 4.1 | 4.9E-2 | ||
| T cell receptor signaling pathway | 5 | 2.3 | 6.5E-2 | ||
| Mmu-mir-21 | KEGG_PATHWAY | Cytokine–cytokine receptor interaction | 5 | 4.3 | 5.0E-2 |
| Jak-STAT signaling pathway | 4 | 3.5 | 5.8E-2 | ||
| TGF-beta signaling pathway | 3 | 2.6 | 9.9E-2 | ||
| Mmu-mir-365 | KEGG_PATHWAY | mTOR signaling pathway | 3 | 2.8 | 2.9E-2 |
| Colorectal cancer | 3 | 2.8 | 6.8E-2 | ||
| Small cell lung cancer | 3 | 2.8 | 7.3E-2 | ||
| Prostate cancer | 3 | 2.8 | 7.3E-2 | ||
| Mmu-mir-465 | KEGG_PATHWAY | Selenoamino acid metabolism | 3 | 2.0 | 2.1E-2 |
| Ubiquitin-mediated proteolysis | 4 | 2.7 | 8.2E-2 | ||
| Mmu-mir-542-3p | KEGG_PATHWAY | Focal adhesion | 4 | 4.0 | 5.7E-2 |
In summary, the focus of this study was to investigate the differential expression profiles of miRNAs in NIH3T3 cells in response to UVB irradiation. Although our investigation is very preliminary, we believe this study provides a basis for further investigation of their function in signal transduction pathways induced by UVB. miRNAs may be new research hotspots for the prevention or treatment of skin cancer caused by UVB.
Acknowledgments
Acknowledgement— This work was supported by the National Natural Science Foundation of China (30570436).




