Photodynamic Action of Benzo[a]pyrene in K562 Cells
Abstract
Benzo[a]pyrene (BaP) is ubiquitously distributed in the environment, being considered the most phototoxic element among polycyclic aromatic hydrocarbon (PAHs). In presence of oxygen, PAHs can act as a photosensitizer by means of promoting photo-oxidation of biological molecules (photodynamic action, PDA). Thus, the present study analyzed the photodynamic action of BaP under UVA irradiation (BaP + UVA) and its oxidative effects on K562 cells. The evaluation of BaP kinetics showed that the highest intracellular concentration occurred after 18 h of incubation. Cell viability, reactive oxygen species (ROS) generation, lipid peroxidation, DNA damage (breaks and DNA–protein cross-link [DNAPC]) were assessed after exposure to BaP, UVA and BaP plus UVA irradiation (BaP + UVA). Cell viability was decreased just after exposure to BaP + UVA. Lipid peroxidation and DNA breaks increased after BaP + UVA exposure, while the DNAPC increased after BaP, UVA and BaP + UVA exposure, suggesting that BaP + UVA effects were a consequence of both type II (producing mainly singlet oxygen) and type I (producing others ROS) mechanisms of PDA.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a product of incomplete combustion of organic matter (1). Among over a hundred different PAH compounds, 16 have been classified as priority pollutants by the United States Environmental Protection Agency. The ubiquitously distributed benzo[a]pyrene (BaP) is well known for its carcinogenicity, which requires metabolic activation that generates electrophilic reactive metabolites. BaP binds to and activates the aryl hydrocarbon receptor (2), being metabolized by the cytochrome P450 1A1, the microsomal epoxide hydrolase and the glutathione-S-transferase α (3). In addition, toxicity of PAHs can be augmented by physicochemical factors. As a result of a multiple aromatic ring in its molecule, PAHs can absorb light energy in the UVA wavelength and, some compounds, in the visible radiations wavelength (4), forming reactive species and metabolites that damage the cellular components. BaP, for instance, is one of the most phototoxic PAHs (5).
Photodynamic action (PDA) is the photo-oxidation of biological molecules in the presence of oxygen and a sensitizing substance (6). During this process, photosensitizers absorb light energy and reach an excited state. In the return to the ground state, excited photosensitizers transfer their energy to the ground-state oxygen, producing reactive oxygen species (ROS) (7). The excess of intracellular ROS may oxidize macromolecules, leading to lipid peroxidation, protein oxidation and DNA base oxidation (8).
There are two principal mechanisms that lead to the death of the cell by photo-oxidation: type I and type II mechanisms. In the type I mechanism, a photosensitizer absorbs a photon of an appropriate wavelength, activates it to an excited singlet state and produces ROS such as superoxide anion and hydroxyl radicals that will react with biological targets. On the other hand, in the type II mechanism, after activation upon light absorption, the photosensitizer energy is transferred to triplet state oxygen (3O2), producing singlet oxygen (1O2), a potent oxidizer that also interacts with biological targets (9).
The UV radiation of the electromagnetic spectrum is divided into three bands: UVA (400–320 nm), UVB (320–290 nm) and UVC (290–200 nm). Effects of UV can be related to the increment of ROS production, inducing oxidation of proteins and membrane lipids (10,11). Structural damage to DNA can also be directly caused by UVB and indirectly by UVA radiation through ROS generation. The UVA and UVB cause damage to DNA through the photodynamic production of hydroxyl radicals causing single- or double-strand DNA breaks, protein–DNA cross-links, DNA–DNA cross-links and the formation of micronuclei (12,13).
UV radiation also enhances the covalent binding of PAHs to DNA (14). The concomitant exposure to PAHs and UV radiation can also increase DNA single-strand cleavage and oxidation of DNA bases, and form DNA covalent adducts mainly through ROS generation (15–17).
Thus, the aim of the present study was to analyze the photodynamic action of BaP under UVA irradiation (BaP + UVA) and its oxidative effect in K562 cells.
Materials and Methods
Cell line and culture conditions. The K562 is a human erythroleukemic cell line. It was obtained from the Tumoral Immunology Laboratory at the Medical Biochemistry Department (Federal University of Rio de Janeiro, Brazil). The K562 cell line was chosen for being parental to K562-Lucena cells, which is multidrug-resistant (MDR) (18), allowing a posterior comparative study between MDR and non-MDR cells. The cells were grown at 37°C in disposable plastic flasks containing RPMI 1640 (Gibco) medium supplemented with sodium bicarbonate (0.2 g L−1), l-glutamine (0.3 g L−1), Hepes (25 mm), β-mercaptoethanol (5 × 10−5 m), fetal bovine serum (10%; Gibco) and 1% of antibiotics and antimicotic (penicillin [100 U mL−1], streptomycin [100 μg mL−1], amphotericin B [0.25 μg mL−1]; Gibco).
Gas chromatographer/mass spectrometer analysis. Concentrations of BaP solutions were monitored using gas chromatographer/mass spectrometer (GC/MS) analysis. Measured concentrations confirmed that the nominal values were within ±10%. Briefly, BaP solutions (1 mL) were spiked with pyrene-d10 (as surrogate) and then sequentially extracted with 2 mL of n-hexane (three times). The extract was then concentrated down to 1 mL before being analyzed using a GC/MS (Perkin Elmer Clarus 500) equipped with an Elite 5-MS capillary column (30 m long, 0.25 mm ID, 0.25 μm film thickness), on a splitless mode and using helium as the carrier gas. Compound identification was based on individual mass spectra of BaP (m/z = 252) and its retention time in comparison to an authentic standard (Sigma-Aldrich). Compound quantification was made using 9,10-dihydroanthracene as the internal standard. Recoveries were 95 ± 3% (n = 2) for pyrene-d10.
Treatment of cells. Benzo[a]pyrene exposure: Cells were grown for 2 days before the experiments were performed. They were then centrifuged, washed with PBS and suspended in RPMI 1640 medium free of β-mercaptoethanol in each of the experiments. They were then incubated for 48 h in a medium with different concentrations of BaP (Sigma-Aldrich) (1.08, 2.17, 4.34 or 8.68 μg mL−1), whereas the control cells were incubated for 48 h in medium with 1% dimethyl sulfoxide (DMSO) in PBS (no BaP). The DMSO, which was used to prepare the BaP exposure solution, was always kept on a concentration (1%) that had no effect on any of the analyzed end points. After being exposed to BaP the cells were incubated at 37°C into 24-well culture plates (19) to allow further control over the cell viability.
UVA exposure after BaP incubation: Cells were incubated for 18 h in a RPMI 1640 medium free of β-mercaptoethanol with the same concentrations of BaP reported above. During exposure to assess the photodynamic action of BaP, the growth medium was replaced by PBS. The cell line (without—control group or with BaP—BaP + UVA) was irradiated with 1 J cm−2 UVA (VL-115 L, 30 W; Vilber Lourmat, France; without contamination with UVB). The spectrum of UVA irradiation ranged from 320 to 380 nm with the peak intensity at 365 nm. The intensity of irradiation was measured immediately before the cell irradiation using a radiometer/photometer (model IL 1400A, International Light, MA). After the irradiation, the cells exposed to 2.17 μg BaP mL−1 were immediately prepared to subsequent tests.
Kinetics of BaP: The K562 cells were incubated with 2.17 μg BaP mL−1 for different lengths of time up to 48 h (0, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 18, 24 and 48 h). At each incubation time, cells were washed twice with PBS and aliquots of 160 μL (five replicates) were placed into an ELISA plate and the fluorescence intensity determined. The entrance of BaP into the cells was measured using a fluorometer (Victor 2, Perkin Elmer), with an excitation and emission wavelength of 340 and 450 nm, respectively.
Assessment of the sensitivity of the K562 cell line to BaP. Cell viability: The cell viability of K562 cell line exposed to BaP (without irradiation) was assessed by trypan blue exclusion immediately, 24 and 48 h after exposure to BaP and for cells exposed to BaP and UVA irradiation, its viability was assessed after 18 h of incubation with BaP (before irradiation), immediately after irradiation with UVA, and 24 h after irradiation (20). Oxidative effects and DNA damage were measured immediately after UVA irradiation in cells previously exposed to 2.17 μg BaP mL−1.
Oxidative effects of benzo[a]pyrene. Assessment of intracellular ROS formation: A suspension of exposed K562 cells (4 × 105 cell mL−1) was washed twice with PBS and incubated for 30 min at 37°C with the fluorogenic compound 2′,7′-dichlorofluorescin diacetate (H2DCF-DA, 40 μm; Molecular Probes) (21). H2DCF-DA passively diffuses through cellular membranes and, once inside, the acetates are cleaved by intracellular esterases. Thereafter, the nonfluorescent compound H2DCF is oxidized by ROS into a fluorescent compound (DCF). Once loaded with H2DCF-DA, the cells were washed twice with PBS. Each treatment was performed in triplicate. Aliquots of 160 μL of each sample (five replicates) were placed into an ELISA plate and the fluorescence intensity determined during 90 min at 37°C, using a fluorometer (Victor 2, Perkin Elmer) with an excitation and emission wavelength of 485 and 520 nm, respectively. ROS levels were expressed in terms of fluorescence area and were obtained by integrating the fluorescence units (FU) over the measurement time (90 min) and expressed as FU min.
Determination of lipid peroxidation: The levels of lipid hydroperoxides (LPO) were determined according to Hermes-Lima et al. (22), with modifications (23). The method is based on the oxidation of Fe(II) by LPO (FOX reactive substances) at acidic pH in the presence of the Fe(III)-complexing dye, xylenol orange. Samples were homogenized (10% wt/vol) in 100% cold (4°C) methanol. The homogenates were then centrifuged at 1000 g, for 10 min at 4°C. The supernatants were used for LPO determinations (580 nm). Cumene hydroperoxide (Sigma-Aldrich) was employed as standard.
DNA damage. DNA–protein cross-link: DNA–protein cross-link (DNAPC) levels were determined using K+/SDS precipitation assay, following the method of Zhitkovich and Costa (24), modified by Schoenfeld and Witz (25). Exposed cells (1 × 107 cells mL−1) were lysed in 500 μL of 0.5% SDS, 1 mm PMSF, 20 mm Tris–HCl, pH 7.5. The DNAPC fraction was digested with 200 μg of proteinase K (15 U mg−1 protein) for 3 h at 50°C. DNA was stained with SyberGold (Fluka) fluorescent dye. The amount of DNA in the samples was determined using the fluorometer (Victor 2, Perkin Elmer), with an excitation and emission wavelength of 485 and 535 nm, respectively.
Single-cell electrophoresis assay: The DNA damage was assessed through the alkaline single-cell electrophoresis (comet) assay, carried out as described by Singh et al. (26) and Tice et al. (27), with slight modifications. An aliquot (50 μL) of cell suspension of each sample (2 × 105 cell mL−1) was mixed with 30 μL of 1.73% low-melting-point agarose and added to fully frozen slides, which had been covered with a layer of 0.65% normal-melting-point agarose. Following the layer solidification, cells in slides were lysed (2.5 m NaOH, 0.1 m EDTA, 0.01 m Tris, 1% sodium sarcocinate, 1% Triton X-100, and 10% DMSO, pH 10) overnight at 4°C. Subsequently, samples were placed in the electrophoresis buffer (300 mm NaOH and 1 mm EDTA, pH 13) for 35 min to allow DNA unwinding. Then, electrophoresis was performed during 25 min at 25 V and 280 mA. Finally, the slides were neutralized with 0.4 m Tris buffer (pH 7.5), stained with 95 μL of Sybergold (10 000×) and analyzed using an epifluorescence microscope Zeiss Axioplan (400× magnification). In 50 randomly selected cells, in duplicated slides, the DNA damage was scored as: undamaged (class 0), presenting short migration of DNA (class 1), mean migration (class 2), long migration (class 3) and complete migration (no nucleus remaining, class 4). The final score was achieved by adding up all the attributed class for each of the 50 cells, resulting in scores ranging from 0 (for no damage) to 200 (for maximum damage).
Statistical analysis. At least three independent experiments were done in triplicates for each exposure experiment. Data were expressed as mean (±SE) and analyzed by ANOVA followed by Tukey multiple-range test. ANOVA assumptions (normality and homogeneity of variances) were previously checked. The significance level (α) was fixed in 0.05.
Results
Kinetics of BaP by K562 cells
The kinetic of BaP accumulation by K562 cells was evaluated using the PAHs fluorescence properties, which allows us to follow the accumulation inside the cells. After being incubated for just 1 h, the cells already showed higher levels of fluorescence (P < 0.05), reaching their highest levels after 18 h and decreasing afterwards (Fig. 1).
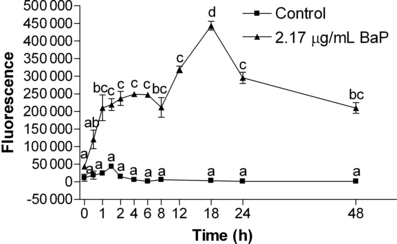
Kinetics of benzo[a]pyrene (BaP) accumulation by K562 cells (measured by fluorescence) after incubation with 2.17 μg BaP mL−1 for different times. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
Cell viability
Cell viability did not show significant difference in the cells treated with different concentrations of BaP (without irradiation) up to 48 h (Fig. 2) when compared with the control group (P > 0.05). However, immediately after UVA irradiation, the cells incubated with 4.34 and 8.68 μg mL−1 BaP showed a significantly lower viability (P < 0.05) when compared with the control group (just irradiated) (control: 92.26 ± 2.33; 4.34 μg mL−1 BaP + UVA: 65.52 ± 6.92; 8.68 μg mL−1 BaP + UVA: 51.10 ± 1.62). After 24 h, all cells died except those of the control group (Fig. 3).
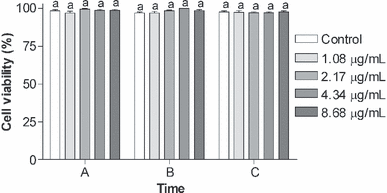
Cell viability (%) of K562 cell line exposed to different concentrations (1.08, 2.17, 4.34 or 8.68 μg mL−1) of benzo[a]pyrene (BaP) in different times: (A) Immediately after exposure to BaP, (B) 24 h after exposure to BaP and (C) 48 h after exposure to BaP. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
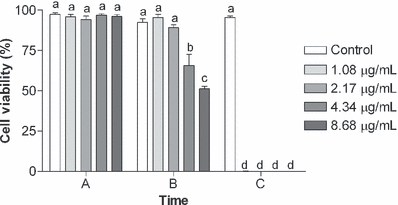
Cell viability (%) of K562 cell line incubated during 18 h with different concentrations (1.08, 2.17, 4.34 or 8.68 μg mL−1) of benzo[a]pyrene (BaP) and irradiated with UVA (1 J cm−2) in the following times: (A) 18 h after incubation with BaP, before UVA irradiation, (B) immediately after UVA irradiation and (C) 24 h after UVA irradiation. The control cells received only UVA radiation. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
Oxidative effects of benzo[a]pyrene
The capability of photodynamic action to generate ROS was assessed by the exposure of K562 cells to BaP (without irradiation), UVA (without BaP) and BaP followed by UVA irradiation (BaP + UVA). Only K562 cells treated with BaP + UVA showed a significant decrease (P < 0.05) in the amount of ROS produced (control: 86.4 × 105 ± 5.2 × 105 FU min; BaP + UVA: 26.7 × 105 ± 1.6 × 105 FU min) (Fig. 4a). Cell viability analyzed after ROS assay also showed a significant (P < 0.05) decrease in the cells exposed to BaP + UVA (control: 98.15 ± 1.85%; BaP + UVA: 47.96 ± 8.49%) (Fig. 4b).
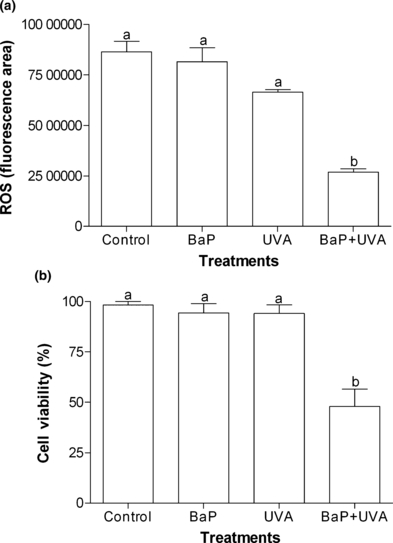
(a) ROS production (fluorescence area) in K562 cell line exposed to benzo[a]pyrene (BaP; 2.17 μg mL−1 for 18 h), or UVA (irradiated with 1 J cm−2) or coexposed to BaP + UVA compared to control cells. (b) Cell viability (%) of K562 cell line after ROS production assay. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
The capability of LPO induction (Fig. 5) and the DNA damage showed by comet assay (strand breaks) (Fig. 6) gave significant difference (P < 0.05) only between the control cells and BaP + UVA exposed cells, while all treatments (BaP, UVA and BaP + UVA) were capable of inducing DNAPC (P < 0.05) when compared with the control cells (Fig. 7).
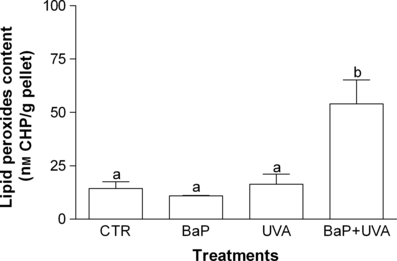
Lipid peroxides content in K562 cell line exposed to benzo[a]pyrene (BaP; 2.17 μg mL−1 for 18 h), or UVA (irradiated with 1 J cm−2) or coexposed to BaP + UVA compared to control cells. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
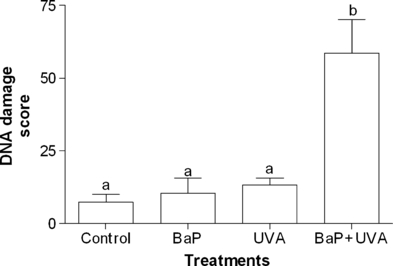
DNA damage (score) in K562 cell line exposed to benzo[a]pyrene (BaP; 2.17 μg mL−1 for 18 h), or UVA (irradiated with 1 J cm−2) or coexposed to BaP + UVA compared to control cells. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
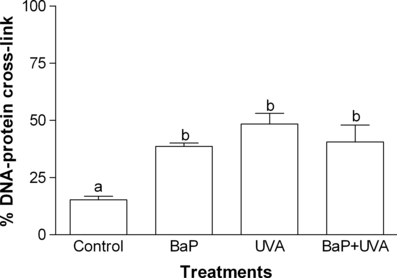
DNA–protein cross-link (%) in K562 cell line exposed to benzo[a]pyrene (BaP; 2.17 μg mL−1 for 18 h), or UVA (irradiated with 1 J cm−2) or coexposed to BaP + UVA compared to control cells. Data are expressed as mean ± 1 SE. Similar letters indicate absence of significant differences (P > 0.05).
Discussion
The investigation of the kinetics of BaP uptake by cells is crucial since the literature demonstrated that PDA assessment of BaP + UVA showed streaking differences related to BaP incubation period, e.g. 1 (28), 2 (29), 16–18 (30) and 24 h (31). As results for K562 cells showed a maximum fluorescence response (highest incorporation of BaP by the cells) after 18 h, this incubation time was the selected one for testing the oxidative effects of BaP-PDA.
Results for cell viability have shown that BaP alone was not toxic for the concentrations and incubation period analyzed (Fig. 2). This result agrees with that obtained by Ibuki and Goto (28), which demonstrated that BaP alone did not present significant toxicity. In the same way, UVA alone, in the environmental dose utilized (unpublished results from our laboratory), has not shown any toxicity for the conditions tested, which corroborate with the results showing that a UVA dose as high as 3 × 105 J m−2 was necessary to reach DL50 in K562 cell line (19). Nevertheless, BaP + UVA coexposure has been reported to induce cytotoxic effects by metabolic activation of BaP (17). Other cytotoxic effects were also reported in several cell lines: DNA damage in human epidermoid carcinoma cells—A431 (15); depletion of cellular adenosine triphosphate, which results in an alteration in cytoskeletal organization and in an increased resistance to trypsinization in nontransformed human MCF-10A cell line (30); induction of apoptosis by singlet oxygen production in lymphoblast cell line (Jurkat cells) (28) and an enhancement of H2O2 production also in A431 cell line (29).
Reactive oxygen species production has also been previously associated to BaP + UVA coexposure (17,29,31). However, rather than promoting an increment, a reduction in ROS production was observed in K562 cells exposed to BaP + UVA (Fig. 4a). The reduction can be explained by the concomitant decrease in cell viability (Fig. 4b), indicating a dysfunction of cellular metabolism. Ibuki and Goto (28) showed lethality in lymphoblast due probably to singlet oxygen production after exposure to BaP + UVA. Thus, the singlet oxygen, a highly reactive substance, could be one of the ROS responsible for the reduction in the cell viability, what may have led to the low ROS concentration observed in the K562 cells exposed to BaP + UVA.
Conversely, an increase in LPO levels was observed after BaP + UVA coexposure (Fig. 5). Despite very few studies having assessed the association between BaP + UVA and LPO levels, this oxidative damage has already been reported as a major contributor to the loss of cell function under oxidative stress conditions (32) and supports the idea of oxidative stress generation as a toxic mechanism of BaP in presence of UVA radiation.
The results obtained for the comet assay (Fig. 6) have proved that the association between BaP + UVA was capable of inducing genotoxicity. DNA damage after coexposure to BaP + UVA has been previously reported by measuring 8-hydroxy-2′-deoxyguanosine (8-OhdG) generation (15,17,31), but only Toyooka et al. (33), utilizing a slightly modified comet assay, also identified DNA breaks. On the other hand, DNA damage measured by DNAPC was not exclusively induced by BaP + UVA coexposure, but also by either BaP or UVA irradiation (Fig. 7). Peak and Peak (34) have also observed DNAPC in human carcinoma cells treated with UVA irradiation, while Cosma and Marchok (35) have demonstrated that high doses of BaP (without irradiation) were capable of producing DNA breaks in rat tracheal epithelial cells (although no DNAPC was observed). In the present study, it was demonstrated that UVA, BaP and BaP + UVA coexposure were capable of DNAPC induction and that coexposure do not cause a synergistic response. However, this coexposure was also capable to provoke DNA breaks, as evidenced by the comet assay results.
Ibuki and Goto (28) considered singlet oxygen as being a major intermediate agent in DNA damage induction under association of BaP + UVA exposure and Shyong et al. (29) demonstrated the formation of H2O2 under this association. Based on that, it could be suggested that effects of coexposure to BaP + UVA are resultant of concomitant type II (producing mainly singlet oxygen) and type I (producing others ROS) mechanisms of BaP photodynamic action.
Finally, considering that BaP is ubiquitously distributed in the environment and levels of UVA exposure are continuously increasing due to ozone layer depletion, it is essential to appraise and understand the effects that this association may produce in different biological organizational levels. Thus, the present work fills up to the study of deleterious effects caused by coexposure to BaP and UVA irradiation in cellular level.
Acknowledgments
Acknowledgements— This work was supported by PRONEX (Process E26-171.217-2003-APQ1) and by FAPERGS (Process 04/0023.1). J. M. M. and G. F. are research fellows from Brazilian CNPQ and L. A. G. is a postdoctoral fellow from CAPES (PRODOC program). The authors thank Dr. Paulo César Abreu for the use of the epifluorescence microscope and Vivian Mary Barral Dodd Rumjanek for internship in her laboratory.




