Modulation of Hypericin Photodynamic Therapy by Pretreatment with 12 Various Inhibitors of Arachidonic Acid Metabolism in Colon Adenocarcinoma HT-29 Cells
Abstract
One proposal to increase the efficiency of photodynamic therapy (PDT) is to accompany photosensitization with other treatment modalities, including modulation of arachidonic acid (AA) metabolism. The aim of this study was to evaluate the effectiveness of a combined modality approach employing 48 and 24 h pretreatment with various inhibitors of lipoxygenase (LOX; nordihydroguaiaretic acid, esculetin, AA-861, MK-886 and baicalein), cyclooxygenase (COX; diclofenac, flurbiprofen, ibuprofen, indomethacin, SC-560 and rofecoxib) and cytochrome P450-monooxygenase (proadifen) pathways, followed by hypericin-mediated PDT. Cytokinetic parameters like MTT assay, adherent and floating cell numbers, viability and cell cycle distribution analysis were examined 24 h after hypericin activation. Pretreatment of human colon cancer cells HT-29 prior to PDT with 5-LOX inhibitor MK-886 as well as 5, 12-LOX and 12-LOX inhibitors (esculetin and baicalein, respectively) resulted in significant and dose-dependent effects on all parameters tested. Pretreatment with diclofenac, flurbiprofen, ibuprofen and indomethacin, the nonspecific COX inhibitors, promoted hypericin-mediated PDT, but these effects were probably COX-independent. In contrast, application of SC-560 and rofecoxib, specific inhibitors of COX-1 and COX-2, respectively, attenuated PDT. Inhibition of P450 monooxygenase with proadifen implied also the significance of this metabolic pathway in cell survival and cell resistance to hypericin photocytotoxicity. In conclusion, our results testify that application of diverse inhibitors of AA metabolism may have different consequences on cellular response to hypericin-mediated PDT and that some of them could be considered for potentiation of PDT.
Introduction
The concept of photodynamic therapy (PDT) is based on preferential accumulation of photosensitizer in neoplastic tissue and its activation with light of appropriate wavelength (1,2). PDT has found its applicability in the treatment of malignant as well as benign tumors, which are localized in several organ systems, including the digestive tract.
Application in PDT of hypericin, a photosensitizer naturally occurring in Hypericum perforatum (St. John’s Wort), has demonstrated powerful photocytotoxic properties in vitro and in vivo alike (2–6). The molecular mechanisms involved generally in PDT, and particularly in hypericin-based PDT, are not completely understood, although it has become clear that cell photosensitization initiates multiple signaling pathways, which ultimately leads to cell death (7–9).
Cellular membrane phospholipids are readily attacked by phospholipases and degraded to free fatty acids after various stimuli. Of major importance is the release of arachidonic acid (AA) and its subsequent conversion. While cyclooxygenases (COX, multienzyme complexes) catalyze the conversion of AA to tromboxanes (A2, B) and prostaglandins (PDG2, PDE2 and 6-ketoPGF1), lipoxygenases (LOX) convert AA to leukotrienes (B4, C4, D4) and hydroxy acids and cytochrome P-450 monoxygenase convert AA to epoxyeicosatrienoic acids, hydroxyeicosatrienoic acids (HETEs) and diols. Some of these generated substances are involved in an acute inflammatory reaction or tumorigenesis. For example, Kobayashi et al. (10) revealed that PDT could stimulate PMNs to generate an especially large amount of leukotriene B4 (LTB4). On the other hand, Panossian et al. (11) described AA release and inhibition of LTB4 production after hypericin treatment on human leukocytes.
The metabolism of AA can be modulated by selective enzyme inhibitors. A lot of reports from our and other laboratories have demonstrated the antiproliferative and proapoptotic potential of various more or less specific COX as well as LOX inhibitors, in a number of human cancer cell lines (e.g. breast, colon, prostate, lung and malignant hemapoietic cells) (12–14).
Controversial results have been observed in cancer cells with modulated metabolism of AA prior to PDT. The studies of Henderson and Donovan (15) and Penning et al. (16) demonstrated the post-PDT release of PGE2 and tromboxane B2 from mouse fibrosarcoma cells and human bladder cancer cells, respectively. Both studies showed that indomethacin inhibited this effect and additionally increased the sensitivity of tumor cells to PDT, suggesting that PGE2 has a protective function. However, application of exogenous PGs increased the cell-killing effect of PDT in glioma cells and human endothelial cells (17). Recently, it has been demonstrated that a selective COX-2 inhibitor, NS-398, enhanced PDT responsiveness in radiation-induced fibrosarcoma tumors without increasing toxicity to normal tissue (18). At the same time NS-398 caused upregulation of COX-2 and induced apoptosis resistance in hypericin-mediated PDT on HeLa cells (19,20). These studies suggest that new production of LTB4, PGE2 and TXB2 may involve the phospholipase and LOX, COX enzyme systems, and hence support the importance of AA metabolism in the tumoricidal effects of PDT. Moreover, literature data imply different effectiveness and cell type specificity of AA metabolism inhibitors. Using human colon adenocarcinoma cell line HT-29, therefore, we modulated AA metabolism with various inhibitors of LOX, COX and P450 pathways prior to PDT with hypericin. We employed low/nontoxic doses of both treatments and simultaneously studied parameters reflecting therapy effectivenes such as cell metabolic activity, cell number, viability and cell cycle progression.
Materials and methods
Cell culture. HT-29 cells (human colon adenocarcinoma) were purchased from American Tissue Culture Collection (ATCC, Rockville, MD). Cells were grown in complete RPMI 1640 medium (Gibco, Grant Island, NY) supplemented with 7.5% NaHCO3 (10 mL/L), penicillin 100 U/mL, streptomycin 100 μg/mL and amphotericin 25 μg/mL (Invitrogen, Carlsbad, CA) and 10% heat-inactivated fetal calf serum (FCS; PAA Laboratories GmbH, Linz, Austria) at 37°C, 95% humidity and in an atmosphere of 5% CO2.
Reagents. Nordihydroguaiaretic acid (NDGA; 4,4-(2,3-dimethyl-1,4-butanediyl)-bis-(1,2-benzenediol); Sigma-Aldrich Corporation, St. Louis, MO) as a general inhibitor of LOX pathways and esculetin (6,7-dihydroxycoumarin; Sigma) as an inhibitor of 5- and 12-LOXs were used. MK-886 (3-[1-(4-chlorobenzyl)3-t-butyl-thio-5-isopropynidol-2-yl]-2,2-dimetyl propionic acid; gift from Merck Frosst Canada, Inc., Pointe Claire-Dorval, Québec, Canada), a specific inhibitor of five LOX activating protein (FLAP) and AA-861 (2-(12-hydroxydodeca-5,10-dinyl)-3,5,6-trimethyl-1,4-benzoquinone; Sigma) a specific 5-LOX inhibitor were used as the inhibitors of the 5-LOX pathway. Baicalein (5,6,7-trihydroxyflavone; Sigma) was used as a specific inhibitor of 12-LOX pathway. Indomethacin (1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-Indole-3-acetic acid; Sigma), ibuprofen (2-(4-Isobutylphenyl)-propionic acid; ICN Biomedicals Inc., Aurora, OH), flurbiprofen (2-(3-fluoro-4-phenyl-phenyl) propanoic acid; ICN Biomedicals, Inc.), diclofenac (2-[2-(2,6-dichlorophenyl) aminophenyl] acetic acid, Almiral, pro injection; Medochemie Ltd, Limassol, Cyprus) were used as a nonselective inhibitors of COX pathways. SC-560 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole; Sigma) as specific COX-1 inhibitor and rofecoxib (4-(4-methylsulfonylphenyl)-3-phenyl-5H-furan-2-one; gift from Merck Frosst Canada Inc.) as specific COX-2 inhibitor were used. Proadifen (SKF525A; α-phenyl-α-propylbenzeneacetic acid 2-(diethylamino)-ethylester; Sigma) was used as an inhibitor of cytochrome P-450 pathway (see Scheme 1).

Arachidonic acid metabolism pathways and specificity of individual inhibitors.
MK-886, AA-861 and baicalein were dissolved in 96% ethanol. NDGA, esculetin, indomethacin, ibuprofen, flurbiprofen, SC-560 and rofecoxib were dissolved in dimethyl sulfoxide (DMSO; Sigma). Proadifen was dissolved in distilled water. All inhibitors were freshly prepared shortly prior to application to the cultures. The final concentration of diluents did not influence the cytokinetical parameters.
Hypericin (4,5,7,4′,5′,7′-hexahydroxy-2,2′-dimethylnaphtodiantron, HPLC grade; AppliChem GmbH, Darmstadt, Germany). A stock solution with exact concentration specified spectrophotometrically was prepared in DMSO and then diluted to working solutions of particular concentrations. The final concentration of DMSO was less than 0.1% and did not influence the cytokinetical parameters. Our previous results demonstrated no cytotoxic effect of hypericin in dark conditions (21).
Because no significant differences in the response to ethanol or DMSO were observed, data from both controls are therefore referred as control.
Hypericin activation. Plates with the cells treated with hypericin for 16 h in the dark were placed on a plastic diffuser sheet 7 cm above a set of 11 white L18W/30 lamps (Osram, Berlin, Germany) with maximum emission range 530–620 nm. The medium containing hypericin was replaced with fresh, hypericin-free RPMI 1640 with 10% FCS immediately before photosensitization. Incorporated hypericin was activated by light at a total dose of 4.4 J cm−2 (fluence rate 4.4 mW cm−2).
Experimental design. HT-29 cells (2 × 104/cm2) were seeded and cultivated 24 h in a complete medium with 10% FCS. The crucial point of the whole experimental design was the time of intracellular hypericin activation. Cells were pretreated with various inhibitors alone for 48 or 24 h prior to PDT. Hypericin was supplemented 16 h prior to PDT (see Scheme 2). The 3-[4,5-dimethylthiazolyl]-2,5-diphenyltetrazolium bromide (MTT) assay, floating and adherent cell number, viability and cell cycle parameters were analyzed 24 h after PDT.
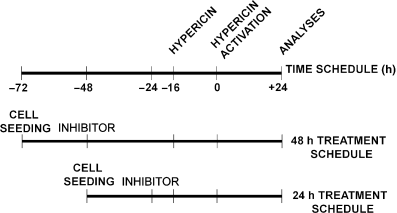
Experimental design. Cells were seeded and cultivated 24 h in a complete medium before first treatment with the relevant inhibitor (−72 in time schedule). Then the cells were pretreated with the relevant inhibitor for 48 h (−48 in time schedule) or 24 h (−24 in time schedule) prior to PDT (point 0 in time schedule). Hypericin was supplemented 16 h prior to PDT (−16 in time schedule). The MTT assay, floating and adherent cell number, viability, apoptosis and cell cycle parameters were analyzed 24 h after PDT (+24 in time schedule).
MTT assay. For MTT assay, cells were seeded in 96-well tissue culture plates (TPP, Trasadingen, Switzerland). MTT (14) was added to cells at analysis time points according to the experimental design (24 h after PDT) and allowed to metabolize. After 4 h incubation the metabolization of MTT was stopped and crystals of formazan were dissolved overnight with 10% SDS (final concentration 3.3%) (Serva, Heidelberg, Germany). Absorbance of dissolved formazan was measured at 584 nm using FLUOStar Optima (BMG Labtechnologies GmbH, Offenburg, Germany) and results were evaluated as the percentage of metabolic activity compared with the control group.
Cell number, floating cells quantification and viability assay. For enumeration of adherent and floating cells or viability assay, cells were seeded in six-well TPP. Adherent and floating cells were counted separately using a Coulter Counter (Model ZF, Coulter Electronics Ltd, Luton, Beds, UK). The number of adherent cells was evaluated as a percentage of control. The number of floating cells was expressed as a multiplication in comparison with control. Total cell viability (adherent + floating) was analyzed by staining with 0.15% eosin using light microscopy and evaluated as the percentage of unstained (viable) cells in a total of 100 cells.
Analysis of cell cycle parameters. For flow cytometric analysis of the cell cycle, floating and adherent cells were harvested together 24 h after PDT, washed in cold PBS, fixed in cold 70% ethanol and kept at −20°C overnight. Prior to analysis, cells were washed twice in PBS and resuspended in staining solution (0.1% Triton X-100, 0.137 mg/mL ribonuclease A and 0.02 mg/mL propidium iodide), incubated in the dark at room temperature for 30 min. Cell cycle analysis was performed on a BD FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) with argon ion laser (488 nm) and fluorescence was analyzed in FL2-A channel. A total of 1.5 × 104 cells were analyzed in each sample. The CellQuestPro (Becton Dickinson) and ModFit 3.0 (Verity Software House, Topsham, ME) softwares were used to generate DNA content frequency histograms and quantify the number of cells in the individual cell cycle phases including subG0/G1 population considered as apoptotic.
Statistical analysis. Results were calculated as mean ± SD of three to nine independent experiments. Statistical significance (P < 0.05) was determined by one-way analysis of variance followed by Bonferroni post-tests. Groups treated with inhibitors or hypericin per se were compared with control, groups pretreated with inhibitors (for 48 or 24 h) prior to hypericin-mediated PDT were compared with groups treated with either agent alone.
Results
Three low concentrations (approximately CC90, CC80 and CC70) of LOX (NDGA, esculetin, AA-861, MK-886 and baicalein), monooxygenase (proadifen) and COX pathways inhibitors (diclofenac, flurbiprofen, ibuprofen, indomethacin, SC-560 and rofecoxib) and hypericin (concentration—CC80) were chosen based on MTT metabolic assay for each agent used alone (data not shown).
Treatment with hypericin alone (0.1 μM) caused significant decrease in metabolic activity, reduction of cell numbers accompanied by increased numbers of floating cells, decreased cell viability and changes in cell cycle. This hypericin concentration caused time-dependent accumulation of cells in the S, G2/M as well as in the subG0/G1 phases accompanied by decreased cell numbers in the G0/G1 phase.
Inhibition of LOX pathway prior to PDT
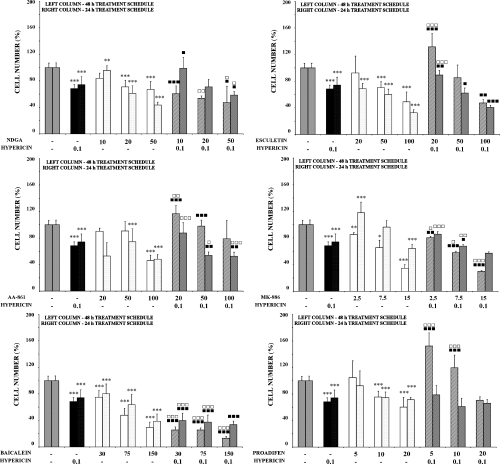
General inhibition of LOX pathway. Exposure of cells to NDGA alone caused dose-dependent reduction of their number (Fig. 1), but without significant changes in metabolic activity, number of floating cells or viability (Tables 1 and 2). Combined treatment only reflected the effect stemming from the corresponding concentration of both agents as single therapies (Fig. 1; Tables 1 and 2). The impact of hypericin’s phototoxicity on the cell cycle was controlled by pretreatment with NDGA (Fig. 2).
Effects of LOX inhibitors and inhibitor of P-450, PDT with hypericin or their mutual combination on cell numbers. The HT-29 cells were not exposed (control) or were treated for 24 h with the relevant inhibitor alone or exposed to PDT with hypericin alone or their mutual combination and analyzed 24 h after PDT. Results of adherent cell numbers are expressed relative to control (100%) and represent mean values ± SD of at least three independent experiments. The groups treated with hypericin alone and the relevant inhibitor alone were compared with control (*, **, ***—P < 0.05, P < 0.01, P < 0.001). The experimental groups given combined treatment were compared with the groups treated with the agents alone (, , —P < 0.05, P < 0.01, P < 0.001 for hypericin alone and for the relevant inhibitor alone: □, □ □, □ □ □—P < 0.05, P < 0.01, P < 0.001).
| Inhibitor (μM) | Hyp (μM) | Metabolic activity (%) | Inhibitor (μM) | Hyp (μM) | Metabolic activity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 48 h schedule | 24 h schedule | 48 h schedule | 24 h schedule | ||||||
| Control | – | – | 100.00 ± 3.5 | 100.00 ± 2.6 | |||||
| Hypericin | – | 0.1 | 75.65 ± 7.6*** | 75.89 ± 8.7*** | |||||
| NDGA | 10 | – | 108.48 ± 10.1 | 96.17 ± 3.0 | Diclofenac | 10 | – | 93.22 ± 4.1 | 106.77 ± 7.1 |
| 20 | – | 95.90 ± 4.6 | 107.00 ± 8.1 | 20 | – | 93.33 ± 10.4 | 104.25 ± 8.8 | ||
| 50 | – | 80.69 ± 1.7 | 95.81 ± 3.7* | 50 | – | 104.46 ± 5.0 | 92.59 ± 7.6 | ||
| 10 | 0.1 | 63.46 ± 7.8 | 84.95 ± 1.8□□□ | 10 | 0.1 | 60.49 ± 8.0□□□ | 57.78 ± 11.9□□□▪▪ | ||
| 20 | 0.1 | 70.48 ± 12.6▪ | 109.56 ± 16.7□□ | 20 | 0.1 | 52.15 ± 12.1□□□▪▪▪ | 49.67 ± 7.5□□□▪▪▪ | ||
| 50 | 0.1 | 56.98 ± 0.5□ | 79.31 ± 13.1□▪ | 50 | 0.1 | 48.96 ± 11.4□□□▪▪▪ | 44.80 ± 4.8□□□▪▪▪ | ||
| Esculetin | 20 | – | 87.35 ± 9.7 | 104.77 ± 8.9 | Flurbiprofen | 20 | – | 95.07 ± 6.3 | 96.40 ± 6.9 |
| 50 | – | 80.35 ± 3.9*** | 101.58 ± 9.6 | 50 | – | 84.70 ± 11.6 | 91.52 ± 4.2 | ||
| 100 | – | 66.07 ± 5.0*** | 80.87 ± 7.8*** | 100 | – | 64.06 ± 14.9*** | 80.96 ± 5.3*** | ||
| 20 | 0.1 | 62.56 ± 8.2□□□▪ | 97.33 ± 1.5▪▪ | 20 | 0.1 | 74.57 ± 21.5 | 66.40 ± 6.6□□□ | ||
| 50 | 0.1 | 55.02 ± 3.5□□□▪▪▪ | 69.98 ± 22.2□□□ | 50 | 0.1 | 67.92 ± 26.4 | 62.89 ± 6.6□□□▪ | ||
| 100 | 0.1 | 36.99 ± 8.5□□□▪▪▪ | 58.72 ± 12.8□□ | 100 | 0.1 | 49.37 ± 24.1 | 55.17 ± 7.7□□□▪▪▪ | ||
| MK-886 | 2.5 | – | 96.94 ± 6.5 | 98.10 ± 10.4 | Ibuprofen | 20 | – | 95.11 ± 9.4 | 103.89 ± 3.5 |
| 7.5 | – | 89.18 ± 5.5 | 92.80 ± 12.5 | 50 | – | 88.57 ± 12.1 | 92.04 ± 5.2 | ||
| 15 | – | 63.46 ± 8.8*** | 76.16 ± 19.8*** | 100 | – | 69.54 ± 11.4** | 81.13 ± 6.6*** | ||
| 2.5 | 0.1 | 74.61 ± 13.0□□□ | 77.74 ± 10.2□□□ | 20 | 0.1 | 71.30 ± 17.4 | 72.33 ± 9.5□□□ | ||
| 7.5 | 0.1 | 61.14 ± 16.8□□□ | 67.36 ± 11.7□□□ | 50 | 0.1 | 65.48 ± 21.5 | 62.57 ± 7.3□□□▪ | ||
| 15 | 0.1 | 45.49 ± 7.0□□□▪▪▪ | 48.50 ± 21.3□□□▪▪▪ | 100 | 0.1 | 60.06 ± 23.8 | 53.33 ± 4.8□□□▪▪▪ | ||
| AA-861 | 20 | – | 101.24 ± 4.6 | 78.91 ± 1.5*** | Indomethacin | 20 | – | 92.31 ± 14.0 | 97.19 ± 8.7 |
| 50 | – | 89.74 ± 12.3 | 61.24 ± 1.1*** | 50 | – | 93.61 ± 9.8 | 97.78 ± 7.1 | ||
| 100 | – | 82.70 ± 4.6* | 88.44 ± 1.7 | 100 | – | 74.66 ± 12.4* | 84.19 ± 4.4*** | ||
| 20 | 0.1 | 95.31 ± 11.2▪ | 100.23 ± 3.1□▪▪▪ | 20 | 0.1 | 69.17 ± 22.0 | 61.28 ± 4.5□□□ | ||
| 50 | 0.1 | 82.31 ± 21.6 | 77.07 ± 1.7 | 50 | 0.1 | 87.07 ± 13.5 | 56.79 ± 12.5□□□▪▪▪ | ||
| 100 | 0.1 | 77.17 ± 5.3 | 106.21 ± 4.6▪▪▪ | 100 | 0.1 | 60.14 ± 25.5 | 43.42 ± 5.0□□□▪▪▪ | ||
| Baicalein | 30 | – | 117.37 ± 3.5** | 126.63 ± 5.9*** | SC-560 | 10 | – | 94.12 ± 9.1 | 82.34 ± 5.0** |
| 75 | – | 138.35 ± 5.4*** | 146.34 ± 5.8*** | 20 | – | 94.99 ± 12.4 | 77.95 ± 0.8*** | ||
| 150 | – | 160.45 ± 0.9*** | 183.21 ± 6.9*** | 50 | – | 66.00 ± 4.4*** | 65.16 ± 0.1*** | ||
| 30 | 0.1 | 87.97 ± 14.0□□□ | 79.48 ± 4.9□□□ | 10 | 0.1 | 84.33 ± 16.8 | 100.99 ± 4.1▪▪▪ | ||
| 75 | 0.1 | 123.47 ± 14.1□□▪▪▪ | 129.91 ± 5.1▪▪▪ | 20 | 0.1 | 87.66 ± 20.8 | 92.98 ± 0.6▪▪ | ||
| 150 | 0.1 | 113.89 ± 4.5□□□▪▪▪ | 150.23 ± 12.1□□□▪▪▪ | 50 | 0.1 | 55.38 ± 10.0▪ | 86.59 ± 1.0□ | ||
| Proadifen | 5 | – | 87.38 ± 1.9** | 100.49 ± 8.3 | Rofecoxib | 1 | – | 117.22 ± 5.3* | 108.37 ± 1.4 |
| 10 | – | 70.28 ± 2.9*** | 96.03 ± 9.0 | 5 | – | 111.99 ± 8.6 | 108.68 ± 0.2 | ||
| 20 | – | 55.71 ± 4.9*** | 76.91 ± 11.6*** | 10 | – | 112.67 ± 6.9 | 99.23 ± 16.8 | ||
| 5 | 0.1 | 60.26 ± 4.1□□□▪▪ | 64.33 ± 14.9□□□ | 1 | 0.1 | 97.01 ± 7.2□▪▪ | 94.41 ± 1.0▪▪ | ||
| 10 | 0.1 | 53.61 ± 7.7□□▪▪▪ | 60.01 ± 10.2□□□ | 5 | 0.1 | 94.63 ± 4.5▪▪ | 83.10 ± 4.0□□ | ||
| 20 | 0.1 | 47.84 ± 9.7▪▪▪ | 53.52 ± 5.2□□□▪▪▪ | 10 | 0.1 | 96.37 ± 9.6▪▪ | 83.17 ± 1.6 | ||
- The HT-29 cells were not exposed (control) or were treated for 24 h with the relevant inhibitor alone or exposed to PDT with hypericin alone or their mutual combination and analyzed 24 h after PDT. Results are expressed relative to control (100%) and represent mean values ± SD of at least three independent experiments. The groups treated with hypericin alone and the relevant inhibitor alone were compared with control (*, **, ***—P < 0.05, P < 0.01, P < 0.001). The experimental groups given combined treatment were compared with the groups treated with the agents alone (, , —P < 0.05, P < 0.01, P < 0.001 for hypericin alone and for the relevant inhibitor alone: □, □ □, □ □ □—P < 0.05, P < 0.01, P < 0.001).
- Hyp = hypericin, NDGA = nordihydroguaiaretic acid, MK-886 = 3-[1-(4-chlorobenzyl)3-t-butyl-thio-5-isopropynidol-2-yl]-2,2-dimetyl propionic acid, AA-861 = 2-(12-Hydroxydodeca-5,10-dinyl)-3,5,6-trimethyl-1,4-benzoquinone, SC-560 = 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole, AA = arachidonic acid, PDT = photodynamic therapy, MTT =3-[4,5-dimethylthiazolyl]-2,5-diphenyltetrazolium bromide.
| Inhibitor (μM] | Hyp (μM) | Floating cells increase | Cell viability | Inhibitor (μM) | Hyp (μM) | Floating cells increase | Cell viability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 h schedule | 24 h schedule | 48 h schedule | 24 h schedule | 48 h schedule | 24 h schedule | 48 h schedule | 24 h schedule | ||||||
| Control | – | – | 1.00 ± 0.1 | 1.00 ± 0.1 | 92 ± 1 | 80 ± 6 | |||||||
| Hypericin | – | 0.1 | 2.80 ± 0.8*** | 3.06 ± 0.7*** | 63 ± 4*** | 61 ± 10* | |||||||
| NDGA | 10 | – | 1.05 ± 0.2 | 1.48 ± 0.6 | 93 ± 6 | 93 ± 5 | Diclofenac | 10 | – | 0.85 ± 0.1 | 1.08 ± 0.4 | 94 ± 7 | 93 ± 3 |
| 20 | – | 1.12 ± 0.1 | 1.52 ± 0.5 | 94 ± 8 | 94 ± 8 | 20 | – | 0.99 ± 0.2 | 1.30 ± 0.3 | 89 ± 13 | 95 ± 6 | ||
| 50 | – | 0.96 ± 0.2 | 1.55 ± 0.6 | 95 ± 6 | 96 ± 2 | 50 | – | 1.01 ± 0.1 | 1.10 ± 0.2 | 91 ± 5 | 93 ± 3 | ||
| 10 | 0.1 | 2.15 ± 0.7▪▪▪ | 2.00 ± 0.4▪▪▪ | 96 ± 5▪ | 97 ± 2▪▪▪ | 10 | 0.1 | 1.70 ± 0.3□□▪▪▪ | 3.52 ± 1.6□□□ | 95 ± 6▪ | 96 ± 1▪▪▪ | ||
| 20 | 0.1 | 2.01 ± 0.8□▪▪ | 2.20 ± 0.5□▪▪▪ | 99 ± 2▪▪ | 96 ± 2▪▪▪ | 20 | 0.1 | 1.49 ± 0.3□□□▪▪▪ | 3.23 ± 1.6□□□ | 92 ± 4▪ | 97 ± 2▪▪▪ | ||
| 50 | 0.1 | 1.76 ± 0.9□□□ | 2.51 ± 0.4□□□▪▪ | 96 ± 5▪ | 96 ± 1▪▪▪ | 50 | 0.1 | 2.01 ± 0.1▪ | 2.78 ± 1.1□□ | 89 ± 4 | 94 ± 0▪▪▪ | ||
| Esculetin | 20 | – | 0.86 ± 0.1 | 1.30 ± 0.3 | 96 ± 4 | 94 ± 4 | Flurbiprofen | 20 | – | 1.01 ± 0.2 | 1.48 ± 0.4 | 91 ± 9 | 84 ± 6 |
| 50 | – | 0.75 ± 0.1 | 1.28 ± 0.3 | 90 ± 3 | 96 ± 4 | 50 | – | 1.00 ± 0.1 | 1.55 ± 0.4 | 89 ± 8 | 85 ± 4 | ||
| 100 | – | 0.86 ± 0.3 | 1.49 ± 0.4 | 92 ± 8 | 94 ± 5 | 100 | – | 0.96 ± 0.2 | 1.66 ± 0.2 | 79 ± 12 | 84 ± 1 | ||
| 20 | 0.1 | 1.81 ± 0.7□□□▪▪▪ | 2.17 ± 0.3□□□▪▪▪ | 97 ± 4▪▪ | 94 ± 5▪▪▪ | 20 | 0.1 | 3.76 ± 0.3□□□ | 5.41 ± 2.0□□□▪▪▪ | 35 ± 3□□□▪▪▪ | 39 ± 18□□□▪▪▪ | ||
| 50 | 0.1 | 1.78 ± 1.1□□▪▪▪ | 2.06 ± 0.5□□□▪▪▪ | 97 ± 2▪▪ | 95 ± 1▪▪▪ | 50 | 0.1 | 5.67 ± 0.5□□□▪▪▪ | 6.14 ± 1.4□□□▪▪▪ | 5 ± 3□□□▪▪▪ | 27 ± 1□□□▪▪▪ | ||
| 100 | 0.1 | 1.71 ± 0.9□□▪▪ | 3.24 ± 0.6□□□ | 99 ± 1▪▪ | 80 ± 4▪▪▪ | 100 | 0.1 | 3.59 ± 1.4□□□ | 7.91 ± 1.5□□□▪▪▪ | 2 ± 0□□□▪▪▪ | 12 ± 4□□□▪▪▪ | ||
| MK-886 | 2.5 | – | 0.35 ± 0.1 | 0.30 ± 0 | 84 ± 2 | 83 ± 8 | Ibuprofen | 20 | – | 1.17 ± 0.2 | 1.31 ± 0.3 | 88 ± 5 | 82 ± 2 |
| 7.5 | – | 0.48 ± 0.2 | 0.42 ± 0.2 | 88 ± 5 | 83 ± 4 | 50 | – | 1.14 ± 0.1 | 1.16 ± 0.4 | 90 ± 4 | 82 ± 7 | ||
| 15 | – | 0.77 ± 0.2 | 0.52 ± 0.1 | 79 ± 12 | 85 ± 7 | 100 | – | 1.27 ± 0.1 | 1.24 ± 0.4 | 84 ± 6 | 91 ± 2 | ||
| 2.5 | 0.1 | 3.72 ± 1.3□□□▪▪▪ | 4.70 ± 1.1□□□▪▪▪ | 73 ± 4 | 49 ± 15 | 20 | 0.1 | 4.57 ± 0.5□□□▪▪▪ | 4.31 ± 2.6□□ | 32 ± 16□□□▪▪▪ | 38 ± 5□□□▪▪▪ | ||
| 7.5 | 0.1 | 4.40 ± 0.9□□□▪▪▪ | 7.47 ± 1.4□□□▪▪▪ | 74 ± 5 | 26 ± 5□□□▪▪▪ | 50 | 0.1 | 5.55 ± 0.2□□□▪▪▪ | 6.24 ± 3.3□□□▪▪ | 20 ± 13□□□▪▪▪ | 31 ± 17□□□▪▪▪ | ||
| 15 | 0.1 | 4.53 ± 0.8□□□▪▪▪ | 7.01 ± 0.9□□□▪▪▪ | 55 ± 7 | 30 ± 13□□□▪▪▪ | 100 | 0.1 | 6.21 ± 0.3□□□▪▪▪ | 7.05 ± 3.3□□□▪▪▪ | 5 ± 1□□□▪▪▪ | 23 ± 1□□□▪▪▪ | ||
| AA-861 | 20 | – | 0.81 ± 0.2 | 1.74 ± 1.0 | 88 ± 2 | 96 ± 4 | Indomethacin | 20 | – | 0.77 ± 0.3 | 1.71 ± 0.4 | 91 ± 6 | 84 ± 6 |
| 50 | – | 1.04 ± 0.1 | 1.64 ± 1.1 | 89 ± 6 | 91 ± 5 | 50 | – | 0.79 ± 0.2 | 1.49 ± 0.6 | 86 ± 7 | 78 ± 6 | ||
| 100 | – | 1.41 ± 0.6 | 2.07 ± 1.2 | 89 ± 8 | 89 ± 3 | 100 | – | 0.73 ± 0.3 | 1.65 ± 0.4 | 90 ± 7 | 87 ± 8 | ||
| 20 | 0.1 | 1.23 ± 0.2▪▪▪ | 2.13 ± 0.7 | 89 ± 3 | 99 ± 2▪▪▪ | 20 | 0.1 | 2.13 ± 0.5□□□ | 6.07 ± 3.1□□□ | 42 ± 1□□□▪▪▪ | 24 ± 9□□□▪▪▪ | ||
| 50 | 0.1 | 1.51 ± 0.2□▪▪▪ | 2.11 ± 0.7 | 93 ± 0 | 93 ± 0▪▪▪ | 50 | 0.1 | 5.26 ± 0.3□□□▪▪▪ | 8.02 ± 5.5□□□▪▪▪ | 16 ± 3□□□▪▪▪ | 15 ± 10□□□▪▪▪ | ||
| 100 | 0.1 | 1.98 ± 0.7▪▪▪ | 2.69 ± 1.0 | 89 ± 4 | 92 ± 3▪▪▪ | 100 | 0.1 | 5.16 ± 0.3□□□▪▪▪ | 8.50 ± 3.8□□□▪▪▪ | 11 ± 6□□□▪▪▪ | 9 ± 6□□□▪▪▪ | ||
| Baicalein | 30 | – | 0.94 ± 0.2 | 1.64 ± 0.6 | 88 ± 4 | 90 ± 1 | SC-560 | 10 | – | 0.68 ± 0.0 | 0.97 ± 0.2 | 94 ± 2 | 93 ± 7 |
| 75 | – | 1.07 ± 0.1 | 1.51 ± 0.9 | 90 ± 4 | 91 ± 1 | 20 | – | 0.39 ± 0.1* | 1.70 ± 0.2** | 87 ± 1 | 83 ± 5 | ||
| 150 | – | 2.16 ± 1.1 | 2.62 ± 2.3 | 81 ± 21 | 94 ± 0 | 50 | – | 0.52 ± 0.1* | 1.62 ± 0.5 | 82 ± 6 | 90 ± 6 | ||
| 30 | 0.1 | 2.70 ± 0.8□□ | 4.65 ± 2.8□□□▪▪▪ | 29 ± 1□□□▪▪▪ | 25 ± 18□□□▪▪▪ | 10 | 0.1 | 0.72 ± 0.1□□▪▪▪ | 1.80 ± 0.5□▪▪▪ | 91 ± 3▪ | 95 ± 11 | ||
| 75 | 0.1 | 3.05 ± 0.2□□ | 4.16 ± 2.2□□□▪▪▪ | 23 ± 6□□□▪▪▪ | 20 ± 7□□□▪▪▪ | 20 | 0.1 | 0.75 ± 0.1□□□▪▪▪ | 2.08 ± 0.3▪▪▪ | 99 ± 5▪▪ | 93 ± 7▪ | ||
| 150 | 0.1 | 3.37 ± 0.3 | 4.60 ± 2.0□□▪▪▪ | 9 ± 0□□□▪▪▪ | 40 ± 14□□□▪ | 50 | 0.1 | 0.94 ± 0.2▪▪▪ | 2.08 ± 0.2▪▪▪ | 96 ± 4▪ | 88 ± 9 | ||
| Proadifen | 5 | – | 0.94 ± 0.1 | 1.22 ± 0.5 | 96 ± 3 | 97 ± 0 | Rofeoxib | 1 | – | 0.82 ± 0.1 | 0.80 ± 0.1 | 97 ± 1 | 98 ± 2 |
| 10 | – | 2.03 ± 1.1 | 1.30 ± 0.6 | 89 ± 10 | 93 ± 3 | 5 | – | 0.78 ± 0.1 | 0.71 ± 0.2 | 93 ± 1 | 98 ± 3 | ||
| 20 | – | 3.27 ± 1.4 | 1.93 ± 0.6 | 91 ± 6 | 86 ± 7 | 10 | – | 0.66 ± 0.1 | 0.74 ± 0.1 | 96 ± 2 | 94 ± 1 | ||
| 5 | 0.1 | 1.80 ± 0.3 | 4.06 ± 2.0□□□ | 97 ± 5▪ | 98 ± 2 | 1 | 0.1 | 1.80 ± 0.2□□□▪▪▪ | 1.53 ± 0.2□□□▪▪▪ | 100 ± 1▪▪▪ | 97 ± 1▪▪ | ||
| 10 | 0.1 | 2.13 ± 0.7□□ | 5.04 ± 1.7□□□▪▪ | 94 ± 3▪ | 89 ± 12▪ | 5 | 0.1 | 1.01 ± 0.1▪▪▪ | 1.33 ± 0.1□□▪▪▪ | 96 ± 5▪ | 98 ± 5 | ||
| 20 | 0.1 | 3.15 ± 1.9 | 4.41 ± 0.8□□□▪ | 92 ± 5▪ | 90 ± 1▪ | 10 | 0.1 | 0.62 ± 0.1▪▪▪ | 1.26 ± 0.1□▪▪▪ | 97 ± 6▪ | 96 ± 3▪ | ||
- The HT-29 cells were not exposed (control) or were treated for 24 h with the relevant inhibitor alone or exposed to PDT with hypericin alone or their mutual combination and analyzed 24 h after PDT. Floating cells were counted separately and results were quantified as increase in floating cell number in comparison with untreated control (1.0). Viability of cells in the experimental group was evaluated as a percentage of live cells. All concentrations are given in μM. Results are expressed as mean values ± SD of at least three independent experiments. The groups treated with hypericin alone and the relevant inhibitor alone were compared with control (*, **, ***—P < 0.05, P < 0.01, P < 0.001). The experimental groups given combined treatment were compared with the groups treated with the agents alone (, , —P < 0.05, P < 0.01, P < 0.001 for hypericin alone and for the relevant inhibitor alone: □, □ □, □ □ □—P < 0.05, P < 0.01, P < 0.001).
- Hyp = hypericin, NDGA = nordihydroguaiaretic acid, MK-886 = 3-[1-(4-chlorobenzyl)3-t-butyl-thio-5-isopropynidol-2-yl]-2,2-dimetyl propionic acid, AA-861 = 2-(12-Hydroxydodeca-5,10-dinyl)-3,5,6-trimethyl-1,4-benzoquinone, SC-560 = 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole, AA = arachidonic acid, PDT = photodynamic therapy.
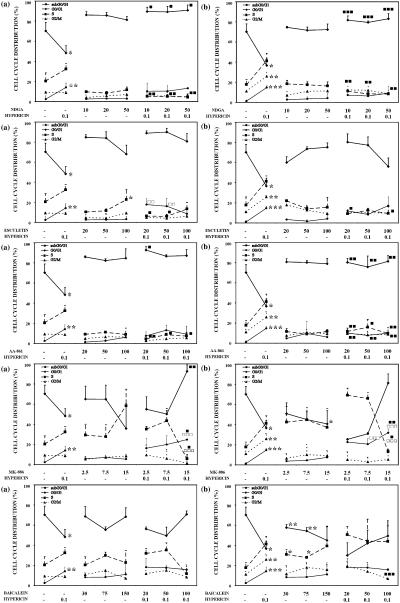
Effects of various inhibitors of AA metabolic pathways, PDT with hypericin or their mutual combination on cell cycle distribution. Control cells or cells treated for 48 h (a) or 24 h (b) with the relevant inhibitor alone or exposed to PDT with hypericin alone or their mutual combination were analyzed 24 h after PDT. Results of DNA content analyzed using ModFit 3.0 software were calculated as the mean values + SD of at least three independent experiments. The groups treated with hypericin alone and the relevant inhibitor alone were compared with control (*, **, ***—P < 0.05, P < 0.01, P < 0.001). The experimental groups given combined treatment were compared with the groups treated with the agents alone (, , —P < 0.05, P < 0.01, P < 0.001 for hypericin alone and for the relevant inhibitor alone: □, □ □, □ □ □—P < 0.05, P < 0.01, P < 0.001).
Inhibition of 5, 12-LOX pathways. Treatment of cells with esculetin alone induced decrease in metabolic activity and cell number in time- and dose-dependent manner (Table 1 and Fig. 1). However, no changes in percentage of floating cells and viability were observed (Table 2). Pretreatment with higher doses of esculetin stimulated the effect of PDT, manifested as floating cells accumulation and reduction of metabolic activity. S-phase accumulation and reduction of the G0/G1 phase of the cell cycle induced by 48 h esculetin treatment was slightly abated in combination with PDT (Fig. 2).
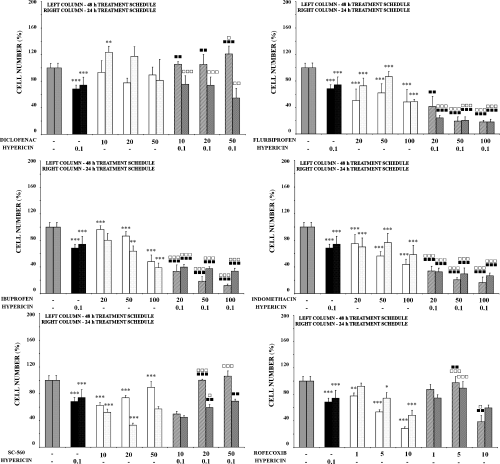
Effects of general and specific COX inhibitors, PDT with hypericin or their mutual combination on cell numbers. The HT-29 cells were not exposed (control) or were treated for 24 h with the relevant inhibitor alone or exposed to PDT with hypericin alone or their mutual combination and analyzed 24 h after PDT. Results of adherent cell numbers are expressed relative to control (100%) and represent mean values ± SD of at least three independent experiments. The groups treated with hypericin alone and the relevant inhibitor alone were compared with control (*, **, ***—P < 0.05, P < 0.01, P < 0.001). The experimental groups given combined treatment were compared with the groups treated with the agents alone (, , —P < 0.05, P < 0.01, P < 0.001 for hypericin alone and for the relevant inhibitor alone: □, □ □, □ □ □—P < 0.05, P < 0.01, P < 0.001).
Inhibition of 5-LOX pathway. Two ways of 5-LOX pathway inhibition were chosen. In the experimental group pretreated (24 h) with AA-861 alone, decrease in metabolic activity and cell number was observed. However, application of PDT caused cell number decrease in both treatment schedules, with only minimal changes to metabolic activity, viability and cell cycle distribution.
Treatment of cells with specific, but not direct 5-LOX, inhibitor MK-886 alone (blocking FLAP activity) caused significant decrease in cell metabolic activity and cell number in both treatment schedules (Table 1 and Fig. 1). Pretreatment with higher doses of MK-886 significantly potentiated the effects of single agents on all parameters detected. Significant changes in cell cycle distribution induced by the highest MK-886 concentration were still more pronounced in cells subsequently treated with PDT (Fig. 2). While MK-886 alone (15 μM) decreased the G0/G1 phase (from 60% to 36%) and increased the population of cells in the S-phase (from 35% to 58%), subsequent PDT caused displacement of cell population from S-phase into G0/G1 (up to 80–90%).
Inhibition of 12-LOX pathway. Opposite effects on metabolic activity (increase) compared with cell number and viability (decrease) after treatment with baicalein alone were observed. Compared with baicalein and hypericin alone, the decrease in cell number and viability and increased percentage of floating cells were significantly potentiated after combination of these two modalities (Table 2 and Fig. 1). Cell treatment with baicalein induced cell cycle arrest in the S-phase in dose-dependent manner, and this impact was even more cumulative in combination with PDT (Fig. 2).
Inhibition of COX pathway prior to PDT
Generally, no significant effects on cell metabolic activity, cell number, viability and percentage of floating cells were detected after treatment with nonspecific COX inhibitors (diclofenac, flurbiprofen, ibuprofen and indomethacin) alone. However, these agents significantly potentiated the effects of PDT with hypericin. Compared with these agents as well as hypericin alone, these effects were significantly higher after combined treatment (Fig. 3 and Tables 1 and 2). Minimal changes were observed in cell cycle distribution after combined treatment (data not shown). Interestingly, the effect of diclofenac in combination with PDT did not correlate with other nonspecific COX inhibitors used.
Interesting results were obtained with specific inhibitors of COX-1 (SC-560) and COX-2 (rofecoxib) pathways. Used alone these agents did not induce significant changes in metabolic activity (Table 1) and viability (Table 2), but dose-dependently they decreased cell numbers (Fig. 3). However, both agents mostly attenuated the phototoxic effect of hypericin in combination with PDT.
Inhibition of cytochrome P-450 (monooxygenase) pathway prior to PDT
Inhibition of P-450 monooxygenase pathway with proadifen alone induced cytotoxic effects in time- and dose-dependent manner when expressed as metabolic activity, and these were more pronounced in combination with PDT, especially when applied as 48 h pretreatment (Table 1). On the other hand, the decreasing effect on cell numbers of proadifen and hypericin as single treatments was eliminated by their combination (Fig. 1). Mutual combination of proadifen (5 and 10 μM) with hypericin increased levels of adherent cells (decreasing percentage of floating cells) and increased viability in comparison with both agents alone (Fig. 1, Table 2). Cell cycle (data not shown) was not significantly affected in comparison with hypericin alone.
Discussion
Arachidonic acid and its metabolites are closely linked to carcinogenesis. The antiproliferative potential of AA metabolism inhibitors has been broadly studied in vivo as well as in vitro (4,5,18). AA and eicosanoids serve as intermediates in growth factor signaling pathways, cell proliferation, cell death and inflammation processes. One modality used to modulate the metabolism of AA in cancer cells is the inhibition of one or more AA metabolic pathways (22). The second way is to use combined treatment using modulators of AA metabolism with other clinically applied therapies like PDT. Recently, the forefront of interest in this field is to replace conventional high-dose regimens with the low-dose administration of agents with minimal side effects (23).
As previously reported results regarding the sensitivity of cancer cells to modulation of AA metabolism (mainly COX inhibition) and the therapeutic effectiveness of PDT seem to be controversial (15–17,20,24), we investigated the impact of inhibition of various AA metabolism pathways before hypericin-mediated PDT using colon adenocarcinoma HT-29 cells. Our attention was focused specifically on the efficiency of low-dose PDT on cells affected in advance (24 or 48 h) with suboptimal concentrations of AA metabolism inhibitors.
Even though inhibition of the LOX pathway by general LOX inhibitor NDGA prior to PDT did not indicate major effects on the evaluated cytokinetic parameters, specific LOX inhibitors esculetin (5- and 12-LOX), AA-861 and MK-886 (5-LOX) or baicalein (12-LOX) produced more distinctive results.
To evaluate the contribution of 5-LOX inhibition to cell cytokinetics, we used different specific 5-LOX inhibitors that interfere with 5-LOX activity through distinct mechanisms. The AA-861 acts as an alternative substrate of 5-LOX by virtue of its redox potential and MK-886 as a FLAP inhibitor (25). When comparing the potentiation of PDT by 5-LOX inhibition with these two agents, more pronounced effects were detected after pretreatment with MK-886. Moreover, we and others have described significant changes in cell proliferation and cell death also after single therapy with MK-886 in several in vitro models (26,27). However, it is also known that the cytotoxicity of MK-886 does not depend only on LOX inhibition, as it can also be observed in cells that do not express FLAP (22,28,29). However, the five series of eicosatetraenoids could reverse the growth inhibitory effect of MK-886 in prostate cancer cells supporting the concept of a 5-LOX-dependent mechanism (30).
Cell cycle transition in groups treated with 12-LOX-specific inhibitor baicalein as a single therapy or as pretreatment to hypericin-mediated PDT presented a pattern rather resembling for instance the work of Leung et al. (31). The S-phase in our experimental model had time- and dose-dependent attributes, and PDT with hypericin enhanced these effects to reach values over 60%. With these solid effects of combined therapy, the 12-LOX-dependent mechanism of baicalein’s action might be in question. However, inhibition of proliferation in human gastric cancer cell lines by baicalein have been reversed by addition of 12-hydroxyeicosatetraenoic acid (12-HETE) (32). Moreover, baicalein induced apoptosis in renal cell carcinomas in time- and concentration-dependent manner (33). For these reasons, we assume that 12-LOX pathways play an important role in cancer cell survival (34), and its inhibition might be an effective co-therapy to hypericin-mediated PDT.
Dual inhibition of 5- and 12-LOX pathways using esculetin evoked only moderate effects when compared with separate inhibition of 5-LOX/12-LOX. Application of esculetin as a single therapy in our experimental design demonstrated effects similar to published data (35–39), with minimal effects after combined application as compared with single therapy.
Inhibition of prostaglandin synthesis is also considered by some authors as a possibility to increase effectiveness of PDT (15–17,20,24). The effect of flurbiprofen, ibuprofen and indomethacin (all three preferentially COX-1 inhibitors) had a very similar pattern in all tested parameters. Metabolic activity of cells was affected in time-/dose-dependent manner as a single therapy; as pretreatment to PDT, however, although still dose-dependent, the 24 h pretreatment schedule was more efficacious. We hypothesize that metabolism of cells pretreated with the above-mentioned inhibitors 24 h prior to hypericin-mediated PDT should not be affected so intensely. As metabolically active cells are more susceptible to different therapeutical approaches, we assume that pretreatment with these agents should not only affect cell proliferation, but also impair the metabolism in time-dependent manner. The number of floating cells confirmed these anticipations, as the effectivity of the 24 h schedule in all three mentioned inhibitors was more expressive in both cases—single and combined therapy. Viability remission was dose- and time- (except indomethacin) dependent with particularly massive impact on cell populations undergoing combined therapy. Cell cycle was affected by these inhibitors only moderately (data not shown), and in summary there is a slight trend towards arresting cells in the G0/G1 phase and towards dose-dependent interpenetration of hypericin’s action. This is in agreement with published data, as many authors mention that the antiproliferative action of ibuprofen or flurbiprofen—e.g. (40)—was not coupled with cell-cycle changes or that indomethacin increased the proportion of cells in the G0/G1 phase and reduced the S-phase proportion in HT-29 cells (41).
Some authors claim that the action of some COX-1 inhibitors might be COX-independent. For instance Grosch et al. (42) proved that S-flurbiprofen as well as R-flurbiprofen, its inactive (in terms of COX inhibition) enantiomer, induced cell-cycle block in the G0/G1 phase and apoptosis in colon cancer cell lines HCT-15, Caco-2, HT-29 or HCT116 (43). Similar data were presented by Janssen et al. (44) with S-/R-ibuprofen and finally Wang and Zhang (45) demonstrated both in vitro and in vivo anti-tumor effect of indomethacin at least partially independent of the COX inhibitory profile. Nevertheless, if the action of these nonsteroidal anti-inflammatory drugs (NSAIDs) is fully or partially independent of COX activity, they still represent a viable modality for chemoprevention of various tumors and for application as pretreatment to hypericin-mediated PDT.
While being only slightly effective on their own, pretreatment with rofecoxib and SC-560, selective inhibitors of COX-2 and COX-1, respectively (46), in general attenuated the impact of PDT in time-dependent manner. Although there are some discrepancies in adherent cell counts, results of other analyses also indicate this trend, which is distinct from the application of other COX inhibitors presented herein (indomethacin, ibuprofen and flurbiprofen). We hypothesize that this is due to lack of COX-independent effects of rofecoxib and applied SC-560 concentration range, respectively.
SC-560 is a structural analog of celecoxib, a selective COX-2 inhibitor. Both are known for COX-independent growth inhibition in a broad range of cancer types (47,48). The growth-inhibitory effect in these studies appeared mostly/exclusively at concentrations no longer selective for either COX isoform. Only 48 h pretreatment with 50 μM SC-560 was statistically lower to hypericin-mediated PDT per se (Table 1). The cells pretreated with lower, more selective SC-560 concentrations even enhanced cell proliferation in comparison with low-dose PDT as well as to the inhibitor by itself. We hypothesize that this might be due to upregulation of stress-inducible pathways stimulated by pretreatment with an inhibitor and further enhanced by low-dose PDT.
Rofecoxib (and also some other selective COX-2 inhibitors) is on the other hand considered as a highly selective COX-2 inhibitor with no COX-independent antiproliferative effect (49). So it seems to be in our experimental model as well. Rofecoxib stimulated metabolic activity when applied alone and attenuated the effect of PDT when applied as pretreatment. Although the attenuation is indirectly proportional to rofecoxib’s concentration, it is still significant. Similarly to SC-560, we assume that this might be due to acceleration of cell survival signaling.
Diclofenac, although considered as a nonspecific COX inhibitor with low gastrointestinal toxicity (50,51), seems to be more specific to COX-2 than to COX-1 (46,51). Diclofenac is also known for its COX-independent mechanisms of cancer proliferation inhibition (52–54); however, in our experimental model it did not affect cell proliferation significantly when applied alone. When applied as pretreatment to PDT, some discrepancies in cell numbers, MTT assay and viability had been observed. As Yamazaki et al. (55) reported anti-apoptotic effects of diclofenac in endoplasmic reticulum stress-induced apoptosis, we assume that these effects might be a result of delayed cell death.
Inhibition of the P450 monooxygenase pathway by proadifen (SKF525A) prior to hypericin-mediated PDT in our experimental model presented a pattern resembling the experimental work of Hoferováet al. (40) carried out on G:5:113 murine fibrosarcoma cells. Lower doses stimulated cell proliferation in single as well as combined therapy, while higher doses reduced cell numbers. MTT assay paradoxically remained lower in the whole concentration range, indicating reduced/corrupted oxidative metabolism, whereas viability remained unaffected when compared with single therapy with an inhibitor, but increased on the other hand when compared with PDT alone. These findings suggest a significant impact of proadifen on cell-death signaling pathways.
We present herein preliminary data summing up the in vitro application possibilities of NSAIDs targeting single pathways of AA metabolism as pretreatment to hypericin-mediated PDT in colon cancer cells. It seems that inhibition of LOX activity, especially 12-LOX with baicalein, strikes cell survival and proliferation in combination with low-dose PDT with hypericin intensively. On the other hand, application of COX inhibitors seems to have two different patterns. Inhibitors with known COX-independent action potentiated PDT, and inhibitors with absent or less-distinct pharmacological effects attenuated PDT, respectively. However, the in vivo effects of these agents should not be ignored. Results of pretreatment with P450 inhibitor proadifen prior to PDT indicate an opportunity for therapeutical application of P450 inhibition in PDT enhancement.
In summary, the application of NSAIDs in cancer therapy is well established at the present time, though not all mechanisms of their action are well understood. Neither treatment with various AA inhibitors nor detailed study of MK-886 influence on HT-29 cells treated with PDT (56) elucidated whether the enhanced photocytotoxic effect of hypericin was evoked by modulation of the corresponding AA metabolic pathway or by pharmacological properties distinct from this action. Evidence indicating more mechanisms independent of AA inhibition is unquestionable; however, this group of pharmacological agents is among the most frequently prescribed ones and requires further studies especially when applied together with other therapeutical modalities. Since a number of paradoxical variations in recorded cytokinetical parameters have been observed, these will be investigated in the future.
Acknowledgements— This work was supported by the Science and Technology Assistance Agency under the contract APVT-20-003704 and by the Ministry of Education of the Slovak Republic grant VEGA 1/2329/05 and by the grant of the Academy of Sciences of the Czech Republic 1QS500040507. The authors wish to thank Viera Balážová, Zdenka Lacková, Rastislav Jendželovský and Lucia Grada-Kuliková for their superb technical assistance. Thanks also to Andrew J. Billingham for proofreading the manuscript. We would like to thank Merck Frosst Canada, Inc. for their kind gift of compounds MK-886 as well as rofecoxib.




