The Scale-up of Spotted Rose Snapper, Lutjanus guttatus, Larval Rearing at Mazatlan, Mexico
Abstract
The scale-up of spotted rose snapper, Lutjanus guttatus, larval rearing is described. Fertilized eggs (480,000) were obtained from a 1-d harvest of a natural spawning captive broodstock acclimatized for 1 yr and 6 mo in two fiberglass tanks (18 m3). Fourteen hours after spawning, 89.6% of the collected eggs were floating, of which 96.2% were transparent with live embryos. Incubation at 25–26 C lasted 21 h, with 90.2 ± 2.1% hatching percentage of normal larvae. The percentage of viable larvae at 48 h after hatching was 79.7 ± 1.9%. Initial stocking density was 10.4 ± 1.0 larvae/L 2 days after hatching (d.p.h.). A total of 22,600 juveniles (1256 ± 170 juveniles/m3) were harvested from six 3-m3 cylindrical fiberglass tanks. Average survival was 12.1 ± 1.1%. Final mean length and weight were 5.5 ± 0.05 cm and 2.24 ± 0.04 g, respectively. Growth expressed in total length was TL = 2.1476e0.0543t (R2 = 0.9911). Final mean biomass and condition factor were 2.8 kg/m3, 12.3% and 1.346. General length-weight ratio was W = 0.05460 LT2.2306.
The snappers (Lutjanidae family) are highly valued as food fishes with strong market demand and high prices worldwide. Production from red snapper fisheries around the world has been increasing for the last 50 yr; however, in the last 10 yr, the mean annual increase in production has been much lower as compiled by the Food and Agriculture Organization of the United Nations (FAO) (FAO 2011), and these fisheries are considered overexploited in many areas (Davis et al. 2000; Watanabe et al. 2005). Culture of some species has been established, mostly with traditional technologies in floating cages, with a total production of 7648 tons and a wholesale value of US$32.4 million in 2009 (FAO 2011). Major culture species are the mangrove red snapper, Lutjanus argentimaculatus, and John's snapper, Lutjanus johnii, from the Indo-Pacific region.
One of the main difficulties for commercial snapper cultivation is the lack of controlled mass production of juveniles which means that most grow-out facilities depend on unsustainable supplies of wild-caught juveniles with seasonal and unpredictable availability (Davis et al. 2000; Watanabe et al. 2005). Therefore, a reliable, cost-effective, and stable culture technology with consistent results for mass production of good quality fry must be developed to meet the growing demand. In the Indo-Pacific region the best results for snapper larval rearing have been achieved with mangrove red snapper (Duray et al. 1996; Leu et al. 2003) and John's snapper (Lim et al. 1985; Schipp et al. 2001). In America, research projects are currently being carried out to develop juvenile mass production techniques, and best results have been found with Mutton snapper, Lutjanus analis, (Watanabe et al. 1998, 2005), red snapper, Lutjanus campechanus (Ogle and Lotz 2006), and yellowtail snapper, Ocyurus chrysurus (Turano et al. 2000).
The spotted rose snapper, Lutjanus guttatus, is a commercially important food fish, highly appreciated in Mexico and other Latin American countries. This species reaches high prices and the demand significantly exceeds fishery supply. Aquaculture research on spotted rose snapper has been mostly focused on induced spawning (Valverde Chavarría and Boza Abarca 1999; Ibarra-Castro and Duncan 2007; Boza-Abarca et al. 2008; Ibarra-Castro and Alvarez-Lajonchère 2009), although there has been progress in improving juvenile mass production (Alvarez-Lajonchère et al. 2010). Herrera-Ulloa et al. (2010) reported the production of 70,000 spotted rose juveniles in 1 yr (this being the first juvenile mass production of this species), considered an important breakthrough after a joint effort of several institutions for 5 yr, which allowed the establishment of a grow-out pilot farm in the Gulf of Nicoya, Costa Rica.
Research with spotted rose snapper started in 2003 at the Research Centre for Food and Development (CIAD) in Mazatlan, Mexico. They used wild mature spawners caught near Sayulita Beach, Nayarit, that were induced to spawn 2 h after capturing (Ibarra-Castro and Duncan 2007). The fertilized eggs were transported for 7–8 h from Nayarit to laboratory facilities at Mazatlan. These trials resulted in the production of only a few juveniles (Alvarez-Lajonchère et al. 2010). In 2005, a captive broodstock population was established at the CIAD Mazatlan facility, and the first larvae obtained through hormone induction (Ibarra-Castro and Alvarez-Lajonchère 2009) enabled the conduction of small-scale experiments to improve larviculture techniques (Abdo-de la Parra et al. 2010). Afterward, natural spawning of high-quality eggs from the captive broodstock (Ibarra-Castro and Alvarez-Lajonchère 2011) enabled scaling-up using experimental larval rearing technology (Abdo-de la Parra et al. 2010) to a new pilot-scale plant (Alvarez-Lajonchère et al. 2007).
This paper shows the results of a study on larval rearing and juvenile production conducted in the 2006 spawning season, with a description of the modifications to the previous experimental procedure, as well as recommended changes to improve mass production technology for increased production efficiency.
Material and Methods
Egg Production
Fertilized eggs were obtained from the natural spawning of captive broodstock (five females with 0.91 ± 0.2 kg body weight [BW] and 14 males with 1.2 ± 0.3 kg BW). The fish were held in spawning tanks (3.5 m diameter by 1.8 m deep, 18 m3) with a flow-through system (8 tank volumes/d), strong aeration and covered by a shade cloth (to block 70% of the sunlight) suspended approximately 2 m above the tanks, at the Research Centre for Food and Development (CIAD), Mazatlan, Mexico (Ibarra-Castro and Alvarez-Lajonchère 2011). Eggs were collected in a 200-L tank containing a 20-L bin screened with 0.5-mm mesh placed under the outflow of the spawning tanks. All eggs were removed from the collector 12–14 h after spawning, at the embryo segmentation stage. The number of eggs floating and sinking were estimated volumetrically with a 500-mL graduated cylinder, based on the equation E = −18.648 D + 16645; P = 0.05, R2 = 0.9013, where E is the total number of eggs in 1 mL and D is the mean egg diameter of 50 eggs (Ibarra-Castro and Alvarez-Lajonchère 2011). Egg viability was recorded as the percentage of floating eggs with live embryos at that stage, from a sample of floating eggs >120. Egg and oil droplet diameters were measured to the nearest 10 µm. Floating eggs were rinsed in ultraviolet (UV)-treated seawater.
Larval and Juvenile Rearing Environment Management
Incubation and larval rearing were carried out in six 3-m3 cylindrical fiberglass tanks (2 m diameter by 1 m deep) with black walls and a white bottom. Initial stocking density was of 15 floating eggs/L, and hatch rate was estimated, 2 h after hatching in each of the six rearing tanks, as the mean of normal live hatched larvae in four 1-L subsamples, taken with plastic beakers before aeration was lowered. The same procedure was applied for estimating viable larval survival (straight larvae with open mouth and pigmented eyes) 48-h post-hatching (h.p.h.). The tanks were located indoors, in a room with a partially translucent fiberglass roof.
Seawater was treated using pressurized sand filters, followed by multi-cartridge filtration (16 µm relative retention) before a continuous UV-lamp exposure (60 mJ/cm2) and finally a fine filtration with line cartridge filters of 5 and 1 µm absolute retention. Water quality parameters during egg incubation, larval rearing, and juvenile nursery stages were: temperature 26 ± 1.0 C, salinity 35 ± 1.0 g/L, dissolved oxygen 5.1 ± 0.6 mg/L (saturation: 77.8 ± 9%), pH 8 ± 0.2 and NH3 of <0.05 mg/L.
Larval rearing tank setup was described by Alvarez-Lajonchère et al. (2007). A water quality and environmental management protocol was established and followed for egg incubation, larval rearing, weaning and first nursery stage (Fig. 1). A daily work plan was developed for environmental control practices, cleaning, feeding, and behavioral observations, following Alvarez-Lajonchère et al. (2002).
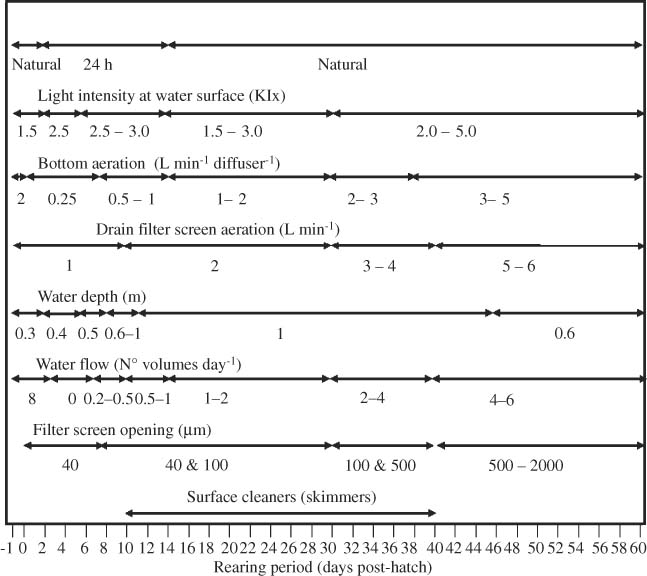
Water quality and environmental management protocol during incubation and larval and juvenile rearing of the spotted rose snapper, Lutjanus guttatus.
As part of the environmental management protocol, two species of microalgae, Nannochloropsis oculata (5 × 105 cells/mL) and Isochrysis sp. (5 × 104 cells/mL), were added to the larval rearing tanks each day immediately after their morning cleaning to maintain the nutrition of any uneaten rotifers and copepods (Fig. 2), following the good results obtained by Alvarez-Lajonchère et al. (2002).
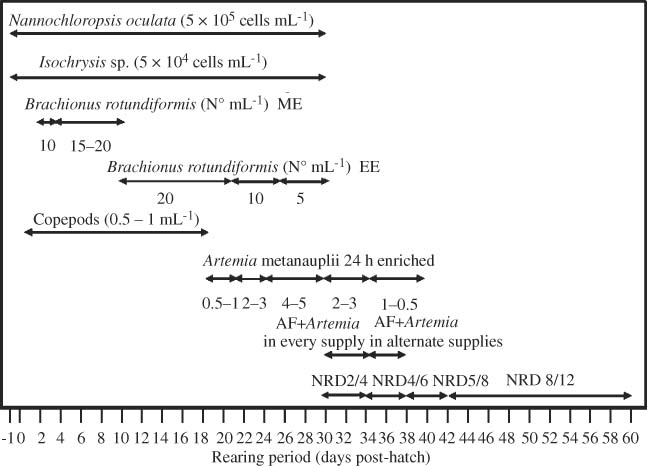
Feeding regimen during larval and juvenile rearing of the spotted rose snapper, Lutjanus guttatus. ME = microalgae enriched rotifers; EE = emulsion enriched rotifers.
Live Feed Production and Larval and Juvenile Feeding Regime
Microalgae, N. oculata and Isochrysis sp., and rotifers, Brachionus rotundiformis, were batch cultured. Algae were initially grown with the modified F/2 medium (Moretti et al. 1999) and with an artificial fertilizer formula (Alvarez-Lajonchère and Hernández Molejón 2001) indoors in translucent 80-L and 700-L cylindrical tanks. The last two culture stages of N. oculata were carried out outdoors under direct sunlight in 1.2-m3 cylindrical–conical fiberglass tanks and 7-m3 cylindrical fiberglass tanks. Rotifers were cultured intensively indoors, using Selco 3000® culture (INVE Aquaculture Inc., Mazatlan, Mexico) in 1.2-m3 cylindrical-conical tanks.
Batches of two copepod species, benthic Harpacticoid, Tisbe monozota, and pelagic Calanoid, Pseudodiaptomus euryhalinus, were maintained under continuous culture conditions (27 ± 1 C, 35 g/L salinity, 12 h:12 h light : dark photoperiod) at the CIAD Laboratory. These copepods were batch cultured starting with the inoculation of 300-L tanks followed by the production scale-up in 700-L, 1.2-m3, and 7-m3 tanks on microalgae (Tetraselmis tetrathele, Isochrysis sp. [T.ISO], Chaetoceros muelleri and N. oculata) at a cell density of 1 × 106 cells/mL, with partial harvests of 25–33% volume/d (Hernández Molejón and Alvarez-Lajonchère 2003; Puello-Cruz et al. 2006).
The larvae were fed rotifers, B. rotundiformis from 2 to 30 d.p.h. and copepod nauplii from 2 to 18 d.p.h. (Fig. 2). Rotifers were enriched with microalgae, N. oculata and Isochrysis sp., 18 h prior to feeding for the first week and with Protein Selco Plus® (INVE Aquaculture Inc.) thereafter, following methods described by Alvarez-Lajonchère and Hernández Molejón (2001).
Rotifers were supplied in three portions, half the day's ration was supplied immediately after the morning cleaning activities (0900–0930 h), 25% at noon (1200–1230 h), and the remainder at 1700 h. Rotifer density in the rearing tank was of 10/mL for early larvae, and it increased to 15–20/mL for 3–21 d.p.h. larvae and later reduced to 10/mL, and finally to 5/mL (Fig. 2). Copepods were fed to the larvae once a day, in the morning (0900–0930 h), at a rate of 0.5–1/mL. Artemia metanauplii, obtained from decapsulated cysts and enriched for 18 h with SuperSelco® (INVE Aquaculture Inc.), were fed from 18 d.p.h. onward using the same pattern as rotifers. The remaining daily rations of enriched rotifers and Artemia metanauplii were stored at low temperature (10–12 C for rotifers and 4–10 C for Artemia metanauplii).
Weaning was started at 30 d.p.h., using a marine weaning ration (NRD from INVE Aquaculture Inc.) mixed with concentrated enriched Artemia metanauplii given on the first 4 d, six or seven times a day, and only mixed on alternate supplies the following 4 d (Fig. 2). Weaning started with small size NRD 2/4® (INVE Aquaculture Inc.) followed by increasing sizes of NRD 4/6®, NRD 5/8®, and NRD 8/12® during the first nursery stage until harvest.
Larvae and Juvenile Sampling and Grading
The larvae were sampled using a plastic 1-L beaker for the first week and hand nets thereafter. After the first 28 days of rearing, a specially designed, in-house built, size grader was operated inside the larval rearing tanks, to segregate the largest individuals, which were at least two to three times the size of most larvae. The grader (Fig. 3) was designed to allow the smaller larvae to pass between transparent acrylic rods (3 mm in diameter and 2 mm apart), which could rotate freely in a stainless steel frame. A shade with 70% absorbance was also fixed to the central polyvinyl chloride (PVC) pipe and used as a crowding screen to concentrate the larvae before they reached the acrylic rods, encouraging the smaller larvae to swim through the grading screen. Large individuals that could not pass through the grader were later picked out with a hand net. Each of the two stainless steel frames had a synthetic fiber strip (Scotch Brite, Super Lustre, S. A. de C. V., México City, México) to ensure strong contact between the frames and the tank wall (Fig. 3).
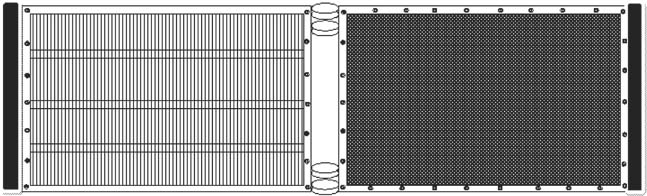
Larval grader used for spotted rose snapper, Lutjanus guttatus, inside the larval rearing tanks. See text for descriptions.
Larval growth in each tank was monitored every 5–10 d by measuring total length (TL) of 15–20 individuals sampled at random from each tank. The larvae and juveniles were anesthetized with 0.15 mL/L of 2-phenoxyethanol (Sigma, Toluca, México) for morphological observations, and measurements were taken using a compound microscope with a calibrated ocular micrometer (to the nearest 10 µm).
All juveniles were harvested at the end of the rearing period (60 d.p.h.). Total number in each tank was estimated by the visual method, following traditional Asian methods (Liu and Kelley 1991; Alvarez-Lajonchère and Hernández Molejón 2001), and transferred to 5-m3 nursery tanks. A brief description of the visual estimation method applied follows: (a) four plastic bowls 60 cm in diameter and 20 cm deep were filled with 15 L of clean seawater and a constant aeration was supplied with an airstone; (b) water depth in the larval rearing tank was lowered to about 40 cm; (c) juveniles were deep sedated with 0.15 mL/L of 2-phenoxyethanol (Sigma); (d) 250 juveniles were individually counted and placed in the first pail, using 300 mL plastic cups, and marked as the standard density control; (e) an approximately similar density was placed in each of the other three pails, by visual appreciation, removing excess water to maintain the initial 15 L volume; (f) when the densities appeared to be the same as in the control pail, juveniles placed by visual appreciation into the three other pails were stocked in nursery rearing tanks and 750 juveniles were recorded as harvested; (g) this procedure was repeated until the larval rearing tank was emptied; and (h) a new control pail was used when each rearing tank was harvested. Variability between the three other bowls ranged from 8 to 10%.
Final survival was estimated at 48 h.p.h. to 60 d.p.h., considering a stock of 15 floating eggs/L with 96.2% viability, mean hatching percentage of 90.2%, and mean survival of 79.7% at 48 h.p.h., for an initial 48 h.p.h. larval density of 10.4%.
Total length of 250 juveniles (anesthetized as described above) was measured with a small piece of graph paper glued under a Petri dish. Wet weights were measured following the methods described by Moretti et al. (1999). General survival was estimated at the end of the study, based on total number of harvested juveniles and estimated number of normal larvae at hatching.
After harvest all juveniles were size graded with a specially designed grader built with free rotating 316 stainless steel rods, 3 mm in diameter and with a 3–4 mm separation (Fig. 4). Total length and wet weight were determined on samples of 100 juveniles that passed through the grader and those that were retained.
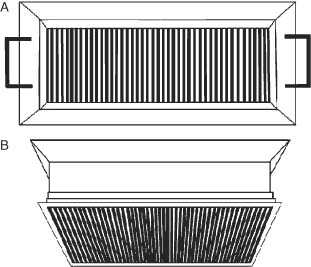
Stainless steel size grader built with free rotating rods, (A) upper view; (B) lateral-inferior view. See text for descriptions.
Specific growth rates (SGRs) were calculated according to the equation SGR = 100 (ln Wt– ln Wo)/t, where Wo and Wt are mean total weight on entering the nursery and mean total weight at harvest, respectively, and t is the number of days. Mean daily growth in TL and total weight, during the entire rearing period and for the nursery period were calculated. Mean condition factor at harvest was calculated from the formula: K = W 100/TL3, where W = weight (g) and TL = total length (cm).
The increase in the variance of size distribution with time was calculated by the increase in the coefficient of variation of mean length (ΔCV) at entering nursery (CV1) and that at harvest (CV2) and is estimated as Δ CV = CV2– CV1(Goldan et al. 1998).
Growth was analyzed by using an exponential regression model. All results are given as mean ± standard error of the mean (SEM) or as otherwise specified.
Results
Egg Production and Quality
Eggs for this trial were chosen from a single night's spawning in the third month after the captive broodstock started natural spawning (Ibarra-Castro and Alvarez-Lajonchère 2011). A total of 480,000 eggs were collected from one spawning tank, 14 h after spawning, of which 89.6% were floating, with a viability of 96.2% (transparent eggs with a live embryo). Approximately 270,000 floating eggs were stocked into six rearing tanks, at an average of 15 floating eggs/L (average diameters of eggs 770 ± 3.2 µm and oil droplet of 130 ± 0.9 µm). Time to hatching at 25–26 C was 21 h, hatching percentage was 90.2 ± 2.1%, and viable larval percentage at 48 h post-hatching was 79.7 ± 1.9%, thus initial larval density was 10.4 ± 1.0 larvae/L. Total length of newly hatched larvae was 2.5 ± 0.12 mm. No abnormal larvae were found at hatching.
Production and Survival of Larvae and Juveniles
Larval mouth was opened at the end of 2 d.p.h. when their yolk sac was almost fully reabsorbed. Rotifers and copepods were observed in guts at 3 d.p.h. Enriched Artemia metanauplii were eaten at 18 d.p.h. on the first day they were supplied. Two critical periods occurred with high larval mortality, the first between 4 and 8 d.p.h., during the pre-flexion stage, and the second during the third and fourth weeks (16–28 d.p.h.), at the start of metamorphosis. No signs of stress were detected, such as loss of swimming control, swimming up and down in the water column or paralyzation, as described for other marine fish species by Alvarez-Lajonchère and Hernández Molejón (2001, p. 238) and Alvarez-Lajonchère et al. (2002, p. 210), which could be associated to sudden death.
After the first week, sampling became more difficult because individuals were scattered and the largest ones that were usually at the bottom of the tank swam very fast and avoided the hand nets. Active head first cannibalism was observed after 12 d.p.h. The largest individuals remained at the bottom of the water column and swam actively to the middle and upper water layers to efficiently capture smaller larvae. Predators were at least 50% and up to 200% larger than their prey. When the difference in size was of approximately 30%, predators could be found dead with their digestive tract obstructed by the prey.
A total of 22,600 juveniles were harvested at 60 d.p.h. from the six rearing tanks. A mean harvest density of 1256 ± 170 juveniles/m3 (range: 933–1450 juveniles/m3) was achieved from the pilot-scale production trial, with an average survival of 12.1 ± 1.1% (range: 9.0–13.9%) after 48 h.p.h. Mean final biomass was 2.8 kg/m3.
Larval and Juvenile Growth
Growth of the larvae and juveniles was very slow during the first 4 wk, reaching a mean TL of 8 mm at 25 d.p.h. (0.22 mm/d), which increased more rapidly thereafter, reaching 55 mm at 60 d.p.h. (1.34 mm/d) (Fig. 5). Growth during the entire rearing period, including the graded fish, is described by the following exponential equation TL = 2.1476e0.0543t (R2 = 0.9911). Mean total length and weight at harvest were 5.5 ± 0.05 cm and 2.24 ± 0.04 g respectively. Mean daily growth and total weight during the entire rearing cycle and in the nursery phase were 0.92 mm/d and 40.7 mg/d, and 1.67 mm/d and 92.7 mg/d, respectively. The increase in the variance of size distribution (total length) during the nursery phase was 12.3%. SGR during the nursery stage was 11.5%. Final mean condition factor was 1.3464 and the general length-weight ratio was W = 0.05460 LT2.2306.
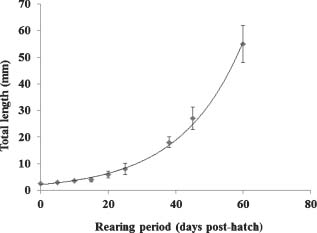
Growth curve of spotted rose snapper, Lutjanus guttatus, larvae and juveniles at water temperatures of 25–27 C. Each point represents the mean± standard error of the mean of 50 fish sampled all rearing tanks.
Length and weight of juveniles that were size-graded at harvest were 5.8 ± 0.25 cm and 2.5 ± 0.13 g for those retained by the grader, which were significantly higher (P < 0.05) than total length and weight, 3.9 ± 0.12 cm and 1.2 ± 0.09 g, of those passing through the stainless steel rods of the grader.
Discussion
Egg quality was high, as were the hatching and 48-h post-hatch survival percentages. Prophylactic treatment of the eggs consisting of a 1-h bath in 10 mL/m3 formalin solution and incubation and hatching of eggs in a separated tank should improve the rearing environment by lowering bacterial levels and avoiding the introduction of unhatched eggs and shells in the larval rearing tank (Ibarra-Castro et al. unpublished data).
Water quality variables were acceptable for the species, although dissolved oxygen and percent saturation levels were lower than those of ocean water where the species naturally spawn. This could negatively influence growth and survival. As the laboratory seawater is from a coastal source (Alvarez-Lajonchère et al. 2007), activated carbon filtration of the incoming water supply would lower the dissolved organic substances and indirectly increase oxygen levels, which could also be increased by using packed columns before the water enters the rearing tank and using foam fractionators during the nursery stage (Huguenin and Colt 2002). The rearing environment could also be improved by lowering salinity (ca. 25 g/L), based on good results in other marine species (Alvarez-Lajonchère and Hernández Molejón 2001), including snappers (Ogle and Lotz 2006).
Survival rates of the spotted rose snapper larvae during the rearing period were improved in this study, compared to those of previous trials conducted in 600-L cylindrical fiberglass tanks (Garcia-Ortega et al. 2005; Abdo-de la Parra et al. 2010), and the average survival rate achieved for this species in Costa Rica (Boza-Abarca et al. 2008; Herrera-Ulloa et al. 2010). High mortality occurred during two critical periods: at the start of the exogenous feeding phase, as reported for spotted rose snapper by Boza-Abarca et al. (2008) and for other snapper species (Lim et al. 1985; Emata et al. 1994; Duray et al. 1996; Tucker 1998); and during metamorphosis, which in itself is a stressor in fish larvae (Kraul et al. 1993). These two critical periods are common in other marine fish species (Tucker 1998). Two of the most successful snapper larval rearing studies did not report mortality during the first critical period; instead they recorded a gradually declining survival until 20–26 d.p.h. (Watanabe et al. 1998; Leu et al. 2003), when strong cannibalism occurred.
As reported for other snapper species, growth of larvae was slow in the first few weeks during the rotifer feeding period (Lim et al. 1985; Doi et al. 1994; Emata et al. 1994; Duray et al. 1996). It was much slower than in most other snappers, but similar to the mutton snapper (Watanabe et al. 1998) and other reports of spotted rose snapper (Boza-Abarca et al. 2008; Abdo-de la Parra et al. 2010). As noted in other studies (Garcia-Ortega et al. 2005) growth notably increased after larvae started feeding on Artemia metanauplii.
Newly hatched snapper larvae are very small, with extremely limited yolk reserves, for which the start of exogenous feeding is critical (Davis et al. 2000). This highlights the importance of first feeding, which may be improved with the use of smaller rotifers (80–100 µm) for the first few days, as well as copepod nauplii and copepodites, which have the size and nutritional value required by larvae of many marine fishes (Tucker 1998), including snappers (Lim et al. 1985; Doi and Singhagraiwan 1993; Schipp and Pitney 1995; Schipp et al. 2001; Leu et al. 2003; Watanabe et al. 2005; Ogle and Lotz 2006).
During this critical period, larval development was characterized by metamorphosis, which is a complex stage where important morphological and physiological changes take place (Lim et al. 1985; Doi et al. 1994; Emata et al. 1994), and where differential growth leads to size hierarchy, resulting in increased cannibalism (Lim et al. 1985; Doi and Singhagraiwan 1993; Emata et al. 1994; Duray et al. 1996; Ogle and Lotz 2006; Boza-Abarca et al. 2008). In this study, strong cannibalism occurred during the second week, much earlier than in other snapper species where it starts on the fourth or fifth week (Lim et al. 1985; Duray et al. 1996; Leu et al. 2003; Ogle and Lotz 2006; Herrera-Ulloa, pers. comm.). Mortality in this period could have also been partially caused by detaining the supply of copepod after 18 d.p.h., and offering Artemia that is less digestible and less nutritious.
Modifications are suggested, which include: an extended supply of rotifers (especially with Brachionus plicatilis), copepods and microalgae (up to 35–40 d.p.h.); higher copepod density (3–5 copepods/mL); Artemia supply could start earlier than 18 d.p.h. if umbrella-stage is used (Nhu et al. 2009), and weaning may be performed earlier with commercially available micro-diets especially formulated for the early weaning of marine fish larvae, following beneficial results in other species (Schipp et al. 2007; Nhu et al. 2010). These changes in the feeding regime would favor the survival of small larvae and stimulate faster growth, reducing larval rearing period (Hecht and Pienaar 1993).
Mean growth of juveniles in this study was slightly lower than that in previous studies (Boza-Abarca et al. 2008; Abdo-de la Parra et al. 2010), possibly because of the higher survival and harvest density achieved in this study. The SGR of the fish during the nursery stage was similar to other species, and the final mean condition factor was similar to other marine fish juveniles of similar sizes produced in hatcheries (Moretti et al. 1999). The ΔCV was higher than that reported for other marine species (Goldan et al. 1998), although this value may be underestimated because of sampling difficulties because the largest individuals that were usually near the bottom of the tank avoided the hand nets by swimming rapidly. This has been observed in length distribution with growth in other snappers (Lim et al. 1985; Doi and Singhagraiwan 1993; Emata et al. 1994; Duray et al. 1996; Watanabe et al. 1998). The size hierarchy due to differential larval growth has been shown to increase cannibalism in many species (Dowd and Clarke 1989; Hecht and Pienaar 1993), including snappers (Lim et al. 1985; Leu et al. 2003).
Grading is very important in minimizing cannibalism in many marine species (Hecht and Pienaar 1993; Goldan et al. 1998; Moretti et al. 1999). Here, size grading conducted inside larval rearing tanks and during the juvenile stage successfully separated the largest individuals. An earlier larval size grading, perhaps in the third week of culture, and afterward at a weekly frequency, could further improve survival and mean growth rate.
Some of the best results in snapper larval rearing have been obtained using mesocosm technologies (Watanabe et al. 1998; Feeley et al. 2000; Schipp et al. 2001), with rearing tank size being one of the most important characteristics. The present technology may be improved if applied in larger cylindrical tanks (7–10 m3).
The first mass production of a spotted rose snapper hatchery, with 70,000 juveniles in 2007 was reported by Herrera-Ulloa et al. (2010). Those results were obtained with spontaneously spawned eggs, as in the present study, but larval rearing tanks were stocked with newly hatched larvae with an initial density of 33 eggs/L, while here the larviculture tanks were stocked with 15 floating eggs/L, 12–14 h after fertilization. The age of juveniles was similar, although mean survival and harvest density were lower than in the present study (Table 1).
| Species | Rearing tanks (m3) | Stocking density (No./L) | Duration (d.p.h.) | Survival (%) | Final density (No./L) | TL (mm) | W (g) | Biomass (kg/m3) | Juveniles (× 103) | Authority |
|---|---|---|---|---|---|---|---|---|---|---|
| Mutton snapper, Lutjanus analis | 30 × 1t | 10.5a | 38 | 14.3 | 1.2 | 22.2 ± 3.6c | 0.31 ± 0.02 | 0.38 | 36.9 | Watanabe et al. (1998). |
| (8.6 2 db) | ||||||||||
| Northern red snapper, | 1 × 12t | 10b | 24 | 16.5 | 1.3 | 12.6 ± 2.8 | — | — | 6.6 | Ogle and Lotz (2006). |
| Lutjanus campechanus | (1–31) | (0.3–9.3) | ||||||||
| Mangrove red snapper, | 3 × 9t | 30b | 55 | 12 | 0.6 | 30.63 | ca. 0.54d | ca. 0.3e | 10.2 | Duray et al. (1996). |
| Lutjanus argentimaculatus | 4 × 6t | 9.9 ± 4b | 50 | 21.1 ± 7 | 2.0 ± 1.0 | 49.4 ± 4.3 | ca. 2.1d | ca. 4.2e | 48.9 | Leu et al. (2003). |
| John's snapper, | 40 × 2t | 2b | 35–40 | 25–35 | 0.5–0.7 | 56 | Schipp et al. (2001). | |||
| Lutjanus johnii | ||||||||||
| Spotted rose snapper, | 2.5 (500 L) × 4tf | 20a | 67 | 1.5 | 0.3d | 2.42 ± 0.84 | 0.7e | 0.6f | Boza-Abarca et al. (2008). | |
| Lutjanus guttatus | 6 × 2t × 9c | 33b | 55–60 | 2 | 0.66g | — | — | — | 70 | Herrera-Ulloa et al. (2010) |
| 3 × 6t | 15a | 60 | 12.1 ± 1.1 | 1.3 ± 0.2 | 55 ± 0.5 | 2.24 ± 0.04 | 2.8 | 22.6 | Present study. | |
| 10.4b |
- t = Tank number; c = cycles.
- aEggs.
- bLarvae.
- cStandard length.
- dEstimated considering TL = FL × 1.018 and W = 0.0280 FL2.844 (www.fishbase.org).
- eCalculated.
- fJ. Boza-Abarca (pers. comm.).
- gA. Herrera-Ulloa (pers. comm.) L. johnii: W = 0.0182 TL2.984; TL = 1.009 FL.
Our results show the second highest harvest density and biomass of all reports for snapper species (Table 1). Results for snapper production are poor compared to species such as gilthead seabream, Sparus aurata, and European Seabass, Dicentrarchus labrax (Moretti et al. 1999), but similar to other important species such as cobia, Rachycentron canadum (Benetti et al. 2008).
Acknowledgments
Authors are indebted to the staff and students of the Reproduction Laboratory at CIAD for their valuable assistance. Thanks are also due to S. Kraul, G. Schipp, N. Rhody, and M. Ribas for their useful comments and English edition of the manuscript. This study was supported by grants 6299-K and 6299-A from the National Aquaculture and Fishery Commission (CONAPESCA).




