Genetic Variations in Populations from Farms and Natural Habitats of Asian Green Mussel, Perna viridis, in Singapore Inferred from Nine Microsatellite Markers
Abstract
The Asian green mussel, Perna viridis, has been widely cultured in Southeast Asia as delicious seafood depending on natural spat. We analyzed the genetic diversity and population structure of three populations (two from Singapore and one from Malaysia) from natural habitats and two from Singapore farms, by genotyping a total of 262 adult individuals using nine polymorphic microsatellites. In all five populations, high allelic (number of alleles A = 11.0–14.4,allelic richness Ar = 10.4–13.9) and gene diversity (expected heterozygosity He = 0.73–0.77) were observed, whereas deficiency of heterozygosity (inbreeding index f = 0.08–0.12) was detected in all populations. Genetic differentiation among populations was low (pairwise fixation index ranged from 0.003 to 0.088), but statistically significant. The usefulness of the information about genetic variations for genetic resource management of the populations in wild habitats and farms to keep genetic variation and setting up a breeding program of Asian green mussel was discussed.
The Asian green mussel, Perna viridis L, is distributed in the Indo-Pacific region (Rajagopal et al. 2006). P. viridis is an oviparous and dioecious species. Sexual reproduction occurs when gametes are released into the water column where fertilization takes place. Its life cycle exhibits a long larval dispersal period and a sedentary adulthood (Rosell 1991). The pelagic larval stage lasts for about 3 wk, providing ample opportunities for larval dispersal and thereby promoting gene flow (Yap et al. 2002; Prakoon et al. 2010). After the spat begin settling, they spend the rest of their lives as sedentary organisms. P. viridis has been widely used as delicious seafood, and wild mussels have been harvested by commercial fishermen for decades (Rosell 1991; Mazuki 1998). In some countries, P. viridis has been cultured (Rajagopal et al. 1998; Prakoon et al. 2010). According to the Food and Agriculture Organization statistics (FAO 2010), in 2007 the annual production of P. viridis was 300,000 mt. In Singapore and Malaysia, culture of P. viridis holds considerable potential in coastal waters (Mazuki 1998). The culture activity started in the Johor Straits in the southern coast of Peninsular Malaysia due to availability of natural seed. However, the cultivation depends heavily on natural spatfall. Spat supply is highly variable and no breeding program for genetic improvement has been initiated yet. For profitable and sustainable aquaculture, and conservation of wild stocks, it is necessary to improve productivity of famed stocks, which can be achieved by selective breeding (Newkirk 1980; Gjedrem 1983). Genetic improvement through selective breeding was very successful in some shellfish species (Boudry et al. 1997), such as in Pacific oyster (Langdon et al. 2003), eastern oyster (Allen et al. 1993), abalone (Kube et al. 2007), and scallop (Ibarra et al. 1999). Aquaculture of these shell species has substantially reduced pressure on wild stocks. Genetic variations in the founder populations are the basis for later genetic improvement (Gjedrem and Baranski 2009). Therefore, it is necessary to know the genetic variations in Asian green mussel from farms and natural habitats to initiate a breeding program for genetic improvement.
Among many bivalve species, mussels have been studied on genetic diversity or population structures by using different biochemical or DNA markers. In the species of the genus Mytilus, a number of studies on genetic diversity and population relationships were conducted (Yamanaka and Fujio 1984; Fairbrother and Beaumont 1993; Raymond et al. 1997). Most of these studies suggested that genetic variations were high and population structuring was not significant. However, there is little information about genetic diversity and population structure on Perna mussels in cultured and wild stocks, although few studies on genetic variation of cultured and wild populations were reported (Yap et al. 2002, 2007; Apte et al. 2003; Prakoon et al. 2010).
In the present study, nine polymorphic microsatellites developed by us previously (Lin et al. 2007) were used to estimate genetic variations and population structure of two populations from farms and three from natural habitats to supply necessary information about genetic variations in different populations for setting up a breeding program to improve the productivity of P. viridis in Singapore.
Materials and Methods
Samples and Isolation of DNA
Two hundred and sixty-two adductor muscle tissue samples of adult mussels from five populations were collected in December 2006 and January 2007. The sample size of the each population ranged from 40 to 63 (Table 1). The three wild populations were from the Straits of Johor (SGUB), Port Klang in Malaysia (MYPK), Singapore island (SGIS) and the two farmed populations were from two coastal fish farms (SGLO and SGFA) in Singapore (Fig. 1 and Table 1). The two farms have been using wild caught spat from nearby locations for the culture of green mussel in rope web for more than 10 yr. However, nothing is known about the genetic diversity of the green mussels in the two farms and the gene flow among farmed and wild populations. Each collected sample was separately stored in absolute ethanol in 4 C freezer until DNA extraction. Genomic DNA was extracted using a method that was previously developed (Yue and Orban 2005) and the concentration of each sample is adjusted to 5 ng/µL. The DNA of each sample was arrayed into 96 well plates and stored in −20 C freezer for further polymerase chain reaction (PCR) amplification of microsatellite DNA.
| Populations | GM03 | GM04 | GM05 | GM07 | GM08 | GM09 | GM12 | GM16 | GM18 | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SGUB (wild), N = 40 | A | 22 | 19 | 21 | 15 | 23 | 7 | 4 | 7 | 6 | 13.77 |
| Ar | 22.00 | 19.00 | 21.00 | 15.00 | 23.00 | 7.00 | 4.00 | 7.00 | 6.00 | 13.77 | |
| Ho | 0.63 | 0.95 | 0.63 | 0.88 | 0.88 | 0.68 | 0.30 | 0.75 | 0.45 | 0.68 | |
| He | 0.90 | 0.90 | 0.95 | 0.90 | 0.90 | 0.74 | 0.36 | 0.75 | 0.57 | 0.77 | |
| F IS | 0.30 | −0.06 | 0.34 | 0.03 | 0.03 | 0.08 | 0.17 | −0.01 | 0.22 | 0.12 | |
| P | 0.01 | 0.84 | 0.06 | 0.46 | 0.152 | 0.74 | 0.39 | 0.71 | 0.02 | – | |
| MYPK (wild), N = 45 | A | 20 | 19 | 23 | 16 | 25 | 5 | 4 | 8 | 4 | 13.77 |
| Ar | 19.30 | 18.40 | 22.17 | 15.51 | 24.15 | 5.00 | 3.99 | 7.78 | 4.00 | 12.36 | |
| Ho | 0.71 | 0.98 | 0.71 | 0.78 | 0.98 | 0.42 | 0.27 | 0.60 | 0.31 | 0.64 | |
| He | 0.92 | 0.90 | 0.94 | 0.89 | 0.92 | 0.57 | 0.28 | 0.71 | 0.43 | 0.73 | |
| F IS | 0.23 | −0.08 | 0.24 | 0.13 | −0.06 | 0.27 | 0.05 | 0.15 | 0.27 | 0.12 | |
| P | 0.01 | 0.08 | 0.29 | 0.24 | 0.47 | 0.19 | 0.02 | 0.26 | 0.02 | – | |
| SGIS (wild), N = 56 | A | 23 | 17 | 23 | 14 | 25 | 7 | 4 | 7 | 5 | 13.88 |
| Ar | 20.28 | 15.75 | 21.73 | 13.42 | 22.38 | 6.38 | 3.99 | 6.87 | 4.69 | 12.83 | |
| Ho | 0.90 | 0.93 | 0.64 | 0.78 | 0.93 | 0.69 | 0.38 | 0.69 | 0.38 | 0.70 | |
| He | 0.91 | 0.89 | 0.94 | 0.89 | 0.90 | 0.68 | 0.35 | 0.74 | 0.58 | 0.77 | |
| F IS | 0.02 | −0.04 | 0.33 | 0.13 | −0.04 | −0.01 | −0.07 | 0.06 | 0.34 | 0.08 | |
| P | 0.10 | 0.46 | 0.00 | 0.10 | 0.20 | 0.68 | 0.27 | 0.24 | 0.00 | – | |
| SGLO (cul), N = 58 | A | 14 | 15 | 18 | 14 | 16 | 8 | 4 | 6 | 4 | 11.00 |
| Ar | 13.51 | 14.44 | 17.30 | 12.00 | 15.16 | 7.26 | 3.71 | 6.00 | 4.00 | 10.44 | |
| Ho | 0.71 | 0.79 | 0.75 | 0.88 | 0.91 | 0.45 | 0.45 | 0.64 | 0.48 | 0.67 | |
| He | 0.87 | 0.84 | 0.93 | 0.87 | 0.89 | 0.49 | 0.38 | 0.73 | 0.54 | 0.73 | |
| F IS | 0.18 | 0.06 | 0.19 | 0.00 | −0.02 | 0.09 | −0.17 | 0.12 | 0.10 | 0.08 | |
| P | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.10 | 0.13 | 0.00 | 0.00 | – | |
| SGFA (cul), N = 63 | A | 22 | 17 | 24 | 16 | 26 | 8 | 5 | 8 | 4 | 14.44 |
| Ar | 18.81 | 15.90 | 22.19 | 15.12 | 23.90 | 7.22 | 4.87 | 7.33 | 4.00 | 13.26 | |
| Ho | 0.78 | 0.84 | 0.81 | 0.92 | 0.94 | 0.76 | 0.30 | 0.51 | 0.35 | 0.69 | |
| He | 0.91 | 0.89 | 0.95 | 0.91 | 0.91 | 0.69 | 0.31 | 0.68 | 0.48 | 0.75 | |
| F IS | 0.14 | 0.05 | 0.15 | −0.02 | 0.01 | −0.11 | 0.04 | 0.25 | 0.28 | 0.08 | |
| P | 0.27 | 0.67 | 0.06 | 0.03 | 0.12 | 0.55 | 0.18 | 0.00 | 0.88 | – | |
- A, number of alleles, Ar, allelic richness; cul, cultured; FIS, inbreeding coefficient; He, expected heterozygosity; Ho, observed heterozygosity; N, number of animals; Overall, the mean over all populations; P, probability of significant deviation from Hardy–Weinberg equilibrium.
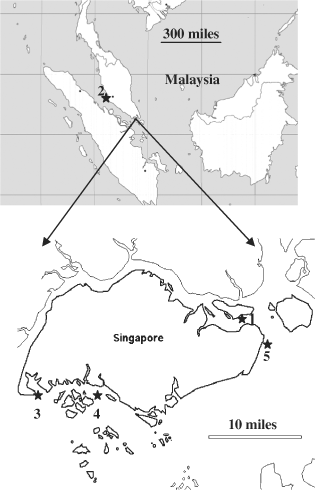
A map showing sampling locations of Asian green mussel. 1, The Straits of Johor (SGUB); 2, Port Klang in Malaysia (MYPK); 3, a population located on the west side of Singapore Island (SGIS); 4, a farm located in the west side of Singapore Island (SGLO); 5, a farm located on the east side of Singapore Island (SGFA).
Genotyping of Microsatellites
Nine microsatellites (Table 1) developed by Lin et al. (2007) were used to genotype 262 individuals from five populations. The forward primer of each pair was labeled with a fluorescent dye (either HEX or 6-FAM) at the 5′ end. PCR amplification for each microsatellite locus was conducted on a PTC-100 PCR machine (MJ Research, Waltham, MA, USA). Each 25 µl PCR reaction contained 10 ng of genomic DNA, 0.5 units of Taq polymerase (Roche, Basel, Switzerland), 1 × Roche Taq PCR buffer containing 1.5 mM MgCl2, 0.2 µM dNTPs, and 200 nM of each primer. PCR was conducted under the following conditions: 2 min denaturation at 94 C; 35 cycles of 30 sec at 94 C, 30 sec at 55 C, and 30 sec at 72 C and a final extension at 72 C for 10 min. PCR products were analyzed on an ABI3730xl DNA sequencer (Applied Biosystems, Foster City, CA, USA). Fragment sizes were analyzed against the ROX-500 size standard using GeneMapper (Applied Biosystems).
Data Analysis
The presence of null alleles can result in false homozygotes and may result in a pattern similar to the Wahlund effect (Wahlund 1928). Therefore, software micro-checker 2.2.3 (van Oosterhout et al. 2004) was used to check for null alleles, stuttering, and allele dropout. The software Genetic Data Analysis (GDA) (Lewis and Zaykin 2000) was used to calculate average number of alleles (A), observed (Ho) and expected heterozygosities (He), and inbreeding index (f) (Lewis and Zaykin 2000). Hardy–Weinberg equilibrium (HWE) was tested for each locus in each population using GDA. Another estimate of allelic diversity: allelic richness (Ar) which is used to control for differences in the number of alleles among populations that differ in sample size, was estimated using the program Fstat 2.9.3 (Goudet 1995). The calculation of all estimates (FST and AMOVA) of population structure and their significance was carried out using Arlequin 3.1 (Excoffier et al. 2005).
Correlation between genetic distance and geographic distance was assessed using the isolation by distance web service (IBDWS version 3.08) (Jensen et al. 2005). Geographic distance referred to the nearest distance between two populations along the coast. Significance of the analysis was examined using Mantel tests as implanted in software IBDWS.
Results
Genetic Variations in Wild and Farmed Populations
The analysis with micro-checker revealed no indications of null alleles, stuttering or allele dropout based genotyping errors in any of the loci except the loci GM03 and GM18. As null alleles at these loci appeared in almost all populations (Table 1), and the analyses performed with and without these two loci returned similar results, the results presented include all nine loci. The average allele number of the nine microsatellites was 17.7 alleles/locus across all five populations. The five populations showed similar allelic richness (Ar = 13.77,12.36, and 12.83 in the wild populations SGUB, MYPK, and SGIS; and Ar = 10.44 and 13.26 in cultured populations SGLO and SGFA [Table 1]). The gene diversity (He) was highest in two wild populations (He = 0.77 and 0.77 in SGUB and SGIS), followed by the farmed population SGFA (He = 0.75), the wild population MYPK (He = 0.73), and the farmed population (He = 0.73). In the five populations the overall inbreeding index ranged from 0.08 for the farmed population SGFA to 0.12 for the wild population MYPK. The wild populations showed slightly higher inbreeding than the farmed populations. The allele frequency at some loci showed significant deviation from HWE in different populations (i.e., GM03 and GM18 in SGUB; GM03, GM12, and GM18 in MYPK; GM05 and GM18 in SGIS; GM03, GM04, GM05, GM09, GM12, and GM18 in SGOL; GM07 and GM16 in SGFA).
Genetic Differentiation among Wild and Farmed Populations
Overall and pairwise genetic differentiation (FST) among the five populations was analyzed. The overall genetic differentiation (FST = 0.039, P < 0.01) was significant. The pairwise FST ranged from 0.003 between SGUB and SGFA, to 0.088 between MYPK and SGIS (Table 2). The FST values were small, but statistically significant (P < 0.05) (Table 2). AMOVA analysis revealed that the most genetic variation (96.12%) came from among individuals within populations while the variation among populations was only 3.88% (Data not shown). The genetic distances correlated well with the geophysical distances (r = 0.89, P < 0.01) (Fig. 2).
| Populations | SGUB | MYPK | SGIS | SGLO | SGFA |
|---|---|---|---|---|---|
| SGUB | – | – | – | – | |
| MYPK | 0.082* | – | – | – | |
| SGIS | 0.068* | 0.088* | – | – | |
| SGLO | 0.019* | 0.077* | 0.020* | – | |
| SGFA | 0.003* | 0.083* | 0.013* | 0.014* |
- * P < 0.05.
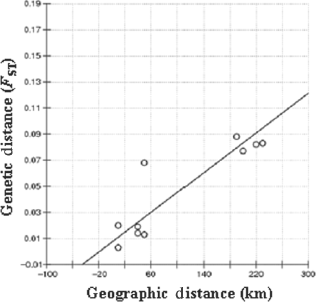
Relationship between genetic distances (FST) and geographic distances (km) in Asian green mussel.
Discussion
Advantages of Polymorphic Microsatellite Markers
The nine microsatellites used in this study, showed high polymorphism with an average of 17.7 alleles/locus, which is similar to those used in examining the populations in Thailand (Prakoon et al. 2010), and is much higher than those microsatellites (2–3 alleles/locus) used for assessing the population in Malaysia (Ong et al. 2009). Low polymorphism of some types of markers, such as allozymes, has limited their ability in identifying population structure (Yap et al. 2002; Liu and Cordes 2004). A number of studies demonstrated that highly variable DNA markers enable scientists to reveal the subtle structure in seemingly panmictic populations (Estoup et al. 1998; Hedrick et al. 2000). Therefore, the high polymorphism of the microsatellite markers used in this study could make the assessment of genetic diversity and populations more effectively. Although at two loci (i.e., BM03 and BM018) null alleles appeared in almost all populations, as the analyzes performed with and without these two loci returned similar results, the results presented here included all the loci.
Genetic Diversity in Five Populations of P. viridis
In this study, the average allelic richness in all five populations was over 10.4/locus and was similar to that in P. viridis in Thailand (Prakoon et al. 2010), suggesting that high allelic diversity is available in populations in the coast of Singapore and Thailand. However, in another study on P. viridis in Malaysia (Ong et al. 2009), the allelic diversity in 10 populations was only 3.1 allele/locus, much lower than the observed in our study. This difference may be due to the use of different microsatellite markers. It is also possible that this difference may reflect the real difference of genetic status of different populations. But, this possibility is very low, as in our study, one population (MYPK) is located in Malaysia, and allelic diversity was 12.4 alleles/locus, much higher than that in populations from the same location (3.0 alleles/locus) (Ong et al. 2009). In this study, the average gene diversity (He) was over 0.73, much higher than that (0.26) in populations located in Malaysia (Ong et al. 2009) and was similar to that in populations located in Thailand (Prakoon et al. 2010), suggesting that there is high gene diversity in all five populations. Taken all together, our data showed that the five Asian populations of the green mussel studied by us are highly variable.
In all five populations, deficiency of heterozygosities was seen. These deficits may be caused by null alleles, Wahlund effect and selection. The analyses performed with and without the two loci (i.e., BMS03 and BMS18) showing null alleles returned similar results. Therefore, deficiency of heterozygosities in all populations is not due to the appearance of null alleles. Similar deficiency of heterozygosities of the same microsatellites was seen in populations of P. viridis in Malaysia and Thailand (Ong et al. 2009; Prakoon et al. 2010), suggesting the deficiency of heterozygosities in wild populations of P. viridis may be related to its life history and marine environment. P. viridis has planktonic larval dispersion (Rajagopal et al. 1998). Larvae from different cohorts originated from different locations may settle at the same site. Previous studies (Johnson and Black 1984, Raymond et al. 1997, Fairbrother and Beaumont 2009) showed that collecting seeds from different locations for the culture will be enough for having a Wahlund effect (Hartl and Clark 1989). It is also possible that selective pressure may work on the deficiency of heterozygotes (Mallet et al. 1985; Sun and Salomon 2003). Certainly this possibility requires examination using more powerful DNA markers located in genes (e.g., immune related genes) (Tirape et al. 2007).
Genetic Differentiations among Wild and Farmed Populations
Both the FST and AMOVA analysis showed that the population differentiation among the five populations was minor, but statistically significant. Similar results were reported in 10 wild populations of P. viridis in Thailand (Prakoon et al. 2010), while the populations in Malaysia did not show significant differentiation (Ong et al. 2009). Low population differentiation is very common in marine mussel species (Ong et al. 2009); this may be because of high capability of dispersal in marine environments (Bayne 1976). The pairwise genetic distances are directly related to the geographic distances, suggesting that dispersal is spatially limited due to the geographical distance and the tendency of individuals to find mates from nearby populations rather than distant populations. Further studies on population structure of Asian green mussel in more locations could bring more insights on its genetic status and population relationships.
Implication of This Study for Aquaculture of Asian Green Mussel
Genetic variations are critically important for survival and adaptation of changing environment for wild populations (Geist and Kuehn 2005) and for genetic improvement of farmed populations (Gjedrem and Baranski 2009). In this study we found that the genetic diversity in the wild and farmed populations was high, while the genetic differentiation among populations was small, but significant. Therefore, there is no immediate danger of low genetic variation in wild populations of Asian green mussel. However, previous studies showed that aquaculture relying on wild caught seeds is not sustainable and had a devastating effect on wild populations (e.g., Naylor et al. 2000). Captive selective breeding for genetic improvement is a way to conserve the wild stocks (Vrijenhoek 1998). The wild and farmed populations of Asian green mussel studied here could be good starting materials for aquaculture and initiating a selective breeding program for genetic improvement. We have noticed that among populations, the genetic differentiation was small but significant, which must be taken into account when initiating a selective breeding program. It is generally believed that a cross between genetically different individuals may generate hybrid vigor (Chen et al. 2010), while it is also possible that outcrossing generates recession (Lynch 1991). Whether crossing individuals from different populations that showed significant genetic differentiation could generate hybrid vigor or recession in Asian green mussel requires testing.
Acknowledgments
This study is funded by the internal funds of the Temasek Life Sciences Laboratory, Singapore. We thank our sequencing facility for technical supports.




