Trophic level and overlap of sea lions (Zalophus californianus) in the Gulf of California, Mexico
Abstract
Stable isotope and scat analyses were used in concert to determine trophic level and dietary overlap among California sea lions from different rookeries in the Gulf of California. Isotopic analysis of the fur of sea lion pups revealed differences in δ15N and δ13C values among rookeries during the breeding season. Mean δ15N and δ13C values varied from 20.2‰ to 22.4‰ and from −15.4‰ to −14.0‰, respectively. The pattern of differences among rookeries was similar between years in most cases. Isotopic variations among rookeries were associated with differences in prey consumption. There was a significant correlation between δ15N value and trophic level, as determined by scat analysis. Joint application of isotopic and scat analyses allowed us to identify how the feeding habits of sea lions vary with location. Our results suggest the presence of spatial structure in available prey as well as the localized use of prey by sea lions across the Gulf of California.
Thirteen California sea lion (Zalophus californianus) rookeries occur in the Gulf of California, with 10 located north of 28°N where sardine and anchovy are most abundant (Aurioles-Gamboa and Zavala-González 1994). Adult females exhibit strong philopatry (Hernández-Camacho 2001), and feeding habits seem to show a regional structure (García Rodríguez and Aurioles-Gamboa 2004). This is particularly true for animals from rookeries that are in close proximity and that overlap in their potential foraging space (Kuhn 2006). Several studies conducted in the Gulf of California have shown that sea lions consume a broad variety of prey and that dietary differences exist among rookeries (Aurioles-Gamboa et al. 1984, Orta-Dávila 1988, Sánchez-Arias 1992, Gutiérrez 2003). These studies have not been conducted at all major rookeries, however, and they were done at different time periods, so differences observed among rookeries might be due to temporal shifts affecting all rookeries. These studies used scat analyses, which offer invaluable, detailed information on prey consumption. Yet quantitative assessment of diet using scat analysis is subject to various well-known biases (da Silva and Neilson 1985, Dellinger and Trillmich 1988, Pierce and Boyle 1991, Cotrell et al. 1996, Tollit et al. 1997, Bowen 2000, Orr and Harvey 2001).
Stable isotope analysis offers less detailed information on dietary composition than scat analysis, but because it provides information on assimilated food, it avoids some of the biases in scat analysis (Tieszen et al. 1983, Hobson et al. 1994, Holst et al. 2001). Moreover, because the turnover rates of elements in consumer tissues vary according to the metabolic rate of those tissues, stable isotope analysis can integrate dietary information over different time periods (Dalerum and Angerbjörn 2005). Stable isotopes of elements in metabolically inactive tissues (e.g., fur, feathers, skin, and nails) do not turn over, and therefore reflect the diet or body chemistry of an individual during a limited period of tissue formation (Tieszen et al. 1983).
Tissues of consumers tend to become enriched in 13C and 15N relative to those of their prey, a process referred to as fractionation or trophic enrichment. The 13C-enrichment per trophic step is roughly + 0.5‰ to + 2‰, based on studies of different tissues of seals and other marine mammals (Kelly 2000, Lesage et al. 2002). The 15N-enrichment ranges from + 2‰ to + 5‰ per trophic step (Schoeninger and DeNiro 1984, Hobson et al. 1996, Kelly 2000).
Both carbon and nitrogen isotope values may vary spatially in primary producers because of regional differences in factors such as nutrient or light levels, types of primary producer, or the isotopic composition of carbon and nitrogen substrates (which might vary with the intensity of upwelling or the magnitude of fluvial or atmospheric inputs). Because of these effects, carbon isotope values differ between inshore vs. offshore and between benthic vs. pelagic food webs, with lower values in offshore/pelagic systems, and higher values in inshore/benthic systems (McConnaughey and McRoy 1979, Rau et al. 1983, Hobson et al. 1994, France 1995). There are latitudinal differences in the nitrogen isotope composition of primary producers at the base of food webs in the Gulf of California, with higher values north and lower values south of the Midriff Region (Fig. 1) (Altabet et al. 1999). In addition, because of strong trophic 15N-enrichement, nitrogen isotope values are a reliable indicator of the relative trophic level of organisms within a food chain (Owens 1987, Kelly 2000).
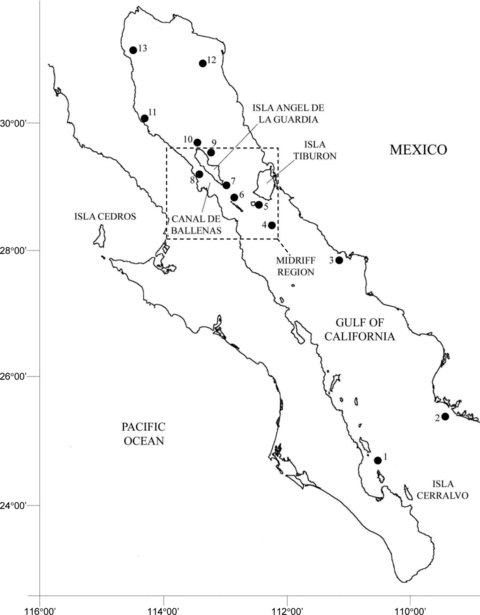
Location of California sea lion rookeries where fur and scat samples were collected: 1. Los Islotes (24°35′N, 110°23′W); 2. Farallón de San Ignacio (25°26′N, 109°22′W); 3. San Pedro Nolasco (26°49′N, 121°12′W); 4. San Pedro Mártir (28°24′N, 112°25′W); 5. San Esteban (28°42′N, 112°36′W); 6. El Rasito (28°49′N, 112°59′W); 7. El Partido (28°53′N, 113°02′W); 8. Los Machos (29°20′N, 113°30′W); 9. Los Cantiles (29°32′N, 113°29′W); 10. Isla Granito (29°34′N, 113°32′W); 11. Isla Lobos (30°02′N, 114°28′W); 12. San Jorge (31°01′N, 113°15′W); 13. Rocas Consag (31°7′N, 114°30′W). The Midriff Region is indicated by dash lines.
Stable isotope analysis of sea lion fur may allow us to examine the spatial structure of foraging by animals from different rookeries. If sea lions from different rookeries forage in different locations, or if they take different types of prey, then isotopic values should differ among sea lion rookeries. One weakness of the isotopic approach is that dietary composition can only be determined at a coarse level (Holst et al. 2001). In our study we remedy this shortcoming by applying both stable isotope and scat analyses to establish the spatial structure of the sea lion foraging throughout the Gulf of California and to assess the trophic level and potential trophic overlap among sea lions at different rookeries.
Methods
Fur and scat samples were collected at different California sea lion rookeries in the Gulf of California, Mexico (Fig. 1). A total of 188 fur samples from sea lion pups were collected at 13 rookeries, primarily during the breeding seasons of 2000 (16–25 July) and 2002 (15–31 July), with a small sample from 2004 (9–22 July). Fur was clipped with scissors at the base from an area of approximately 5 × 5 cm on the middorsal region.
In our study, we analyzed fur from suckling California sea lion pups (approximately 2-mo old), assuming that they would accurately record differences in the foraging patterns in their mothers (see Aurioles-Gamboa et al. 2006 for a similar application). To interpret maternal dietary patterns from pup fur, the isotopic fractionations associated with mother-to-offspring nutrient transfer during pregnancy, lactation, and weaning must be known. Unfortunately, these fractionations are still poorly understood. Theoretically, if milk protein has a nitrogen isotope value similar to other maternal tissues, then suckling offspring should have 15N-enriched values indicating that they are feeding one trophic level higher than their mother. This expected pattern has been observed in a number of species, including California sea lions (Newsome et al. 2006). Because of the smaller magnitude of trophic level 13C-enrichment, and the fact that milk is rich in 13C-depleted lipids, the fractionation from mother to suckling infant is difficult to predict a priori, and appears to be negative in pinnipeds (Newsome et al. 2006). Here, we provide further constraints on these fractionations through a comparison of isotope values for fur between adult females and suckling pups at one rookery. The fur samples from eight adult females were collected from the Los Islotes rookery in April 2003. We did not attempt to match mother-pup pairs, and we recognize that the fur sampled from adult females likely formed after the 2002 breeding season. However, given the difficulty of capturing adult females, it was not possible to sample them in previous seasons or at other rookeries.
Scat samples were collected from 11 rookeries during the breeding season of 2002 (15–31 July). Most of the scat samples were from mothers with pups, as we were collecting at breeding areas dominated by adult females.
Stable Isotope Analysis
Fur samples were rinsed with distilled water and then fully dried at 80°C for approximately 12 h. Lipids were removed using the Microwave Assisted Extraction (MAE) protocol (microwave oven model 1,000 MARS 5 x CEM) with 25 mL of a (1:1) solution of chloroform/methanol (Bligh and Dyer 1959). Samples were subsequently dried and ground into a homogeneous fine powder. Stable carbon and nitrogen isotope measurements were performed on approximately 1.2 mg subsamples of homogenized tissue loaded into tin foil boats using a continuous flow isotope ratio monitoring mass spectrometer (20-20 PDZ Europa, Cheshire, U.K.) after sample combustion to CO2 and N2 at 1,000°C in an on-line elemental analyzer (PDZ Europa ANCA-GSL) (Stable Isotope Lab., University of California, Davis, CA). Ammonium sulfate (δ15N = 1.33‰) was used as a secondary standard for nitrogen, and sucrose (δ13C =−23.83‰) was used for carbon. The analytical error indicated by replicate measurements of secondary standards was ± 0.2‰ for both nitrogen and carbon.
Isotopic composition was expressed in the δ notation, as the deviation from standards in parts per thousand (‰) according to the following equation: δ15N or δ13C =[(Rsample/Rstandard) − 1]× 1,000, where R is the ratio of 15N/14N or 13C/12C for the sample and the standard, respectively. The standards are atmospheric N2 (AIR; δ15N = 0.004‰) for nitrogen and Vienna Pee Dee Belemnite limestone (V-PDB; δ13C = 0.011‰) for carbon.
Student's t-tests were performed for differences in δ15N and δ13C values in sea lion fur between the pups sampled in July 2002 and the adult females sampled 8 mo later. Multivariate analyses of variance (MANOVA), followed by post hoc, pairwise F-tests were performed for differences in δ15N and δ13C values in sea lion fur among rookeries for each year (2000 and 2002). We compared MANOVA results between 2000 and 2002 to assess inter-annual variation.
Scat Analysis
Individual scats were immersed in a detergent solution for 48 h and then screened through a series of sieves with mesh widths of 2.0, 1.19, and 0.71 mm2. Fish otoliths, cephalopod beaks, and other prey remains (i.e., fish bones and scales, eye lenses of fish and squid, and crustacean fragments) were extracted from the sieves. Cephalopod beaks were stored in 70% alcohol; all other items were stored dry in vials. Fish and cephalopod species were identified by otoliths and beaks, respectively. Otolith identification was determined to the lowest possible taxon, using photographs and illustrations (Fitch and Brownell 1968), as well as the reference collection from the Centro Interdisciplinario de Ciencias Marinas-Instituto Politécnico Nacional (CICIMAR-IPN), La Paz, B.C.S., Mexico. Cephalopod beaks were identified by Unai Markaida (ECOSUR).1
We used cumulative prey diversity curves to determine if an adequate number of scat samples were collected to characterize the diets of animals at a rookery. In order to estimate a mean cumulative prey diversity curve and its SD, based on the Shannon-Wiener (H') Index (Krebs 1999), we followed the approach proposed by Ferry and Cailliet (1996), Ferry et al. (1997), and modified by Cruz-Escalona and Turren (CICIMAR-IPN),2 implementing a Matlab routine, which computes 500 random permutations from the original data. If the prey diversity curve reached an asymptote, we assumed that we had an adequate sample size.
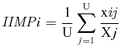

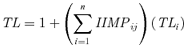
Pearson's correlation was used to relate the δ15N value for each rookery in the 2002 breeding season with TL values. Statistical tests were performed using the Statistica version 6.0 or JMP.
Results
Isotope Fractionation Between Adult Females and Pups
The fur of California sea lion pups (approximately 2-mo old) at Los Islotes was 15N-enriched in relation to fur from adult females by 2.1‰± 0.1‰ (Student's t-test: t16=−15.81, P < 0.001) and 13C-depleted by 0.8‰± 0.2‰ (Student's t-test: t16= 7.23, P < 0.001).
Spatial and Temporal Dietary Variation Based on Stable Isotope Ratios
California sea lion fur showed significant separation in δ15N and δ13C values among rookeries sampled in 2000 (MANOVA: Pillai's Trace test, P < 0.0001). Post hoc F tests revealed significant differences between most pairs of rookeries (Table 1). Most cases of non-significant differences occurred between rookeries that are in the same region of the Gulf of California (i.e., San Pedro Mártir-El Partido and San Esteban and El Partido-Los Machos). Mean values ranged from 20.1‰± 0.3‰ to 21.6‰± 0.4‰ for δ15N and from −15.4‰± 0.2‰ to −14.3‰± 0.2‰ for δ13C (Appendix I). Los Cantiles and Isla Granito had higher δ15N values than the other rookeries, whereas San Esteban, Los Machos, San Pedro Mártir, and El Partido had higher δ13C values (Fig. 2).
| Islotes | Mártir | Esteban | Partido | Machos | Cantiles | Granito | |
|---|---|---|---|---|---|---|---|
| (A) 2000 | |||||||
| Islotes | – | – | – | – | – | – | |
| Mártir | Y | – | – | – | – | – | |
| Esteban | Y | N | – | – | – | – | |
| Partido | Y | N | 0.41 | – | – | – | |
| Machos | Y | Y | Y | N | – | – | |
| Cantiles | 0.0008 | Y | Y | Y | Y | – | |
| Granito | Y | Y | Y | Y | Y | 0.31 | |
| Islotes | Ignacio | Nolasco | Mártir | Esteban | Rasito | Partido | Cantiles | Granito | Lobos | Consag | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (B) 2002 | |||||||||||||
| Islotes | – | – | – | – | – | – | – | – | – | – | |||
| Ignacio | Y | – | – | – | – | – | – | – | – | – | |||
| Nolasco | Y | N | – | – | – | – | – | – | – | – | |||
| Mártir | Y | Y | Y | – | – | – | – | – | – | – | |||
| Esteban | Y | Y | Y | N | – | – | – | – | – | – | |||
| Rasito | Y | Y | Y | N | Y | – | – | – | – | – | |||
| Partido | Y | Y | Y | N | 0.005 | Y | – | – | – | – | |||
| Cantiles | 0.18 | Y | Y | Y | Y | Y | Y | – | – | – | |||
| Granito | Y | Y | Y | Y | Y | Y | Y | 0.0004 | – | – | |||
| Lobos | Y | Y | Y | Y | Y | Y | Y | N | Y | – | |||
| Consag | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |||
- Y indicates that the post hoc F test of differences in mean value was statistically significant (P= 0.05); N indicates that the test was not significant (P > 0.05). The 15 pairs that were analyzed in both 2000 and 2002 are underlined. When the post hoc F tests yielded different results in 2000 and 2002, P values are supplied.
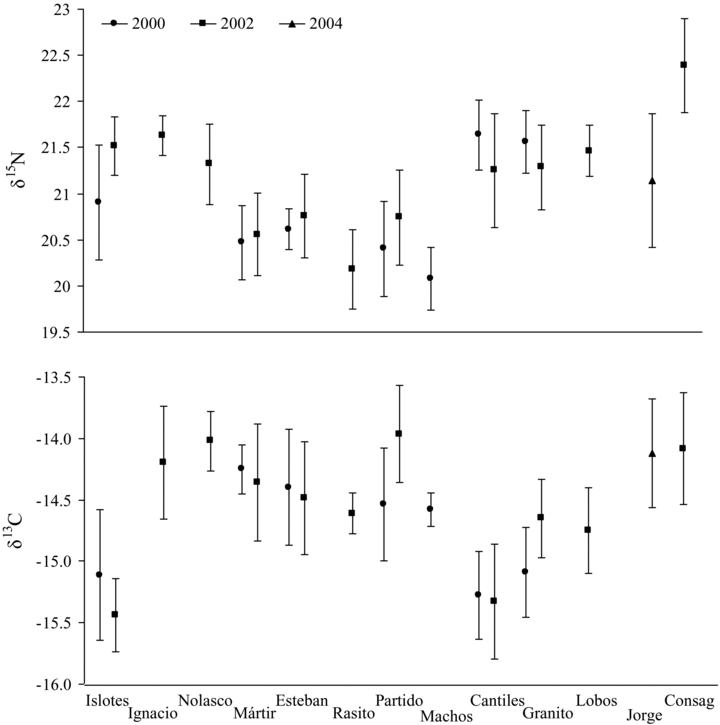
Inter-annual variations in δ15N and δ13C values (mean ± SD, in ‰) in fur of California sea lion pups collected during the breeding seasons of 2000, 2002, and 2004.
For 2002 we examined a larger set of rookeries and, again, found significant separation in δ15N and δ13C values (MANOVA: Pillai's Trace test, P < 0.0001). Most post hoc F tests revealed significant differences between rookeries (50 of 55) (Table 1). Three of the non-significant pair wise comparisons were between San Pedro Mártir and a set of closely spaced rookeries (El Partido, El Rasito, and San Esteban) and one was for a relatively closely spaced pair, Los Cantiles-Isla Lobos. In one case, however, very distant rookeries had statistically indistinguishable δ15N and δ13C values (Farallón de San Ignacio-San Pedro Nolasco). The lowest and highest mean δ15N values were 20.2 ± 0.4 and 22.4 ± 0.5‰ and mean δ13C values were −15.4‰± 0.3‰ and −14.0‰± 0.2‰ (Appendix I). δ15N values were higher at locations south of 28°N (Los Islotes, Farallón de San Ignacio, and San Pedro Nolasco) and north of 29°20'N (Los Cantiles, Isla Granito, Isla Lobos, and Rocas Consag), and lower at locations between 28° and 29°N (San Pedro Mártir, San Esteban, El Rasito and El Partido). δ13C values were high at all sites south of 29°20'N, except for Los Islotes, (i.e., Farallón de San Ignacio, San Pedro Nolasco, San Pedro Mártir, San Esteban, El Rasito and El Partido). Immediately north of 29°20'N (i.e., at Los Cantiles) values drop and then rise again progressively at the northern-most rookeries (Fig. 2). Measurements taken at San Jorge rookery in 2004 fit this pattern.
The temporal consistency of these isotopic patterns was assessed in two ways. First, inspection of Figure 2 and Table 1 suggested that for most rookeries for which measurements were taken in both 2000 and 2002, there was a strong overlap in both δ13C and δ15N values. In most cases, the mean value of a measurement in 1 yr was within roughly one SD of the mean in the other year. The largest shift in mean δ15N values (0.6‰) occurred at Los Islotes whereas the largest shifts in mean δ13C values occurred at El Partido (0.5‰) and Isla Granito (0.4‰). Our second test was to compare the results of post hoc F tests between the years (Table 1). For the 15 pairwise F tests that were conducted in both 2000 and 2002, the results were the same for all but three cases (El Partido-San Esteban, Los Cantiles-Los Islotes and Isla Granito-Los Cantiles).
Diet Composition
Of the 274 scat samples collected, 98.0% contained fish remains, 19.5% mollusk remains, and 7.3% crustacean remains. From the total scat samples, 155 (56.6%) contained identifiable hard parts of prey: 802 otoliths and 84 cephalopod beaks (damaged structures were not included) (Appendix II). Because the sample size was small and no identifiable preys were recovered from the scats at the Isla Granito rookery, this location was not included in these analyses.
Overall, sea lions fed on 52 different fish species (of which 42 were identified at least to the family level) and five cephalopod species. The diet was dominated by serranids (six species), ophidiids (four species), and haemulids and sciaenids (three species). The Carangidae, Engraulidae, Merluccidae, Paralichthyidae, Scorpaenidae, and Sebastidae families were represented with two species, and the remaining families with only one species. When data from the 10 rookeries are averaged, six prey species had IIMP values ≥ 5%: the midshipman (Porichthys spp.), the Pacific anchoveta (Cetengraulis mysticetus), the Pacific jack mackerel (Trachurus symmetricus), the Pacific sardine (Sardinops sagax), the northern anchovy (Engraulis mordax), and the squid (Leachia spp.) (Appendix III).
Spatial Dietary Variation Based on Scat Sample Analysis
The cumulative prey diversity curves for Los Islotes, Farallón de San Ignacio, San Pedro Nolasco, San Pedro Mártir, El Rasito, El Partido, Isla Lobos and Rocas Consag approached an asymptote, indicating in each case that we had adequate scat samples to describe sea lion diets. San Esteban and Los Cantiles, with low numbers of scats, did not reach an asymptote (Fig. 3). Thus, any conclusions regarding diet composition for these rookeries should be viewed with caution.
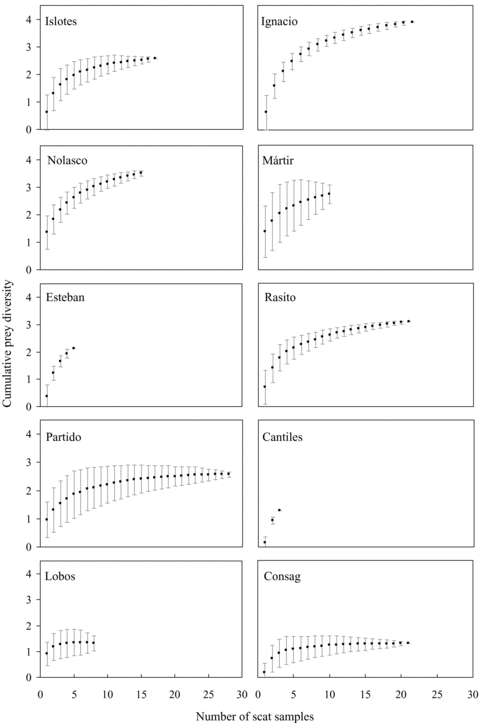
Mean cumulative prey diversity curves and its SD for California sea lions determined from scat samples collected at 10 rookeries in the Gulf of California during the breeding season of 2002. The cumulative prey diversity based on the Shannon-Wiener (H') Index (Y ) is plotted against the number of scat samples (X).
To compare diets among rookeries we considered only the prey items with IIMP values = 10% at any one rookery (18 species). Among these prey species, the rookeries in the south of the Gulf of California (Los Islotes and Farallón de San Ignacio) were represented mostly by prey with demersal habits, whereas prey with pelagic habits were more common in the central and northern regions (San Pedro Nolasco, San Pedro Mártir, San Esteban, El Rasito, El Partido, Los Cantiles, Isla Lobos, and Rocas Consag) (Fig. 4).
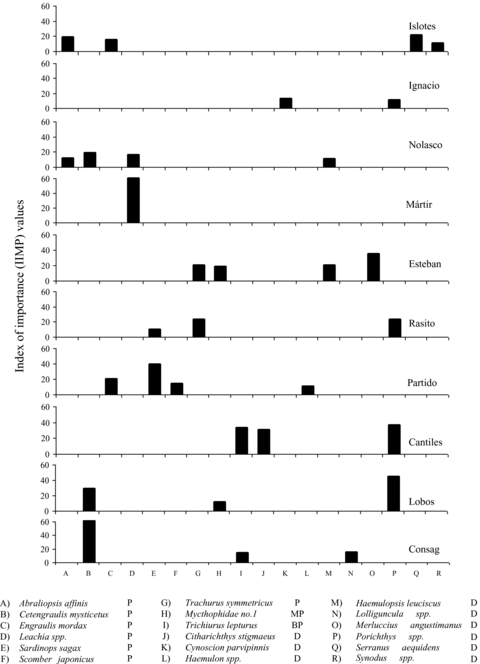
Index of importance (IIMP) values of the principal prey species (≥ 10%) identified from California sea lion scats collected during the breeding season of 2002 (P = pelagic; MP = mesopelagic; BP = benthopelagic; D = demersal; BD = bathydemersal).
We also observed differences in the proportions of fish and cephalopods among the rookeries. Both fish and cephalopods were found in the scats from Los Islotes, Farallón de San Ignacio, San Pedro Nolasco, San Pedro Mártir, El Rasito, and Rocas Consag, whereas the scats collected from the remaining rookeries contained no cephalopods. Among the rookeries that had cephalopods, San Pedro Mártir had the highest percentage (44.6%). At the rest of the rookeries, cephalopods made up less than 20% of the prey items (Appendix III).
Trophic Level
To determine the trophic level for each rookery, we only used prey items with IIMP values = 5% at any one rookery. The TLs calculated for rookeries ranged between 3.54 and 4.95 with an overall mean value of 3.95 (Appendix II). Correlations between TL data and δ15N value in 2002 were weak and not significant (Pearson's correlation: r= 0.36, 8 df, P < 0.30). After excluding two rookeries that had very few scats containing prey hard parts (San Esteban and Los Cantiles), which might have yielded anomalous TL estimates, the correlation was much stronger (Pearson's correlation: r= 0.85, 6 df, P < 0.005).
Trophic Overlap
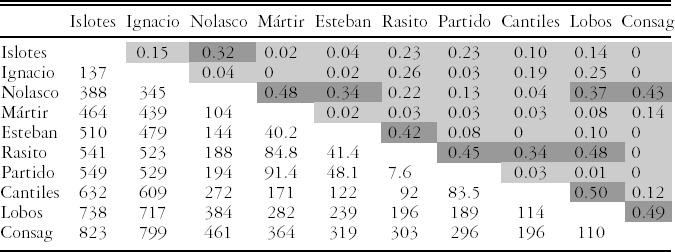
We used data for all prey to calculate trophic overlap using the Morisita-Horn index. No trophic overlap was found between the majority of the rookeries (Cλ < 0.29) (Table 2). Values between 0.30 and 0.65, which indicate a low degree of trophic overlap, were obtained for 11 pairs of rookeries. No significant correlation was found between Cλ values and the distance between the rookeries (Pearson's correlation: r=−0.23, 43 df, P= 0.13).
 |
Discussion
Isotope Fractionation Between Adult Females and Pups
The 15N-enrichment between fur from suckling pups and adult females was expected, as pups effectively forage on their mothers, who synthesize milk protein as they do other body proteins. The 13C-depleted values in pups were also expected due to the 12C-enrichment in the lipid-rich milk diet of the pups relative to the piscivorous diet of older individuals. Adult female-to-suckling pup fractionations of this magnitude and direction have been observed in tooth dentin and bone growth series from California sea lions and another otariid, the northern fur seal (Callorhinus ursinus) (Newsome et al. 2006), and offsets of this magnitude and direction are reported for other taxa as well (Hilderbrand et al. 1996, Jenkins et al. 2001, Polischuk et al. 2001). Thus while the fractionation between suckling pups and adult females was only examined at the Los Islotes rookery, we are confident that these fractionations are consistent within the species and, therefore, that we can use δ15N and δ13C values from the fur of 2–3-mo-old pups to characterize the diets of their mothers.
Inter-annual Isotope Variation
We found little difference in δ15N and δ13C values within rookeries between 2000 and 2002, suggesting inter-annual consistency in diet or foraging areas. According to Hernández-Camacho (2001), the California sea lion is highly philopatric to breeding and haul-out sites, as are numerous other pinnipeds (e.g., the northern elephant seal, Mirounga angustirostris, Reiter et al. 1981; Weddell seal, Leptonychotes weddellii, Croxall and Hiby 1983; harbor seal, Phoca vitulina, Yochem et al. 1987; Antarctic fur seal, Arctocephalus gazella, Boyd et al. 1990, Lunn and Boyd 1991; monk seal, Monachus schauinslandi, Gilmartin et al. 1993; northern fur seal, Callorhinus ursinus, Gentry 1998; southern elephant seal, Mirounga leonina, Bradshaw et al. 2003). In the case of the California sea lion, adult females in particular appear to stay near their rookeries. This may be due to the high cost of dispersion and the energetic requirements of gestation and lactation (Greenwood 1983, Clutton-Brock 1989). Adult female sea lions give birth to one pup per year and nurse it for one year or longer (Peterson and Bartholomew 1967, Newsome et al. 2006). As a consequence, nursing females must forage relatively close to their rookery sites. This in turn would tend to connect females from particular rookeries to local resources with particular environmental characteristics (Santamaría del Ángel and Álvarez-Borrego 1994).
Diet and Trophic Level
Despite the limitations of small sample size, trophic level determined for sea lions at the different rookeries by scat analysis correlated well with trophic level estimates from nitrogen isotope analysis. Although each technique had biases and uncertainties, the combination of the two approaches made it possible to characterize the diets of the California sea lion with greater precision.
Nitrogen isotope values and scat analysis suggest a clear separation between rookeries in the trophic level of their prey. Less 15N-enriched values were found mainly in the Midriff Region, especially at the San Pedro Mártir, San Esteban, El Rasito, El Partido, and Los Machos rookeries. There, the diet was mainly represented by lower trophic level prey, such as the Pacific sardine, northern anchovy, Pacific anchoveta, and cephalopods such as the squids, Leachia spp. and Abraliopsis affinis, which are abundant in this region (Markaida and Sosa-Nishizaki 2003). Conversely, the rookeries located south of the Midriff Region (Los Islotes, Farallón de San Ignacio, San Pedro Nolasco), and those north of it (Los Cantiles, Isla Granito, and Isla Lobos) had more 15N-enriched values. This is probably due to the consumption of prey such as the deep water serrano (Serranus aequidens), Pacific jack mackerel, speckled sanddab (Citharichthys stigmaeus), midshipman, bigeye scad (Selar crumenophthalmus), North Pacific hake (Merluccius productus), largehead hairtail (Trichiurus lepturus), lizardfish (Synodus spp.), California flounder (Paralichthys californicus), and shortfin weakfish (Cynoscion parvipinnis). These prey should all have higher δ15N values than clupeid and engraulid fish, since they largely forage at a higher trophic level (Garcia-Rodriguez and Aurioles-Gamboa 2004).
An anomalous case is that of the Rocas Consag rookery. It has the highest δ15N value (22.4‰± 0.5‰) of any rookery. Yet scat analysis indicates that sea lion diets there are dominated (61.2%) by Pacific anchoveta. This filter-feeding species occupies a low trophic level, which should lead to lower δ15N values. This inconsistency may relate to the location of this site near the mouth of the Colorado River. The site might experience significant 15N-enrichment at the base of the food web due to nutrient contributions from the Colorado River that are cascading up to label higher trophic levels (Aguíñiga-García 1999). If correct, this interpretation suggests that δ15N values are influenced by oceanographic conditions that exist in each region, as well as the type of diet consumed.
Trophic Overlap
When the distribution of two or more species of otariids overlaps, the species tend to utilize different food resources and, therefore, have a low degree of dietary overlap (Everitt et al. 1981, Antonelis et al. 1990, Green et al. 1990, Dellinger and Trillmich 1999, Aurioles-Gamboa and Camacho-Ríos 2007). Furthermore, recent research on the foraging ecology of some marine mammals has shown that individuals within a species feeding under similar conditions may specialize on particular prey or foraging strategies, regardless of age, sex, and morphology (Ford et al. 1999, Estes et al. 2003).
In this study, isotopic data for the fur of California sea lions from across the Gulf of California suggest some trophic segregation among rookeries, probably due to the use of different foraging areas and the consumption of different types of prey. Spatial variation in the diet of California sea lion was also observed by García-Rodríguez and Aurioles-Gamboa (2004). However, with the combination of both isotopic and scat analyses we were able to estimate dietary structure at a wider geographic range.
According to the studies of Durán-Lizárraga (1998) and Kuhn (2006), California sea lions usually conduct feeding trips of 40–50 km from their rookeries. These estimates are greater than the distances that separate some of the rookeries in the Gulf of California, such as El Rasito from El Partido (8 km) or Isla Granito from Los Cantiles (16 km). Even so, differences in isotopic values and inferred diet composition were found among these rookeries. The foraging range for animals at these rookeries may be smaller than previously reported due to local oceanographic factors that influence prey availability. Álvarez-Borrego (1983) noted that the Midriff Region, particularly in the Canal de Ballenas, has the highest nutrient concentration of the entire Gulf of California due to constant upwelling forced by strong tidal mixing. It is possible that the differences in the diet between closely spaced rookeries are a reflection of the high productivity and availability of food near the rookeries, which results in shorter feeding trips compared to other areas (García-Rodríguez and Aurioles-Gamboa 2004).
The El Rasito and El Partido rookeries had a low degree of dietary overlap based on scat analysis (Cλ= 0.45). δ13C values also differed significantly between these rookeries (MANOVA: P= 0.012), but δ15N values did not (MANOVA: P= 0.142). Animals from these rookeries had diets that contained a number of the same prey species, but these prey occurred at different IIMP values, suggesting the differential use of the resources within the same geographic region or the use of different foraging areas. In the case of Isla Granito and Los Cantiles, although we were unable to compare their trophic overlap (because of a lack of scat data from Isla Granito), the δ15N and δ13C values showed a pattern similar to that between El Rasito and El Partido, suggesting again that sea lions may be using different foraging areas.
In summary, whereas similar isotopic values between rookeries cannot be interpreted as evidence for similarity in diet, differences do indicate distinct feeding patterns and trophic segregation. Together with conventional dietary approaches, stable isotope analysis should become a routine tool for characterizing diet and how this might vary in space and time (Hobson et al. 1994).
Footnotes
Acknowledgments
We acknowledge the support given by the Fondo Mexicano para la Conservación y la Naturaleza for a research cruise, Africam Safari, Puebla México, for providing the assistance with anesthesia and equipment, Francisco García-Rodríguez for his help with the identification of otoliths and Juan Fuentes for his assistance in the sample preparation and lipid extraction. Special thanks to Anthony J. Orr who provided useful comments on an early draft and to three reviewers for constructive comments. This research was funded by grants from UC-MEXUS-CONACYT (2004) and SEP-CONACYT 2004-C01-46086. All sampling was done under permits No. SGPA/DGVS. – 0575 from the Dirección General de Vida Silvestre de la SEMERNAT for the project “Evaluación de la interacción de las pesquerías y el lobo marino Zalophus californianus y la estructura del complejo Leptospira interrogans en las Colonias reproductoras del Golfo de California,” supported by Consejo Nacional de Ciencia y Tecnología-SEMARNAT (1230).
Appendix
| Site | Sampling date | Age class | n | δ15N | δ13C |
|---|---|---|---|---|---|
| Summer 2000 | |||||
| Islotes | 16–25 July | Pups | 10 | 20.9 ± 0.6 | −15.2 ± 0.5 |
| Mártir | 16–25 July | Pups | 10 | 20.5 ± 0.4 | −14.3 ± 0.2 |
| Esteban | 16–25 July | Pups | 8 | 20.6 ± 0.2 | −14.4 ± 0.5 |
| Partido | 16–25 July | Pups | 11 | 20.4 ± 0.5 | −14.5 ± 0.4 |
| Machos | 16–25 July | Pups | 10 | 20.1 ± 0.3 | −14.6 ± 0.1 |
| Cantiles | 16–25 July | Pups | 12 | 21.6 ± 0.4 | −15.4 ± 0.2 |
| Granito | 16–25 July | Pups | 9 | 21.6 ± 0.3 | −15.1 ± 0.4 |
| Summer 2002 | |||||
| Islotes | 15 July | Pups | 10 | 21.5 ± 0.3 | −15.4 ± 0.3 |
| Ignacio | 16 July | Pups | 10 | 21.6 ± 0.2 | −14.2 ± 0.5 |
| Nolasco | 18 July | Pups | 10 | 21.3 ± 0.4 | −14.0 ± 0.2 |
| Mártir | 19 July | Pups | 10 | 20.6 ± 0.4 | −14.4 ± 0.5 |
| Esteban | 20 July | Pups | 10 | 20.8 ± 0.5 | −14.5 ± 0.5 |
| Rasito | 21 July | Pups | 10 | 20.2 ± 0.4 | −14.6 ± 0.2 |
| Partido | 22 July | Pups | 10 | 20.7 ± 0.5 | −14.0 ± 0.4 |
| Cantiles | 23 July | Pups | 9 | 21.3 ± 0.6 | −15.3 ± 0.5 |
| Granito | 23 July | Pups | 10 | 21.3 ± 0.5 | −14.7 ± 0.3 |
| Lobos | 24 July | Pups | 10 | 21.5 ± 0.3 | −14.8 ± 0.4 |
| Consag | 26 July | Pups | 9 | 22.4 ± 0.5 | −14.1 ± 0.5 |
| Spring 2003 | |||||
| Islotes | April | Adult females | 8 | 19.4 ± 0.2 | −14.6 ± 0.1 |
| Summer 2004 | |||||
| Jorge | 18 July | Pups | 10 | 21.1 ± 0.7 | −14.1 ± 0.4 |
| Total | 196 | ||||
| Site | Sampling date | Scats | Scats with prey hard parts | Otoliths | Beaks | S | TL |
|---|---|---|---|---|---|---|---|
| Islotes | 9–30 July | 22 | 17 | 182 | 21 | 14 | 3.93 |
| Ignacio | 6 and 16 July | 30 | 23 | 42 | 4 | 18 | 4.04 |
| Nolasco | 18 July | 20 | 16 | 75 | 20 | 15 | 3.59 |
| Mártir | 19 July | 13 | 11 | 26 | 31 | 15 | 3.43 |
| Esteban | 20 July | 17 | 5 | 11 | — | 5 | 4.39 |
| Rasito | 21 July | 36 | 21 | 140 | 2 | 14 | 3.54 |
| Partido | 22 July | 46 | 29 | 178 | — | 15 | 3.66 |
| Cantiles | 23 July | 16 | 3 | 17 | — | 3 | 4.95 |
| Granito | 23 July | 14 | — | — | — | — | — |
| Lobos | 24 July | 19 | 9 | 50 | — | 6 | 3.99 |
| Consag | 26 July | 41 | 21 | 81 | 6 | 5 | 4.07 |
| Total/Average | 274 | 155 | 802 | 84 | 3.95 |
| Family | Prey species | Common name | TL | Islotes | Ignacio | Nolasco | Mártir | Esteban | Rasito | Partido | Cantiles | Lobos | Consag | Average IIMP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fish | ||||||||||||||
| Argentinidae | Argentina sialis | North Pacific argentine | 3.1 | - | - | - | 8.25 | - | 2.38 | - | - | - | - | 1.06 |
| Aulopidae | Aulopus bajacali | Eastern Pacific flagfin | 3.07 | 8.11 | - | - | - | - | - | - | - | - | - | 0.81 |
| Batrachoididae | Porichthys spp. | midshipman | 3.71 | 6.72 | 10.65 | 2.57 | - | - | 22.54 | 0.66 | 36.11 | 44.44 | - | 12.37 |
| Carangidae | Selar crumenophthalmus | bigeye scad | 4.1 | 0.59 | 5.56 | - | - | - | - | 1.15 | - | - | - | 0.73 |
| Trachurus symmetricus | Pacific jack mackerel | 3.56 | - | - | 2.45 | 1.14 | 20 | 23.36 | 9.20 | - | - | - | 5.61 | |
| Clupeidae | Sardinops sagax | Pacific sardine | 2.43 | 2.97 | - | - | 2.27 | - | 10.09 | 39.42 | - | - | - | 5.47 |
| Cynoglossidae | Symphurus spp. | —– | 3.28 | - | - | - | - | - | - | 0.66 | - | - | 8.21 | 0.89 |
| Engraulidae | Cetengraulis mysticetus | Pacific anchoveta | 2.67 | - | - | 18.75 | 9.09 | - | - | - | - | 28.70 | 61.23 | 11.78 |
| Engraulis mordax | northern anchovy | 2.96 | 15.29 | 1.11 | 9.61 | - | - | 7.14 | 20.29 | - | - | - | 5.35 | |
| Gobiidae | Bollmania spp. | goby | 3.22 | - | - | - | - | - | - | - | - | 5.56 | - | 0.56 |
| Haemulidae | Anisotremus davidsonii | xantic sargo | 3.54 | - | 3.24 | - | - | - | - | - | - | - | - | 0.32 |
| Haemulon spp. | grunt | 3.3 | - | - | - | - | - | - | 10.51 | - | - | - | 1.05 | |
| Haemulopsis leuciscus | white grunt | 3.03 | - | - | 10.63 | - | 20 | - | - | - | 2.78 | - | 3.34 | |
| Labridae | Bodianus diplotaenia | Mexican hogfish | 3.44 | 0.24 | - | - | 0.70 | - | - | - | - | - | - | 0.09 |
| Macrouridae | Caelorinchus scaphopsis | shoulderspot grenadier | 3.07 | - | - | - | 1.40 | - | - | - | - | - | - | 0.14 |
| Malacanthidae | Caulolatilus princeps | Ocean whitefish | 3.01 | 3.59 | - | - | - | - | - | - | - | - | - | 0.36 |
| Merlucciidae | Merluccius angustimanus | Panama hake | 3.44 | - | - | 6.25 | - | 35 | 7.30 | - | - | - | - | 4.86 |
| Merluccius productus | North Pacific hake | 4.35 | - | - | 3.31 | - | - | - | - | - | - | - | 0.33 | |
| Moridae | Physiculus nematopus | charcoal mora | 3.4 | - | 6.94 | - | 0.70 | - | 3.06 | - | - | - | - | 1.07 |
| Myctophidae | Myctophidae no.1 | lanternfish | 3.01 | - | - | 6.51 | 2.27 | 18.33 | 3.81 | 0.16 | - | 11.11 | - | 4.22 |
| Ophidiidae | Brotula spp. | brotula | —– | - | 2.78 | - | - | - | - | - | - | - | - | 0.28 |
| Chilaria taylori | spotted cusk-eel | 4.07 | - | - | - | - | - | - | 0.33 | - | - | - | 0.03 | |
| Lepophidium prorates | prowspine cusk eel | 3.15 | - | - | - | - | - | - | - | - | 7.41 | - | 0.74 | |
| Ophidiidae no.1 | cusk eel | 3.51 | - | 6.48 | - | - | - | - | 0.49 | - | - | - | 0.70 | |
| Paralichthyidae | Citharichthys stigmaeus | speckled sanddab | 3.69 | - | - | - | 3.22 | - | - | 1.81 | 30.56 | - | - | 3.56 |
| Paralichthys californicus | California flounder | 4.5 | - | 5.56 | - | - | - | - | - | - | - | - | 0.56 | |
| Sciaenidae | Cynoscion parvipinnis | shortfin weakfish | 4.5 | - | 12.96 | - | - | - | - | - | - | 1.30 | ||
| Roncador stearnsii | spotfin croaker | 3.31 | - | 5.09 | - | - | - | - | - | - | - | - | 0.51 | |
| Sciaenidae no.1 | —– | —– | - | 4.56 | - | - | - | - | - | - | - | - | 0.45 | |
| Scombridae | Scomber japonicus | chub mackerel | 3.09 | - | - | 0.69 | - | - | 5.44 | 13.85 | - | - | - | 2.00 |
| Scorpaenidae | Pontinus furcirhinus | red scorpionfish | 3.34 | - | 5.56 | - | - | - | - | - | - | - | - | 0.56 |
| Scorpaenidae no.1 | —– | —– | - | - | - | - | - | 2.38 | - | - | - | - | 0.24 | |
| Sebastidae | Sebastes exsul | buccaneer rockfish | 3.08 | - | - | - | 1.40 | - | - | - | - | - | - | 0.14 |
| Sebastes macdonaldi | Mexican rockfish | 3.11 | - | - | - | 4.55 | - | - | - | - | - | - | 0.45 | |
| Serranidae | Diplectrum pacificum | inshore sand perch | 3.99 | - | 8.33 | - | - | - | - | - | - | - | - | 0.83 |
| Hemanthias peruanus | splittail bass | 2.66 | 0.59 | - | - | - | - | - | - | - | - | - | 0.06 | |
| Pronotogrammus eos | bigeye bass | 3.04 | 5.88 | 1.39 | - | - | - | - | - | - | - | - | 0.73 | |
| Pronotogrammus multifasciatus | threadfin bass | 3.1 | 4.85 | - | 4.38 | 2.27 | - | 4.76 | - | - | - | - | 1.63 | |
| Serranidae no.1 | —– | —– | - | - | - | - | - | - | 1.15 | - | - | - | 0.11 | |
| Serranus aequidens | deepwater serrano | 3.33 | 20.82 | - | - | - | - | - | - | - | - | - | 2.08 | |
| Synodontidae | Synodus spp. | lizardfish | 4.53 | 11.51 | 4.17 | - | - | 6.67 | - | 0.16 | - | - | - | 2.39 |
| Trichiuridae | Trichiurus lepturus | largehead hairtail | 4.45 | - | - | - | - | - | - | - | 33.33 | - | 13.89 | 4.72 |
| Cephalopods | ||||||||||||||
| Alloposidae | Haliphron atlanticus | Seven-arm octopus | 3.2 | - | - | - | - | - | 4.76 | - | - | - | - | 0.48 |
| Cranchiidae | Leachia spp. | squid | 3.2 | - | - | 16.25 | 60.34 | - | - | - | - | - | - | 7.66 |
| Enoploteuthidae | Abraliopsis affinis | squid | 3.2 | 18.18 | - | 11.10 | 1.27 | - | - | - | - | - | - | 3.06 |
| Loliginidae | Lolliguncula spp. | squid | 3.2 | - | - | - | - | - | - | - | - | - | 14.29 | 1.43 |
| Octopodidae | Octopus spp. | octopus | —– | - | 4.44 | - | - | - | - | - | - | - | - | 0.44 |




