Estimating age of harbor seals (Phoca vitulina) with incisor teeth and morphometrics
Abstract
We compared annuli counts from sets of canine, postcanine, and incisor teeth from 450 subsistence-harvested harbor seals, submitted blind to a laboratory. Postcanine and incisor ages were highly correlated with canine age estimates (r= 0.985 and r= 0.984, respectively), as were postcanine and incisor teeth (r= 0.984). Age estimates from teeth of 23 known-aged seals were highly correlated; canine teeth r= 0.987; postcanine r= 0.996; incisor r= 0.992, although age for all tooth-types was underestimated for a 29-yr-old seal. Incisor estimates were variable; comparison of age estimates from two incisors/individual (n= 42) was r= 0.992 if only high-quality age estimates were used and r= 0.705 if lower-quality estimates were used. Morphometrics and incisor-based ages of 164 live-captured seals were explored to derive a method of estimating ages of harbor seals when age estimates are needed immediately; 39 seals were of known age. Curvilinear length, mass, and axial girth were most predictive of age for females, and curvilinear length and mass for males (equations for morphometrically calculating ages are given). Morphometric-based age estimates were highly correlated with known ages (r= 0.896) and incisor-based estimates (r= 0.904) and discrepancies between known and morphometric-based ages were small for younger seals. Morphometric-based age estimates also accurately distinguished between young and mature individuals.
Obtaining age estimates for wild, live-captured animals is essential for studies of age-specific survival, age structure of populations, or age-related physiology. Ages of wild mammals can be estimated by counting annual growth rings in the cementum of teeth (Bowen 1982, Dietz et al. 1991, Arnbom et al. 1992, Bernt et al. 1996, Childerhouse et al. 2004). For most species, including pinnipeds, canine teeth have been recommended as providing the best estimate of age (Scheffer 1950, Mansfield and Fisher 1960, Bowen et al. 1983, Dietz et al. 1991, Mansfield 1991, Bernt et al. 1996), but extraction of canines from live animals is extremely deleterious (Biknevicius and Van Valkenburgh 1996). Postcanine (premolar) teeth have been used successfully to estimate age in many species and may be the best choice for estimating age of live individuals (Bowen 1982, Arnbom et al. 1992, Troy et al. 1999, Childerhouse et al. 2004). Although extraction of postcanines in seals leaves only a small wound that does not require suturing and causes no long-term detrimental effects (Arnbom et al. 1992), the incisor is smaller than the postcanine with a shorter root, thus its extraction is less invasive. In some species, cementum annuli of incisor teeth provide a reasonable estimate of age (Arnbom et al. 1992, Bernt et al. 1996); however, variability of age estimates is greater with incisors (Bernt et al. 1996). Dietz et al. (1991) validated the accuracy of age estimates of harbor seals using cementum annuli in canine teeth. Incisors also have been used to estimate age of harbor seals (Phoca vitulina, Lydersen and Kovacs 2005) based on their use in gray seals (Halichoerus grypus, Bernt et al. 1996). But no study, to our knowledge, has explored the accuracy of using counts of cementum annuli in incisors to estimate harbor seals ages.
Our objectives in this study were twofold: (1) to determine the accuracy of estimating the age of harbor seals from counts of cementum annuli of incisor teeth, and (2) to develop a method of using morphometrics to estimate ages of harbor seals when extraction of teeth is impractical or when reasonably accurate age estimates are needed immediately. Reliable instantaneous age estimates are often desirable for research projects that use radio telemetry because, depending on the hypotheses being tested, researchers may preferentially place telemetry equipment on reproductively mature individuals, or alternatively, on young-of-the-year and yearlings.
Methods
Age Estimates from Teeth
To extract teeth from live-captured harbor seals, we induced anesthesia by administering isoflourane through a face mask until seals were sufficiently anesthetized to pass an endotracheal tube. Once the seal was intubated, we continued administering isoflourane until general anesthesia and sufficient analgesia were obtained for tooth extraction. The mouth was held open with a mouth gag typically used for dental work in veterinary medicine, and a tooth elevator, also used in veterinary medicine for dental work in dogs, was inserted into the gum along each side of the incisor and used to gently rock the tooth back and forth. Once the tooth was loose it was grasped with a tooth extractor, placing the tips of the extractor at the base of the incisor, and the tooth was gently pulled straight out. Following tooth extraction, an antiseptic solution was used to cleanse the area and direct pressure was applied for approximately one minute to stop blood flow and promote clotting.
In addition to extracting incisor teeth from live-captured seals, we obtained teeth from 450 subsistence-harvested seals acquired via a biosampling program conducted by the Alaska Native Harbor Seal Commission (ANHSC). Skulls from these seals were housed at the University of Alaska Museum (UAM) Frozen Tissue Collection in Fairbanks. Teeth were extracted by UAM personnel and provided to the Alaska Department of Fish and Game (ADF&G) for this study. For subsistence-harvested seals, we obtained a combination of at least two teeth from each individual, including canine, postcanine, and incisor teeth. Canine teeth were extracted for most individuals, the postcanine tooth was taken from only a subset of sampled seals, and 57 seals had two incisors removed to assess variability of age estimates from incisors.
Four of the subsistence-harvested seals and 17 live-captured seals had been previously captured and tagged as pups or yearlings by ADF&G and were thus of known ages when taken during a subsistence hunt or recaptured by ADF&G. An incisor, postcanine, and canine tooth also were obtained from two known-age harbor seals that died from natural causes at the Alaska SeaLife Center (ASLC). We submitted teeth from all seals to a well-established laboratory (Matson's Laboratory LLC, Milltown, MT) with >30 yr experience estimating ages by counting cementum annuli from teeth of other species. All teeth submitted to the laboratory were randomly numbered such that the laboratory had no method of determining which teeth came from which seal. In addition to estimating age for each tooth, the laboratory provides a rating of their confidence in the reliability of age estimates, with a Certainty Code of A being higher than B.
Laboratory Methods
At Matson's Laboratory, teeth were cleaned in a hot water bath (at least 80°C, but never hot enough to boil), followed by firm wiping with nylon mesh to remove dirt. Teeth were treated with acid to remove calcium, leaving behind a collagen matrix. Samples were then rinsed in water to remove acid, treated with isopropyl alcohol to remove water, and toluene to remove alcohol. Next, samples were treated in melted paraffin, allowing paraffin to penetrate into tooth tissue, followed by placement into paraffin embedding molds (Fisher Scientific), which hardens while cooling. Teeth in paraffin blocks were sectioned at 14 microns using a rotary microtome, mounted on glass slides, and dried on warming plates. Slides were treated with toluene to remove paraffin, then hydrated and dried. Giemsa blood stain (Ricca Chemical Company, Arlington, TX; Cat. No. 3250-16) was used to stain the annuli purple and the other tissue blue and pink. A cover slip was affixed to the slide using resin, and annuli were counted at 40×, 60×, 100×, or 160× using a compound microscope with transmitted light. One person with >30 yr experience read all samples. Teeth were aged assuming a 1 June birthday, and that annulus was formed in winter. The first annulus was not always visible at the tip; in most samples the first annulus clearly visible at the tip was the second year annulus, formed in the second winter of life. Further details about methods used by Matson's Laboratory are proprietary.
Age Estimates from Morphometrics
Morphometrics were obtained from 164 live-captured, anesthetized seals from which incisor teeth were also extracted. Body mass was measured to the nearest 0.1 kg, and standard length, curvilinear length, axial girth, maximum girth, and hip girth were measured to the nearest cm. Age was determined from the annuli of an incisor tooth adjusted for calendar date by adding the tooth age plus the percentage of the year from 10 June (assumed birth date for all seals) to the capture date; this assumes that the tooth annuli count accurately reflects seal age.
Based on these morphometrics, we developed the predictive equation  ; we used linear regression to fit the transformed equivalent (Montgomery and Peck 1982) ln (age) = ln (β0) +β*1x, where ln(age) is the natural logarithm of age, β0 and β1 are the regression coefficients, and x is the predictor variable. Initially we fit models with each predictor separately with either a single linear term, or linear and quadratic terms. Next, we used the best of the individual models, based on the bias-corrected version of Akaike's Information Criteria (AICc, Burnham and Anderson 1998), and added the other predictors, again determining the best model based on AICc. Analyses were conducted separately for females and males. We also assessed using a single break point based on curvilinear length for classifying seals as immature or adult. This break point was the curvilinear length that best separated younger seals from adults and was determined graphically.
; we used linear regression to fit the transformed equivalent (Montgomery and Peck 1982) ln (age) = ln (β0) +β*1x, where ln(age) is the natural logarithm of age, β0 and β1 are the regression coefficients, and x is the predictor variable. Initially we fit models with each predictor separately with either a single linear term, or linear and quadratic terms. Next, we used the best of the individual models, based on the bias-corrected version of Akaike's Information Criteria (AICc, Burnham and Anderson 1998), and added the other predictors, again determining the best model based on AICc. Analyses were conducted separately for females and males. We also assessed using a single break point based on curvilinear length for classifying seals as immature or adult. This break point was the curvilinear length that best separated younger seals from adults and was determined graphically.
Accuracy of Age Estimation Methods
We assessed the consistency of age estimates derived from counts of cementum annuli in teeth by examining the distribution of the discrepancies among age estimates from the same seal. In addition, we calculated Pearson correlation coefficients based on the paired age estimates. We examined the accuracy of the age estimates from known-age seals; six based on canines, six from postcanines, 15 from incisors, and 33 from morphometrics (15 morphology-based estimates were from the same animals at different ages).
In previous years researchers in our group had assigned an age class (“field age”) based on visual examination of seals following their capture. To assess our accuracy of subjective estimation of age classifications, we compared our field ages (pup, yearling, subadult, adult [=4 for females, =6 for males]) for a larger data set (n= 544 seals captured from 2003 through 2006) with tooth-based age estimates from incisors of 216 of those seals. To evaluate whether morphometrically derived ages were a better alternative than visual (field) aging, we also compared morphology-based estimates to tooth-based estimates of age for all seals among the 544 that had data for both methods.
Distinguishing Between Immature and Reproductively Mature Seals
We determined how well morphometrically derived age estimates predicted whether seals were mature (i.e., female age < 4 vs. age ≥ 4; male age < 6 vs. age ≥ 6). We estimated misclassification probability as the number of seals assigned to the incorrect age class, divided by the total number of seals classified. These ages were selected as cut-off points based on a study conducted by Lydersen and Kovacs (2005) that determined average age of sexual maturity for harbor seals by measuring estradiol levels in females and testosterone in males. Other studies based on necropsies of harbor seals noted that both sexes typically reach sexual maturity at ages similar to those reported by Lydersen and Kovacs (2005), and average age of first reproduction for female harbor seals throughout their distribution is generally between 4- and 5-yr old (Pitcher 1977, Pitcher and Calkins 1979, Härkönen and Heide-Jørgensen 1990, Bjørge 1992, Lydersen and Kovacs 2005, Hauksson 2006).
Results
Seal Observations After Tooth Extraction
Four seals were recaptured 1 yr after tooth extraction and showed no ill effects as a result of the extraction. An additional 118 individuals were radio tracked following tooth extraction and no mortalities were detected. The average number of days between tooth extraction and last-known location was 443 d (SE = 33, range 4–1,324), although our telemetry results were confounded by indications of transmitter loss or failure, resulting in few relocations for some individuals before radio signals were censored.
Consistency of Age Estimates Among Teeth
Age estimates from canine and postcanine teeth from the same seal generally agreed well, with no systematic bias (i.e., median discrepancy = 0 and symmetric maximum discrepancies) and 90% of the discrepancies were ≤ 1 irrespective of the quality (i.e., Certainty Code provided by Matson's Laboratory) of the age estimates (Table 1). Comparisons involving incisor-based age estimates varied with the Certainty Code of the estimates. If both teeth in a comparison were rated with a Certainty Code of A, the distribution of the discrepancies was similar to comparisons of canine and postcanine estimates (i.e., 0 median discrepancy, 90% of discrepancies ≤ 1; Table 1). Comparisons involving incisor-based estimates were more variable when at least one of the estimates was Certainty Code B; although the median discrepancy was still 0, the 90% range extended beyond 1. In a few instances, the most extreme discrepancies were when age estimates from incisors were much lower than from other teeth (Table 1). Correlations between age estimates from different teeth generally followed the pattern seen with the discrepancies; r= 0.984 for “A” Certainty Codes, with lower Certainty Codes for incisor estimates leading to lower correlations (r= 0.705) than other comparisons (Table 1).
| Typea | Certaintyb | n | r | Median | 90% rangec | Maximumd |
|---|---|---|---|---|---|---|
| ca – pc | A | 197 | 0.985 | 0 | −1, 1 | −5, 4 |
| ca – pc | AB | 38 | 0.930 | 0 | −1, 1 | −4, 3 |
| ca – in | A | 363 | 0.984 | 0 | −1, 1 | −4, 8 |
| ca – in | AB | 86 | 0.759 | 0 | −1, 4 | −3, 12 |
| pc – in | A | 188 | 0.984 | 0 | −1, 1 | −3, 6 |
| pc – in | AB | 31 | 0.914 | 0 | −3, 2 | −4, 4 |
| in – in | A | 42 | 0.992 | n/ae | ≤1 | 4 |
| in – in | AB | 15 | 0.705 | n/a | ≤4 | 5 |
- aca = canine, pc = postcanine, in = incisor; values are based on the age from the first type of tooth minus the age from the second type of tooth.
- bA = both age estimates of the highest (i.e., A) certainty code, AB = at least one estimate in the pair of lower (i.e., B) certainty code.
- cThe inclusive range that contains 90% of the observed differences in age estimates.
- dThe maximum positive and negative differences in age estimates.
- eValues for incisors are based on the absolute value of the differences between age estimates (i.e., all values ≥ 0), hence the median is uninformative, the 90% range is given as a one-sided interval, and there is a single maximum discrepancy.
Comparison of Tooth Ages with Known Age of Seals
Although the sample size was small, age estimates from teeth of known-aged seals (Table 2) were highly correlated with their actual ages (canine r= 0.987; postcanine r= 0.996; incisor r= 0.992). These correlations were based on use of all estimates of ages provided for all teeth submitted regardless of Certainty Code. Discrepancies between estimated and known ages were generally larger for older seals, especially for the 29-yr-old captive seal. Although often considered to be the most reliable, canine-based age estimates were not always the most accurate (Table 2). Discrepancies between estimated and known ages were most often negative, indicating that annuli were missed and ages underestimated. A partial explanation for the observed discrepancies likely was because tooth-based estimates, unlike known ages, were not adjusted for the time of year when the sample was acquired.
| Sex | Known age | Canine age (r= 0.987) | Postcanine age (r= 0.996) | Incisor age (r= 0.992) | Morphology-based age (r= 0.896) |
|---|---|---|---|---|---|
| F | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| M | 2.5 | 3 (0.5) | 2 (−0.5) | 3 (0.5) | |
| M | 3.5 | 3 (−0.5) | 3 (−0.5) | 3 (−0.5) | |
| M | 3.5 | 5 (1.5) | 4 (0.5) | 3 (−0.5) | |
| F | 6.5 | 3 (−3.5) | 4 (−2.5) | 5 (−1.5) | |
| F | 29 | 25 (−4.0) | 21 (−8.0) | 17 (−12.0) | |
| F1 | 0.04 | 0.42 (0.38) | |||
| F | 0.05 | 0.29 (0.24) | |||
| F2 | 0.05 | 0.38 (0.33) | |||
| F3 | 0.05 | 0.43 (0.38) | |||
| F4 | 0.05 | 0.47 (0.42) | |||
| F5 | 0.05 | 0.50 (0.45) | |||
| F4 | 1.05 | 1 (−0.05) | 0.55 (−0.50) | ||
| F6 | 1.08 | 1 (−0.08) | 0.74 (−0.34) | ||
| F6 | 1.71 | 1.50 (−0.21) | |||
| F1 | 1.86 | 1 (−0.86) | 1.39 (−0.47) | ||
| F2 | 4.05 | 3 (−1.05) | 3.46 (−0.59) | ||
| F3 | 4.05 | 4 (−0.05) | 5.69 (1.64) | ||
| F5 | 4.71 | 11.07 (6.36) | |||
| M1 | 0.05 | 0.09 (0.04) | |||
| M2 | 0.05 | 0.09 (0.04) | |||
| M3 | 0.05 | 0.22 (0.17) | |||
| M4 | 0.06 | 0.12 (0.06) | |||
| M5 | 0.07 | 0.11 (0.04) | |||
| M6 | 0.87 | 1 (0.13) | 0.93 (0.06) | ||
| M2 | 1.04 | 0.63 (−0.41) | |||
| M5 | 1.04 | 1.05 (0.01) | |||
| M7 | 1.04 | 0.37 (−0.67) | |||
| M | 1.05 | 0.48 (−0.57) | |||
| M3 | 1.05 | 1.13 (0.08) | |||
| M4 | 1.05 | 0.30 (−0.75) | |||
| M8 | 1.06 | 0.18 (−0.88) | |||
| M9 | 1.08 | 1 (−0.08) | 0.63 (−0.45) | ||
| M6 | 1.89 | 1.28 (0.61) | |||
| M9 | 2.05 | 1.81 (−0.24) | |||
| M7 | 2.05 | 1.91 (−0.14) | |||
| M1 | 3.08 | 4.76 (1.68) | |||
| M3 | 3.87 | 3 (−0.87) | 6.34 (2.47) | ||
| M8 | 4.08 | 3 (−1.08) | 6.12 (2.04) |
- F = female, M = male; superscript numbers (e.g., F1, M1) indicates same animal recaptured at a later date.
- Estimated ages are based on counts of cementum annuli of canine, postcanine, and incisor teeth, and from morphometrics. Correlation coefficients are comparisons with known ages. Numbers in parentheses are differences between known age and estimated age. Correlation between morphology-based and incisor-based estimates for known-age seals was r= 0.904.
Morphometric-based Estimates
The morphometrics we obtained all were highly correlated (r > 0.81), so only one to three predictors (curvilinear length, mass, and axial girth) were needed in the regression models. Curvilinear length likely was a better predictor than standard length because, while curvilinear length involves running a tape measure the length of the spine during which any curvature in the spine can be matched and measured, standard length involves holding the tape on a parallel plane to (above) the seal, with an observer at either end sighting down the tape to the tip of the nose and the tip of the tail. The latter measurement introduces three possible sources of measurement error: measuring plane not parallel; straight-line distance may not incorporate curvature in the spine of the seal; observer sighting-angle from tape to end-point of the seal is angled rather than perpendicular.
For each sex there were two models with very similar AICc values (Table 3); each model differing from its pair by an additional variable. To assess the accuracy of predictions based on these morphometric equations, we used the model for each sex with the smaller median residual. The prediction accuracy of these models was moderate for older animals where the predictors changed little for a wide range of ages, once a seal reached adult (maximum) body size.
| Sex | Intercept | Curved length | (Curved length)2 | Mass | (Mass)2 | Axial girth | AICc | r 2 | Median residual | Rresidual error |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | −5.2765 | 0.03587 | 0.02875 | 223.9 | 0.702 | 0.133 | 0.4308 | |||
| (0.8577) | (0.0096) | (0.01005) | ||||||||
| −2.6927 | 0.03114 | 0.05593 | −0.03537 | 223.4 | 0.709 | 0.095 | 0.4199 | |||
| (1.7586) | (0.0099) | (0.01901) | (0.0211) | |||||||
| Male | −19.727 | 0.2362 | −0.00062 | 105.6 | 0.837 | 0.017 | 0.3518 | |||
| (4.6345) | (0.06719) | (0.00024) | ||||||||
| −22.629 | 0.2874 | −0.00086 | 0.000092 | 105.2 | 0.846 | 0.042 | 0.3333 | |||
| (4.8116) | (0.07175) | (0.00027) | (0.000053) |
- 1Coefficients are for the linearized model, and the resulting prediction needs to be exponentiated. For example, the equation for the first model for females is: age = exp(−5.2765 + 0.03587 * length + 0.02875*mass), where “exp” indicates the exponential function.
For our known-age animals, most of which were younger, correlation between morphology-based ages vs. known ages and incisor ages was r > 0.90 (Table 2). Morphology-based age estimates were within 1 yr of the known age for seals ≤ 3-yr old (Table 2), however, older pups and yearlings, especially males, could not be reliably classified into the correct age group (Table 4). Seals > 3-yr old had larger discrepancies between morphology-based age and known age (Table 2).
| (a) Females | |||||
|---|---|---|---|---|---|
| Field age | |||||
| 0 | 1 | 2–3 | > 4 | ||
| Tooth age | 0 | 26 | 18 | 5 | 0 |
| 1 | 1 | 32 | 18 | 2 | |
| 2–3 | 0 | 3 | 10 | 6 | |
| > 4 | 0 | 0 | 2 | 32 | |
| (b) Males | |||||
| Field age | |||||
| 0 | 1 | 2–5 | > 6 | ||
| Tooth age | 0 | 9 | 11 | 0 | 0 |
| 1 | 1 | 7 | 5 | 0 | |
| 2–5 | 0 | 1 | 8 | 7 | |
| > 6 | 0 | 0 | 0 | 12 | |
| (c) Females | |||||
| Morphology age | |||||
| 0 | 1 | 2–3 | > 4 | ||
| Tooth age | 0 | 41 | 8 | 0 | 0 |
| 1 | 7 | 38 | 6 | 2 | |
| 2–3 | 0 | 5 | 12 | 2 | |
| > 4 | 1 | 0 | 3 | 31 | |
| (d) Males | |||||
| Morphology age | |||||
| 0 | 1 | 2–5 | > 6 | ||
| Tooth age | 0 | 18 | 2 | 0 | 0 |
| 1 | 7 | 5 | 1 | 0 | |
| 2–5 | 0 | 1 | 4 | 11 | |
| > 6 | 0 | 0 | 0 | 12 | |
Identifying Adults
We have three methods that can be used to separate younger seals from adults (i.e., female age < 4 vs. age ≥ 4; male age < 6 vs. age ≥ 6; Lydersen and Kovacs 2005) in a field situation: predictive morphometric-based equations, length-based cut points, and subjective “field” classification. In predicting whether seals were mature, morphometric-based age estimates produced reasonable predictions of incisor-based ages for females with a misclassification probability of 0.051 using the equation (Table 4) or a misclassification probability of 0.045 for a cut point of 142 cm (Fig. 1). For males, a single cut point with a curvilinear length of 162 cm (Fig. 2) was superior (misclassification probability of 0.033) to the predictive equation (misclassification probability of 0.180), which had difficulty correctly assigning older subadults (Table 4); adult males were correctly classified by both methods.
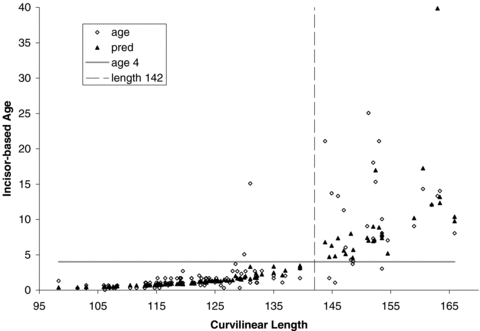
Curvilinear length of female harbor seals live-captured in Alaska relative to incisor-based age estimates; predicted values are based on curvilinear length, mass, and axial girth. Vertical dashed line delineates cut-point for adult (i.e., sexually mature) vs. immature harbor seals.
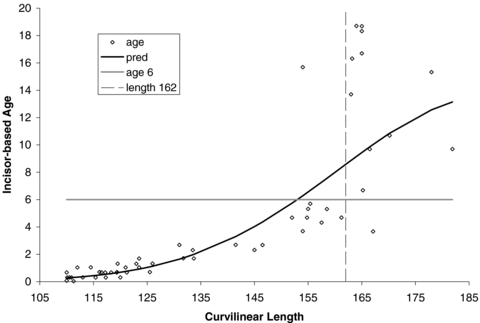
Curvilinear length of male harbor seals live-captured in Alaska relative to incisor-based age estimates. Vertical dashed line delineates cut-point for adult (i.e., sexually mature) vs. immature harbor seals.
Utility of Subjective “Field” Age Classes
Subjective “field” classification of age was only moderately successful, often classifying seals as one age class older than indicated by the incisor-based age estimate (Table 4a, b). This pattern was evident for both sexes, although slightly more pronounced for males. It should be kept in mind that age estimates from teeth tend to be slightly low, so some of the difference between field and tooth-based age estimates could be due to errors in the tooth estimates, which would contribute to the observed pattern. Morphology-based estimates are somewhat more accurate than field classifications for females (Table 4c), but still have high misclassification probabilities for yearling and subadult males (Table 4d).
Discussion
The initial report from Matson's Laboratory, evaluating results from all three tooth types stated that “Among the three tooth types, the canine is likely to be the most accurately aged by cementum because annuli are the most distinct and most consistently occur in a regular pattern.” Others also have stated that canine teeth are the best tooth to use for accurate age estimation for most species (Scheffer 1950, Mansfield and Fisher 1960, Bowen et al. 1983, Dietz et al. 1991, Mansfield 1991, Bernt et al. 1996).
Based on our small sample of known-age seals, however, the canine-based age estimates were not always the most accurate, except possibly for very old animals. Indeed, as the laboratory that we used gained more experience at estimating age from harbor seal teeth they reported that all tooth types examined had distinct annuli, but smaller teeth were difficult to histologically section and canine teeth of harbor seals were too large to be efficiently processed. Therefore, when extracting teeth for age estimates during necropsies, postcanine teeth may be the best choice for aging harbor seals. However, given the generally good agreement between canine-based age estimates and those from incisors and postcanines, and the considerably smaller size of incisors compared to postcanines, we recommend using incisors for age estimates of live-captured harbor seals to minimize trauma to seals during tooth extraction.
As was noted by Bernt et al. (1996), we found variability in age estimates with incisors, as indicated by a lower correlation when comparing age estimates from two incisors extracted from the same individual. Nonetheless, consistency of age estimates from the two incisors was reasonable and, when both teeth yielded age estimates that the laboratory had high confidence in, estimates were highly correlated and observed differences for 90% of the comparisons were within one year of each other (Table 1). Although we only have known ages for 15 harbor seals to compare the accuracy of estimating ages with incisors, the discrepancies between age estimates obtained from incisor teeth and actual age are low, and the correlation of those estimates is high (Table 2). Our study provides evidence that counts of cementum annuli in incisor teeth provide reasonably accurate estimates of age for harbor seals.
It should be recognized, however, that tooth-based age estimates can occasionally differ substantially from the true age (usually an underestimate) and this apparently is more common for teeth with Certainty Codes < A. We therefore also recommend using a laboratory or lab technicians experienced in aging teeth with cementum annuli to improve reliability of age estimates. As the laboratory that we used gained more experience in reading harbor seal teeth, a higher proportion of teeth that they examined received Certainty Codes of A (increasing from 77% for incisors in the first batch, to 90% and 97% with later batches of incisors).
Similar to the findings of Bernt et al. (1996) for gray seals, our results (Table 2) indicate that aging with incisors may result in underestimated ages for older animals, likely as a result of an allometric relationship—errors in counts of annuli will be larger with more annuli present to be counted. Unfortunately, we had only one older, known-aged individual and annuli counts of all three tooth types underestimated the true age of this captive-raised seal (Table 2). Because large numbers of cementum annuli are more easily counted in larger teeth, if accuracy of age estimates for older live-captured seals is essential to a particular study, extracting the larger postcanine tooth may result in more reliable estimates of age than an incisor. For estimates of age for younger live-captured harbor seals, particularly when attempting to distinguish between adults and younger animals that likely have not reached sexual maturity, our results indicate that cementum annuli from incisors provides reasonably accurate age estimates, and little is gained by the more invasive extraction of postcanine teeth.
When estimates of age are needed rapidly in the field, or when tooth extraction is impractical, visually assigning ages to distinguish between yearlings, subadults, and adults is subject to researcher variability and may result in inconsistent age classification for harbor seals. Our comparison of results of our assignment of “field ages” to harbor seals compared with age estimates from incisors (Table 4) clearly demonstrates that using a method of systematically estimating ages for younger animals is superior.
To separate adults from younger seals, we recommend using cut points of curvilinear length of 142 cm for females and 162 cm for males; however, these specific values should be tested before being applied in other regions. Similar to the results of Lydersen and Kovacs (2005), we found that predicting actual age of adult seals based on morphometrics was difficult because individual length varies widely for adults of the same age (1, 2) and changes little over time once maximum growth has been reached, and mass fluctuates seasonally making it of less use in predicting age. Predicting age for younger seals resulted in smaller discrepancies compared with known ages than for older animals, but because the age categories we used for young seals were very narrow (i.e., 1–2 yr), errors occurred when assigning individuals into age classes (Table 4). This is not surprising given that growth rates are variable in seals during their early years; affected by maternal characteristics during gestation and lactation (Bowen et al. 2001a, b), weaning weight (Muelbert et al. 2003, Harding et al. 2005), and variability in initial foraging success. Thus our estimates of age for young seals more often resulted in misclassification of younger seals into the wrong age class, while older seals were generally correctly classified as having reached maturity.
Corpe et al. (1996) suggested using measurements of hair widths to distinguish between young animals (≤1 yr) and older animals and used age estimates from cementum annuli counts of incisor teeth of 14 individuals to verify accuracy of aging with hair, but did not have known-aged individuals to verify accuracy of incisor estimates. They reported that mean hair width of seals in their first year (prior to their first molt) ranged from 0.10 to 0.13 mm, while mean hair width of seals that had undergone a molt (i.e., were >1-yr old) was 0.16–0.21 mm. Although that method would allow distinguishing between young-of-the-year and older seals while in the field, preparation of samples and measurement of hair width are time consuming, compared with obtaining morphometrics and inserting them into a formula, already set up on a spreadsheet.
Using morphometrics and our equations to estimate ages of harbor seals provides a rapid, reasonably reliable, objective, and consistent method of aging these animals (Tables 2, 4; 1, 2), with results somewhat better for females than for males. We further tested the utility of morphometric aging when we noted better agreement between morphometric age estimates and incisor-based ages than for field ages and incisor ages for seals not included in the original data set from which we derived the morphometric-based equations (Table 4).
We did, however, discover that morphometrically derived ages may not be reliable for distinguishing older pups from yearlings (Table 4), at least not for males when only curvilinear length is used to estimate ages. We would therefore recommend utilizing more than one objective method of estimating age for very young seals. In addition to differences in hair widths, Corpe et al. (1996) reported that all seals that had narrower hair width (i.e., those that were <1 yr) also had flexible white tissue along the full length of the undersurface of the nails on their foreflippers, which extended beyond the tip of the nail. If researchers need to distinguish between yearlings and young-of-the-year that have been weaned for several months, they may wish to consider using our morphometric equations and examining the nails on the foreflippers for presence of soft, white tips (Corpe et al. 1996), although we did not field-test the latter method.
Acknowledgments
We wish to thank Alaska Native hunters for their participation in the Alaska Native Harbor Seal Commission (ANHSC) biosampling program providing samples to the University of Alaska Museum (UAM) Frozen Tissue Collection, and Vicki Vanek (ADF&G) and Danielle Saverese of ANHSC for organizing and shipping those samples to UAM. We thank Brandy Jacobsen, Sylvia Brunner, Jonathan Fiely, Jennifer Ostler, Charlene Fortner, and Jacob Graham of UAM for cataloging and extracting teeth from subsistence-harvested seals, and Kalin Kellie, Letty Hughes, and Shawna Karpovich for organizing the teeth for blind submission to the laboratory. We also thank Ms. Karpovich for incorporating the morphometric equations into our relational database to automatically calculate morphometric ages. We thank Gary Matson of Matson's Laboratory for technical assistance and for developing the model for aging harbor seal teeth. We would also like to thank James Bailey DVM, Marty Haulena DVM, Pam Tuomi DVM, Rachel Berngartt DVM, Suzanne Conlon, and Millie Gray for veterinary services including extracting teeth from live-captured seals, and Anne Hoover-Miller of the Alaska SeaLife Center (ASLC) for providing teeth from deceased, captive harbor seals. Additionally we wish to thank the many individuals from the Alaska Department of Fish and Game (ADF&G), the National Marine Fisheries Service (NMFS)/National Marine Mammal Laboratory, the National Park Service, the ASLC, and students from various universities that participated in our field trips and assisted with the live-capture of harbor seals and obtaining morphometrics. This work was conducted under NMFS Scientific Research Permits Nos. 358-1585 and 358-1787 and was funded by annual grants to the ADF&G for harbor seal investigations in Alaska, allocated by the U.S. Congress and administered through the Alaska Region of NMFS/National Oceanic and Atmospheric Administration.




