CRANIAL DESCRIPTION AND GENETIC IDENTITY OF THE HOLOTYPE SPECIMEN OF TURSIOPS ADUNCUS (EHRENBERG, 1832)
Abstract
Two species of bottlenose dolphins are currently recognized by most cetologists: the pan-tropical and temperate Tursiops truncatus and the endemic Indo-Pacific T. aduncus. The latter was described from a specimen from the Red Sea, with nothing in the description that would allow referral of the specimen to one or the other of the two species. Because both species occur in the northern Indian Ocean, it was possible that the holotype specimen was actually a common bottlenose dolphin, not of the Indo-Pacific species. The holotype skull was thought lost but has been found in the Berlin Museum. We describe the skull and examine its affinities through comparison of a partial mtDNA control-region sequence with sequences from South Africa and through application of classification functions from a discriminant analysis of the two putative species from Taiwan. The mtDNA sequence is identical to that of South African specimens referred to the Indo-Pacific species, and the multivariate likelihood assignment associates the skull with Taiwanese specimens referred to that species. These results ensure the correctness of use of the name T. aduncus for the species.
Most cetologists currently recognize two species of bottlenose dolphins: Tursiops truncatus (Montagu, 1821) and T. aduncus (Ehrenberg 1832) (Rice 1998, Perrin et al. 2002, Culik 2004, IWC (International Whaling Commission) 2004, Mead and Brownell 2005). This follows a long period of uncertainty during which one, two, or several species were variously accepted (Hershkovitz 1966; van Bree 1966a,b; Ross 1977, 1984; Ross and Cockcroft 1990; Rice 1998; Perrin and Reeves 2004). T. truncatus is thought to be generally of a larger form, usually unspotted ventrally, that inhabits temperate to tropical waters of all oceans, whereas T. aduncus is considered to be usually smaller, with a longer and more slender beak, usually more teeth, usually spotted below, and occurring in more coastal warm-temperate to tropical waters of the far-western Pacific and Indian Oceans (Natoli et al. 2004). There is little question that these two forms exist and that they are partially sympatric and genetically and morphologically distinct in areas for which rigorous morphological and genetic comparisons have been carried out (Gao et al. 1995, Wang et al. 1999, 2000a,b, Natoli et al. 2004 for China; Hale et al. 2000; Möller and Beheregaray 2001; Kemper 2004 for Australia). In a phylogenetic analysis of the mtDNA cytochrome b gene of delphinids, LeDuc et al. (1999) found “T. aduncus” (specimens from Natal, South Africa, and Indonesia) not to be a sister species of T. truncatus, but rather more closely related to Stenella frontalis. These lines of evidence make a strong case for the existence of two species (or possibly three; see Natoli et al. 2004), although an adequately thorough global analysis of both molecular and morphological relationships has not yet been carried out (see Discussion). However, the wide use of the name T. aduncus for an endemic Indo-Pacific species has been based solely on the fact that it is the oldest nominal species described from the Indian Ocean and adjacent waters (True 1914, Hershkovitz 1966). The original description (as Delphinus aduncus) does not give diagnostic details that would allow distinguishing between the two species, and a holotype specimen was not designated. Thus, as both species occur in the Indian Ocean, the possibility has remained that D. aduncus was based on the more cosmopolitan common bottlenose dolphin rather than the Indo-Pacific endemic. The description is in Hemprich and Ehrenberg (1832)1 (translation from True (1914)):
Delphinus aduncus H. et E. Expansus sexpedalis, Pinna dorsi 1? Capite 17 –pollicari, Rostro depresso elongato, utrinque utrinquesecus 25-dentibus conicis validis armato, ad postremos dentes 2″6 ½″′ lato ad medios 1″ 9″′, mandibulae longioris apice 8 ½″′ lato sursum adunco. In insula Belhosse, Hemprichianarum una, a mari proiectum, corruptum vidi.1
Delphinus aduncus H. & E. Length six feet, dorsal fin 1? Head 17 inches, beak elongate and depressed, armed with 25 strong, conical teeth in each jaw on each side; at the last tooth 2″ 6 ½″′ broad, and at the middle 1″ 9″′. Lower jaw the longer by 8 ½″′, with the broad extremity curved upward. In the island Belhosse [with?] Hemprich I saw one decayed specimen which had been cast up by the sea.1
In an alternative translation (provided by D. Nicolson), vidi as the past perfect means “I have seen,” and Hemprichianarum may be a colloquial term “of the Hemprichies” coined by the members of the expedition for the group of islands being explored.
The description is a footnote to a sentence in the text referring to the specimen (translation by D. Nicolson),
Delphini nondum descripti1 longe rostratum caput attuli, alterius Delphini catulum ore obtuso, pinna unica, recens captum apud Arabes vidimus, frustra appetiimus (D. malayanus?).
Dolphin not yet described1 long-beaked head, other dolphin young with blunt mouth, single fin, we saw recently captured by Arabs, which we desired but in vain (D. malaynanus?).
It was long thought that no holotype specimen existed, that the name was based only on an animal seen stranded (Hershkovitz 1966). However, in an itinerary of the Hemprich and Ehrenberg expedition published by Stresemann (1954; p. 142) based on unpublished contemporary letters, it was noted that “Von Fischen—of fishes” (or possibly a misprint for “Von Fischer–from fisherman”)…a skull of “Delphinus rostratus? Schadel” was collected in the Dahlak Archipelago of Ethiopia in the Red Sea during 12–16 April 1825. The itinerary was “…AM 12ten [April]…Inseln Megaede…Am 13ten—Insel Belhosse…Am 14ten Hauakil, am 16ten Dhalac.” Thus, Belhossse Island was visited during the period in which the specimen was collected. Belhosse Island lies at 15°20’N, 40°40’E.2 The apparent explanation of the identification of the collected specimen in a letter as Delphinus rostratus is that it was initially tentatively identified (note question mark) as a rough-toothed dolphin Steno bredanensis (Delphinus rostratus is a junior synonym), but this was reconsidered when the report of the expedition was written, perhaps even when a draft report was being revised (hence the description in a footnote rather than in the text where the specimen was first mentioned). The fact that Hemprich and Ehrenberg tried but failed to obtain a specimen of another species from the fishermen (mentioned in text, above) suggests that they would have collected the stranded specimen of the species in question here.
Specimens from the expedition were deposited in the Berlin Museum. In 1978 a Tursiops skull was found in the Berlin collection and identified (by PJHvB) as the lost holotype specimen based on its antiquity (battered and soiled, and absent from museum catalogs compiled in the early 20th century; apparently lost in the collection for more than 100 yr) and a label, “Insel Belhosse.” The aim of the present study was to compare genetic and skull characters of the holotype specimen with those of specimens that have been referred to the putative common and Indo-Pacific species, to determine whether the use of the name T. aduncus is justified for the Indo-Pacific species.
Materials and Methods
The holotype skull is ZMB66400 in the Zoologisches Museum Berlin (ZMB), Humboldt Universität zu Berlin Museum für Naturkunde. Most of the measurements were taken in the museum (by WFP) as described in Perrin (1975) and Wang et al. (2000a), to the nearest millimeter. The measurements “posterior width of vomer” and “tip of rostrum to apex of prenarial triangle” were taken by WFP from photographs.
In a multivariate assessment of the holotype skull, we applied the results of a discriminant analysis by Wang et al. (2000a) of the two types of bottlenose dolphin in Chinese waters. A discriminant analysis throws orthogonal axes through n dimensions (measurements) so as to maximize between-group variance and minimize within-group variance for preselected groups. The analysis by Wang et al. (2000a) of eighteen cranial characters showed highly significant differences between the two groups (Wilks’λ= 0.06; ∼F18,37= 35.23; P < 0.0001), which were separated by Mahalanobis distance of 87.4 (F18,37= 35.23; P < 0.0001). A posteriori classifications were 100% correct. We applied the classification functions from the analysis (Table 1) to the measurements of the holotype. For an “unknown,” the group score with the highest absolute value assigns it to that group. For the holotype, we take this as a likelihood-based index of affiliation with one or the other of the two types of bottlenose dolphin.
| Cranial character | Coefficients for Indo-Pacific species | Coefficients for common species |
|---|---|---|
| Rostrum width at base | −4.365 | 1.597 |
| Rostrum width at ¼ length | 2.543 | −0.930 |
| Preorbital width | −3.298 | 1.207 |
| Width of external nares | 3.370 | −1.233 |
| Postorbital width | −6.198 | 2.268 |
| Length of upper tooth row | −2.948 | 1.078 |
| Width of internal nares | −3.709 | 1.357 |
| Posterior width of vomer | −0.389 | 0.142 |
| Tip of rostrum to apex of prenarial triangle | 9.995 | −3.657 |
| Constants | −17.550 | −2.485 |
- *Based on discriminant analysis (Wang et al. 2000a). To classify, add sum of products of measurements and coefficient to constant; higher absolute score indicates most likely classification. From Wang et al. 2000a (character rubrics modified).
A tooth from the holotype skull was loaned to the Southwest Fisheries Science Center (SWFC). The tooth was drilled to obtain powder in a “clean room” used only for extracting DNA from bones and teeth. DNA extractions were performed using the method of Höss and Pääbo (1993), modified as described in Hofreiter et al. 2004. Morin et al. (in press) describe sterile techniques, use of extraction and polymerase chain reaction (PCR) controls, and replication of samples.
Amplification of the DNA was done in a PCR clean room set up exclusively for bone DNA work. PCR protocol was followed as in Morin et al. (2006). Because DNA extracted from bone tends to be degraded, two sets of primer pairs (heavy and light strands) of approximately twenty-nine bases were used for PCR and sequencing. The primer pairs TRO(L15812)-5′-CCT CCC TAA GAC TCA AGG AAG-3′ and A3(H16097)-5′-AAT ACG RGC TTT AAC T-3′ (developed at SWFSC) were used. For the second 200 bp of the sequence, D(H1645)-5′-CCT GAA GTA AGA ACC AGA TG-3′ (Rosel et al. 1994) and A3r(L16076)-5′-GAT AAG TTA AAG CTC GTA TT-3′ (developed at SWFSC) were used. The heavy and light strand positions listed after the primer names refer to base positions in the fin whale sequence (Arnason et al. 1991). Standard protocols were used for sequencing (Sambrook et al. 1989) on an Applied Biosystems 3100 sequencer (Appliea Biosystems, Foster City, CA, USA). Sequences were aligned and pieced together using the program Sequencer (v4.1, Gene Codes Corp., Ann Arbor, MI, USA).
To ensure sequence accuracy, the above procedures were repeated using DNA extract obtained in a second extraction from tooth powder obtained by drilling the tooth again on a separate day.
The sequence obtained was compared to the Reference Sequence Library compiled at SWFSC for species identification. The Library contains over 120 cetacean mtDNA control region (D-loop) sequences (360–400 bases) representing all cetacean genera (except Lipotes) and most species. The genus Tursiops is represented by seven T. truncatus sequences from four ocean basins and two sequences referred to T. aduncus from the South Atlantic and South Pacific oceans. The computer program MacMatch (developed by Perkins and Dizon, SWFSC) compares the sequence in question to the holdings, base by base, for every sequence in the Library, generating a match percent and basepair difference.
Results
Morphological Analysis
The holotype skull (Fig. 1, 2) is of a mature animal; the premaxillae and maxillae are fused for approximately 10 cm of the distal end of the rostrum. It exhibits two character states noted by Wang et al. (2000a) as distinct from those in the common species of bottlenose dolphin. The prenarial triangle is shorter in the Indo-Pacific species than in the common species (distance from tip of rostrum to apex of triangle longer). This is also evident in lateral view; a slight elevation of the rostrum is more proximally placed than in the common species. In addition, in dorsal view there is a narrowing of the premaxillae in the same region.
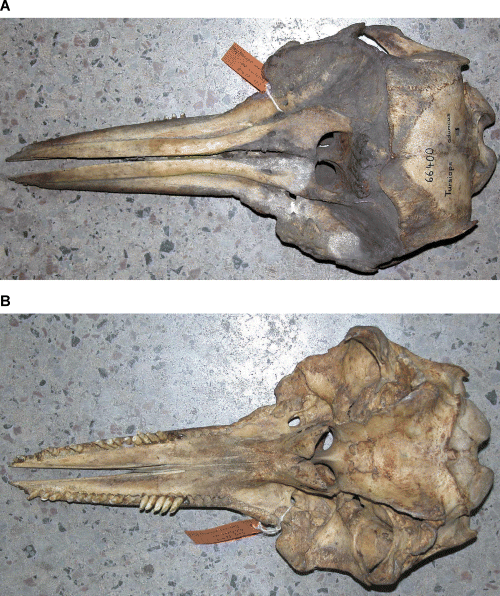
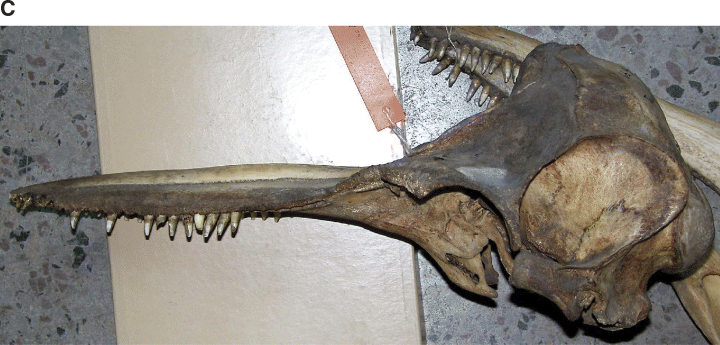
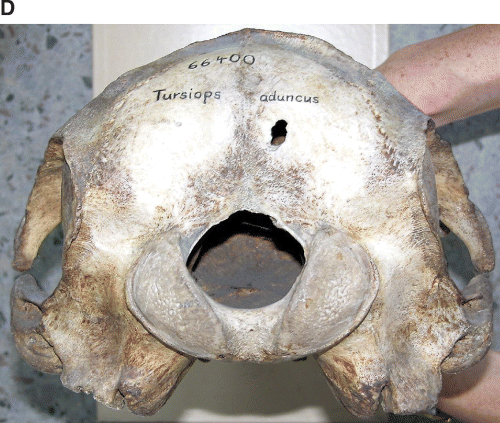

Photographs of holotype specimen of Delphinus aduncus (Ehrenberg, 1832). A, dorsal; B, ventral; C, left lateral; D, occipital; E, mandible (dorsal); F, left ramus (lateral).
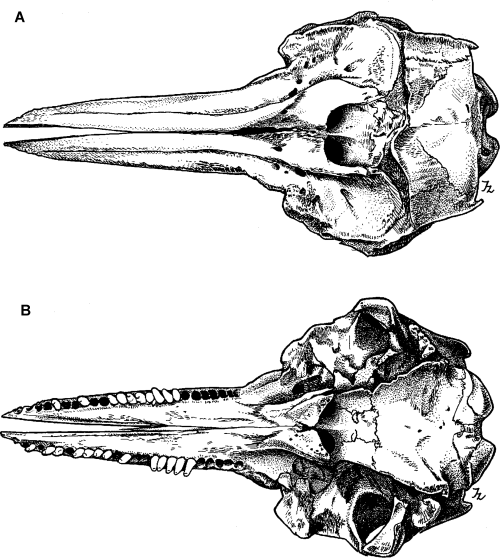
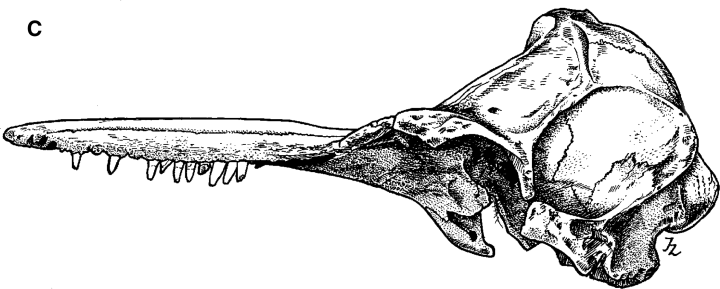
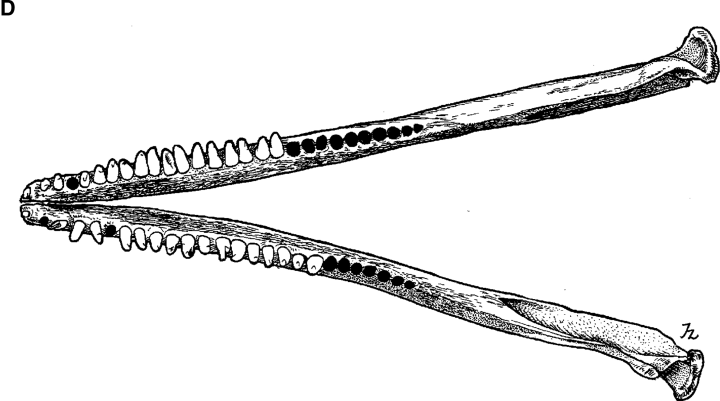
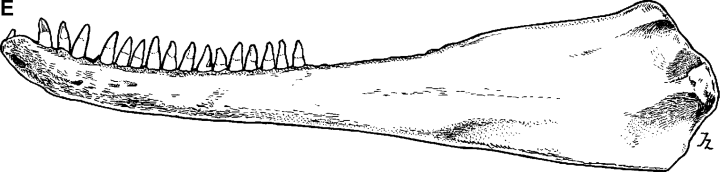
Drawings of holotype specimen of Delphinus aduncus (Ehrenberg, 1832). A, dorsal; B, ventral; C, left lateral; D, mandible (dorsal); E, left ramus (lateral).
Tooth counts of the holotype skull for upper left, upper right, and lower left are at or just below the upper end of the ranges recorded for both species from Taiwan and South Africa and above the range for the common species from South Africa (Table 2). For lower right, the holotype count is above that for the common species from Taiwan and South Africa and within the range for the Indo-Pacific species from both regions. The total count is above the range for the common species. Wang found mean counts to be higher for the Indo-Pacific species (25.2 and 25.8 for upper left; 102.0 and 102.9 for total) than for the common species (23.9 and 24.2; 93.9 and 93.1). Judging from the condition of the holotype skull, some anterior teeth may have been lost during preparation or storage.
| Measurement/count | Holotype of T. aduncus | Common species: Taiwan (min–max) | Indo-Pacific species: Taiwan (min–max) | Common species: S. Africa (min–max) | Indo-Pacific species: S. Africa (min–max) |
|---|---|---|---|---|---|
| Condylobasal length | 479 | 395–561 (50) | 451–529 (18) | 504–578 (9) | 433–507 (33) |
| Length of rostrum | 276 | 204–320 (49) | 258–317 (18) | 283–335 (9) | 250–297 (33) |
| Rostrum width at base | 117 | 98–154 (49) | 103–134 (19) | 127–158 (9) | 101–125 (33) |
| Rostrum width at ¼ length | 75 | — | — | — | — |
| Rostrum width at ½ length | 60 | 55–103 (46) | 56–71 (18) | 73–106 (9) | 56–75 (32) |
| Rostrum width at ¾ length | 46 | 45–80 (47) | 41–61 (17) | 56–85 (9) | 34–60 (33) |
| Width of premaxillae at ½ length | 31 | — | — | — | — |
| Tip of rostrum to external nares | 316 | 244–375 (49) | 298–366 (14) | 337–387 (9) | 294–343 (33) |
| Preorbital width (est.) | 194 | 172–263 (49) | 177–230 (18) | 230–277 (9) | 180–220 (32) |
| Postorbital width | 234 | 187–287 (50) | 200–245 (14) | 254–301 (9) | 202–251 (32) |
| Width of external nares | 54 | 50–68 (50) | 54–70 (18) | 55–68 (6) | 50–61 (33) |
| Zygomatic width | 232 | 189–290 (50) | 209–251 (13) | 257–313 (9) | 46–52 (30) |
| Maximum width of premaxillae | 84 | 77–107 (50) | 77–100 (18) | 88–108 (9) | 77–90 (33) |
| Parietal width | 166 | — | — | — | — |
| Braincase height | 124 | — | — | — | — |
| Length of temporal fossa | 108 | — | — | — | — |
| Height of temporal fossa | 74 | — | — | — | — |
| Length of orbit | 62 | — | — | — | — |
| Length of antorbital process | 42 | 41–72 (49) | 40–51 (14) | 53–71 (8) | 38–53 (21) |
| Tip of rostrum to internal nares | 327 | — | — | — | — |
| Width of internal nares | 64 | — | — | — | — |
| Length of upper tooth row | 231 | 172–278 (49) | 209–266 (19) | 154–277 (6) | 208–245 (31) |
| Length of lower tooth row | 237 | 188–279 (51) | 228–268 (18) | 238–269 (5) | 212–248 (30) |
| Length of ramus | 411 | 341–481 (51) | 386–461 (17) | 426–498 (9) | 373–422 (30) |
| Height of ramus | 87 | 61–104 (51) | 77–93 (17) | 90–110 (9) | 72–90 (30) |
| Posterior width of vomera | 30 | — | — | — | — |
| Tip of rostrum to apex of prenarial triangleb | 199 | — | — | — | — |
| Width of tooth at alveolus | 6.0 | — | — | — | — |
| Number of teeth | |||||
| Upper left | 27 | 21–27 (54) | 23–27 (20) | 23–25 (9) | 24–28 (33) |
| Upper right | 27 | 20–27 (54) | 24–28 (20) | 22–25 (9) | 24–28 (33) |
| Lower left | 26 | 19–27 (54) | 23–28 (19) | 22–24 (9) | 23–28 (29) |
| Lower right | 28 | 20–27 (54) | 24–28 (19) | 21–24 (9) | 23–29 (30) |
| Total | 108 | 80–106 | 96–111 | 88–96 | 97–111 |
- aTermed “Width of alisphenoid at suture with basisphenoid (WAS)” in Wang et al. (2000a).
- bTermed “Tip of rostrum to apex of premaxillary convexity (TPC)” in Wang et al. (2000a).
The holotype skull falls within the ranges of both species for the Taiwan series and for the South African series of the Indo-Pacific species and outside of the lower end of the range for all measurements for the small South African series of rather large skulls of the common species. In the likelihood-based assessment using nine measurements from the two Taiwan series considered simultaneously (Table 1), the holotype skull had absolute scores of 781 for the Indo-Pacific species and 52 for the common species, associating it strongly with the former.
Genetic Analysis
A 399-base sequence of the mtDNA control region was obtained by aligning and piecing together two sequence fragments (Table 3, GenBank DQ517442). There was a 50% overlap between the fragments, ensuring that primer sequence did not interfere with accurate calling of the bases. The sequence generated from the second extraction was identical to that from the first.
| GAAAAAGCCT | ATTGTACAAT | TACCACAACA | TCACAGTACT | ACGTCAGTAT | TAAAAGTAAT | TTGTTTTAAA | AACATTTTAC | TGTACACATT |
| ACATACACAT | ACACATGTAC | ATGCTAATAT | TTAGTCTCTC | CTTGTAAATA | TTCATATATA | CATGCTATGT | ATTATTGTGC | ATTCATTTAT |
| TTTCCATACG | ATAAGTTAAA | GCTCGTATTA | ATTATCATTA | ATTTTACATA | TTACATAATA | TGCATACTCT | TACATATTAT | ATATCCCCTT |
| CAATTTCATC | TCCATTGTAT | CCTATGGTCG | CTCCATTAGA | TCACGAGCTT | AATCACCATG | CCGCGTGAAA | CCAGCAACCC | GCTCGGCAGG |
| GATCCCCCTT | CTCGCACCGG | GCCCATATCT | CGTGGGGGT | |||||
The MacMatch program yielded a 100% match to the T. aduncus sequence in the SWFSC Reference Sequence library from the Atlantic coast of South Africa (from the Port Elizabeth Museum). T. truncatus sequences in the library were 15–17 bases different from the holotype sequence. The sequence is also the same as one of the haplotypes (haplotype 2) found in the small Indo-Pacific form in a study of South African Tursiops by Smith-Goodwin (1997). In her study, seventy-four inshore-form Tursiops sequences were analyzed and yielded ten haplotypes. Haplotype 2 occurred in twenty-nine of the seventy-four cases, making it the most prevalent haplotype of the Indo-Pacific form in the region.
Conclusions and Discussion
The two lines of evidence, genetic and morphological, indicate that the holotype specimen of T. aduncus (Ehrenberg, 1832) is properly referred to the Indo-Pacific species. A critical result is that the holotype corresponds exactly in the mtDNA sequence examined with sequences of specimens from South Africa considered by practicing cetologists to belong to the putative species T. aduncus. The affiliation of the holotype with Indo-Pacific bottlenose dolphins from China in the multivariate assignment test is also strong evidence. The raw morphological data from South Africa, although suffering from the small number of adult skulls available to Ross and Cockcroft (1990), are consistent with the genetic and multivariate results. The status of the name as the senior synonym for the species is assured by the lack of earlier nominal species from its range (Hershkovitz 1966).
It must be noted that Natoli et al. (2004) concluded, based on analysis of both mtDNA sequences and microsatellites for 269 specimens from seven geographic regions, that South African and Chinese bottlenose dolphins of the Indo-Pacific form may belong to different taxa. In both neighbor-joining and parsimony mtDNA trees, the South African Indo-Pacific-form specimens were a sister clade to the common bottlenose specimens, the two together forming a sister clade to the Chinese Indo-Pacific-form specimens. The close association of the holotype specimen of T. aduncus with the South African Indo-Pacific-form specimens and its geographic origin suggest that if it is concluded based on further studies that a separate species inhabits the Indian Ocean, that taxon will bear the name T. aduncus and a different name would have to be designated for a western Pacific/Southeast Asian species. This would require determination of the boundary between two such species and a review of the holotype specimens of the nominal species described from the Pacific and Indian Oceans (e.g., 12 listed in Hershkovitz 1966) presently in the synonymies of T. truncatus and T. aduncus that might be referred to either of the two new divisions of the Indo-Pacific dolphins. Further studies of additional and larger coordinated morphological and genetic datasets will be necessary to settle these questions.
Footnotes
Acknowledgments
Oliver Hampe kindly allowed access to the holotype skull in the Berlin Museum. Robert Asher, the Curator of Mammals, arranged for the loan of a tooth so that DNA could be extracted. Dan Nicolson provided translations of Latin text. J. Zaagman drafted Figure 2. Jay Barlow, Phillip Morin, Richard LeDuc, John Y. Wang, and Robert L. Brownell Jr. reviewed the manuscript and offered useful suggestions for its improvement. We thank these people and others who helped with the study. The mtDNA sequence from the holotype specimen has been submitted to GenBank (DQ517442). The tooth from the holotype specimen was imported to the United States and returned to Germany under SWFSC CITES exchange permit 690343 (US057).




