α1-Adrenergic Receptor Stimulation of Cell Motility Requires Phospholipase D-Mediated Extracellular Signal-Regulated Kinase Activation
Abstract
Phospholipase D is suspected to play a role in tumorigenesis, and the inhibition of phospholipase D has been associated with changes in several cellular events including invasion and migration. We report here that the specific α1-adrenergic receptor agonist, phenylepherine, signals to a growth factor pathway in a manner that requires phospholipase D activity in CCL39 fibroblasts. Phenylepherine increased extracellular signal-regulated kinase phosphorylation eightfold and promoted stress fiber formation threefold. Stress fiber formation was blocked when extracellular signal-regulated kinase activation was inhibited. Stimulation of CCL39 fibroblasts by phenylepherine increased the rate of wound healing fourfold in a wounding assay, while treatment with the MEK inhibitor, PD98059 reduced the closure of phenylepherine-induced wound healing to control levels. Addition of 1-butanol but not 2-butanol inhibited extracellular signal-regulated kinase activation by phenylepherine, presumably by blocking the formation of phosphatidic acid. Exogenously added cell permeable phosphatidic acid increased extracellular signal-regulated kinase activation in a time- and dose-dependent manner as well as stimulated the formation of stress fibers. 1-butanol also significantly inhibited the ability of phenylepherine to stimulate stress fiber formation and wound healing. Taken together, these results indicate a novel role for phospholipase D in the activation of the extracellular signal-regulated kinase growth factor pathway to stimulate early cellular events induced by phenylepherine.
Phospholipase D (PLD) is ubiquitously expressed and activated by a wide variety of both growth factors and G protein-coupled receptor agonists (1–4). In mammalian cells, PLD catalyzes the hydrolysis of phosphatidylcholine to produce phosphatidic acid (PA) (PtdBut, phosphatidylbutanol) and choline. Phosphatidic acid is responsible for the signaling actions of PLD. Two distinct signaling modes have been identified for PA. A putative-binding domain for PA has been identified for several proteins including: Raf, the kinase suppressor of Ras, the mammalian target of rapamycin, and other important signaling intermediates (5,6). Additionally, PA can be further metabolized to form diacylglycerol resulting in the activation of protein kinase C. PLD has the unique ability to utilize a primary alcohol in the place of water creating a metabolically dead phosphatidyl alcohol. The dead-end alcohol-lipid, phosphotidylbutanol (PtdBut), is easily identified in enzymatic assays of PLD and is often used to block the formation of PLD metabolites (1–4,7,8). Two mammalian isoforms of PLD have been identified, PLD1 and PLD2 (3,4). Both isoforms of the enzyme have been implicated in critical mitogenic signaling cascades. The downstream effects of PLD activity have also been found to be involved in the early steps of cell growth and tumor migration (1,3,4). The activity of PLD in several cancer cell lines has been found to be higher than controls cells, and the inhibition of PLD often is accompanied by a decrease in changes in cytoskeletal re-arrangement, cell growth, invasion and migration. PLD activity is required for Ras and tyrosine kinase transformation (9,10). The mitogenic and oncogenic role of PLD makes it a potential therapeutic target for cancer treatment (4).
Sixteen isotypes of α-adrenergic receptor subtypes have been identified in human tissue (11). α1-Adrenergic receptors are classically identified with cardiovascular disorders. An additional, less well-known role for α1-adrenergic receptors is in cell migration events. α1-Adrenergic receptors have been demonstrated to be involved in tumorigenesis in prostate cells (12) and can induce cell proliferation when expressed in fibroblasts (13,14). Recent work in our laboratory has found that phenylephrine (PE) specifically activates both extracellular signal-regulated kinase (ERK) and the sodium hydrogen exchanger in CCL39 fibroblasts (15,16). Therefore, it was our intent to determine the biological role of α1-adrenergic receptors in these cells.
Cell motility requires the formation of focal adhesions and the polymerization of actin monomers in migrating cells. Activation of Gα13-coupled receptors leads to both increased cell migration and stress fiber formation (17). The relationship between stress fiber formation and migration in CCL39 fibroblasts was further established in a series of experiments investigating the somatostatin signal transduction (18). In this work, inhibition of RhoA by somatostatin 1 receptor activation leads to decreases in actin stress fiber formation, focal adhesions and cell migration. The best characterized regulators of actin structures are the small G proteins, RhoA, Rac and CDC42. However, ERK has also been associated with stress fiber formation. An ERK-dependent re-arrangement of actin stress fibers has been identified in endothelial lung cells (19). Because of the link between PLD and ERK or stress fiber formation in several cell lines, it is rational to speculate that PLD could lead to the activation of ERK, and regulate the organization of the actin cytoskeleton and cellular migration in CCL39 cells.
The purpose of this study was to further investigate the biological effect of α1-adrenergic receptor stimulation of the ERK pathway, to better characterize the upstream activator of ERK in CCL39 cells, and to determine a role for PLD in α1-adrenergic-directed cell migration.
Experimental Procedure
Cell culture
Chinese hamster lung fibroblasts (CCL39, ATCC) were cultured onto 35-mm dishes with high glucose Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich, St Louis, MO, USA) and 10% heat-inactivated fetal bovine serum (FBS, Gibco-BRL, Carlsbad, CA, USA) at 37 °C in a 5% CO2 incubator. Prior to agonist stimulation, the cells were allowed to grow to approximately 70–80% confluence. Cells were then growth-arrested for 12–14 h with high-glucose DMEM containing 0.5% serum with antibiotics and antimycotics. One hour prior to agonist treatment, the cells were rinsed with PBS and incubated in serum-free high-glucose DMEM without antibiotics and antimycotics for Western blotting, PLD assays, and stress fiber experiments. Cells were then treated with specific agonists, inhibitors, or activators as indicated. Where indicated, short-chain cell permeable phosphatidic acid (scPA, Avanti Polar Lipids, Alabaster, AL, USA) was prepared by drying the chloroform scPA and resuspending the lipid in PBS by vortexing and sonication.
Western blotting
Following the incubation periods, the cells were washed twice with chilled PBS and lyzed in 200-μL Laemmli sample buffer containing 0.1-mm orthovanadate and 1-mmβ-glycerophosphate. The samples were boiled and DNA sheered with five passes through a 27-gauge needle. The protein content of lyzed samples was analyzed by the Non-Interfering Protein Assay (GenoTechnology, St Louis, MO, USA). Lysate was resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Proteins were then transferred to polyvinylidene difluoride membrane by using a semi-wet electroblotting apparatus. Phosphorylated ERK (p-ERK) was detected by immunoblotting the membrane using p-ERK mouse monoclonal IgG (Cell Signaling Technology, Danvers, MA, USA) in TTBS (10-μm Tris and 150-mm NaCl, pH 8.0 with 0.05% Tween 20) containing 5% dry milk. Total ERK was detected by immunoblotting using non-phospho/total ERK rabbit polyclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bound antibodies were detected by using a secondary antibody, horseradish peroxidase-conjugated IgG (Santa Cruz Biotechnology) in TTBS with 5% dry milk. Proteins were detected by using an enhanced chemiluminescence detection reagent (Santa Cruz Biotechnology). Densitometric analysis of Western blots were quantified by using uscanit software.
Cell Based ERK ELISA Assay
A sensitive and quantitative assay of ERK phosphorylation (fast activated cell-based ELISA ERK1/2 kit; Active Motif, Carlsbad, CA, USA) was used in studies with scPA. Cells were grown to 70% confluence on 96 well plates, washed, and then incubated with 200 μL of 0.5% serum containing media for 12 h. Cells were stimulated with scPA at 37 °C for the indicated time and or concentration. The reaction was terminated by removal of media and addition of 100 μL of 1% formaldehyde in PBS. Phospho-ERK levels were detected by using phospho-specific antibodies as directed by the manufacture. The signals were normalized for cell number by the addition of 100 μL of crystal violet and colorimetric detection.
Wound healing assay
For ligand induced wound healing assays, CCL39 cells were seeded into 35-mm dishes and allowed to grow to confluence in high-glucose DMEM containing 10% serum, antibiotics, and antimycotics. Prior to starting the wound assay, cells were incubated for 12–14 h in serum-depleted (0.5% fetal calf serum) high-glucose DMEM. Wounding was created by scraping a sterile 10-μL pipette through the cell monolayer. Residual cells were removed by washing three times with PBS. Media and as indicated agonist and butanol were replaced at 12 h. Migration was measured in the presence of the indicated agents in high-glucose DMEM. Micrographs were taken with an Olympus IX 70 equipped with an Orca CCD camera in phase contrast mode at 100× magnification. Images were taken at 0, 12 and 24 h after stimulation. The media was replaced following the measurement. For analysis, each wound was measured at five locations each at two individual fields. Wound healing was determined as the percent of wound remaining open at the time of measurement.
Stress fiber formation determination
Treated and control cells grown on glass coverslips were fixed with 3% paraformaldehyde at 4 °C for 30 min, and permeabilized with 0.4% Triton X-100 for 10 min. Actin stress fibers were stained with 0.5-μg/mL flourescein isothiocyanate-phalloidin (Sigma Corp., St Louis, MO, USA) for 60 min. The cells were treated with prolong anti-fade (Molecular Probes, Eugene, OR, USA) prior to mounting the glass slides and analyzed on an inverted Olympus IX70 microscope in fluorescence mode. Cells displaying significant and strong stress fibers (well-formed fibers which are organized and stretched through the majority of the cell) were counted in four to five random fields for each slide. Percent of cells displaying stress fibers was determined as the total number of cells with stress fibers versus the total number of cells observed in the observed fields.
PLD assay
The in vivo PLD activity assay was performed by using the transphosphatidylation assay as described previously (20). Cells were serum-deprived and labeled with 2 μCi/mL [9,10-3H] myristic acid for 16–18 h prior to agonist stimulation. Unincorporated [3H] myristic acid was removed by washing with PBS and the cells were incubated in serum-free high-glucose DMEM media for 1 h. For the final 10 min of pre-incubation, 0.3% 1-butanol was included. After agonist activation, the cells were scraped, lipids extracted using an acidified chloroform methanol extraction and resolved by thin layer chromatography (TLC) (20). Standards for PA and PtdBut were visualized with iodine and bands co-migrating with the authentic standards were scraped into scintillation vials. PLD activity was determined as the incorporation of radioactivity into PtdBut.
Results
Pheneylephrine stimulation of stress fiber formation and wound healing requires ERK activity
Activation of growth factor pathways by G-protein-coupled receptors (GPCR) can lead to the regulation of important mitogenic events (21,22). While the stimulation of the α1-adrenergic receptor pathway is best described through the study of metabolism and contractility in muscle cells, the effect of α1-adrenergic receptors in non-cardiac tissues has not been thoroughly investigated. The specific α1-adrenergic receptor agonist, PE, can mediate cell growth pathways in cardiac myocytes and vascular muscle cells (23,24), induce contractility and mitogenesis in prostatic stromal cells (12), stimulate monocyte migration (25), and induce growth-promoting pathways when expressed in fibroblasts (14). Because we have already demonstrated that PE can stimulate signaling pathways in CCL39 fibroblasts (15,16), we now determined the ability of α1-adrenergic receptors to activate growth factor pathways in the same cell line. To do this, the requirement for activation of ERK in two downstream biological events, stress fiber formation and wound healing, was investigated. As expected (16), the addition of 50-μm PE for 10 min resulted in a significant increase (approx. eightfold) in the phosphorylation of ERK in resting fibroblasts (Figure 1). There is a growing evidence that ERK activation is coupled with the formation of actin stress fibers (26,27). Therefore, we next determined the ability of PE to cause the formation of stress fibers in an ERK dependent manner. PE (50 μm)-stimulated cells showed a significant increase in the number and organization of stress fibers when compared with control cells (Figure 2A). PE stimulation of the fibroblasts increased the number of cells displaying stress fibers from 22% ± 1.3% to 74% ± 3.1%. This was a 3.4-fold increase over the unstimulated, control cells. The formation of stress fibers generally took at least 20 min for strong actin organization to occur (data not shown). Pre-incubation of starved cells with the MEK inhibitor PD98059 prior to PE stimulation resulted in the attenuation of the actin filamental formation when compared with stimulated cells and dropped the number of cells displaying stress fibers to below cells treated with only PD98059 (Figure 2B), 9% ± 2.3%.
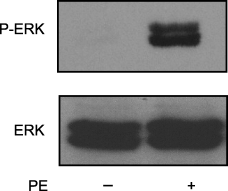
Phenylephrine (PE) stimulates phospho-extracellular signal-regulated kinase (ERK) levels in CCL39 fibroblasts. CCL39 cells were grown in 35-mm culture dishes to 70% confluency and serum-deprived (0.5% serum) overnight. Cells were treated with vehicle (control; PBS) or 50-μm PE for 10 min. Cells were harvested by scraping into 100-μL Lamelli sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer containing phosphatase inhibitors. After lysis, 20 μg of total protein was loaded in each lane and resolved by 10% SDS–PAGE. Active ERK levels were determined by Western blotting with a phospho-specific antibody. Total ERK levels were measured by Western blot with a non-specific ERK antibody. Results are representative of five separate experiments.
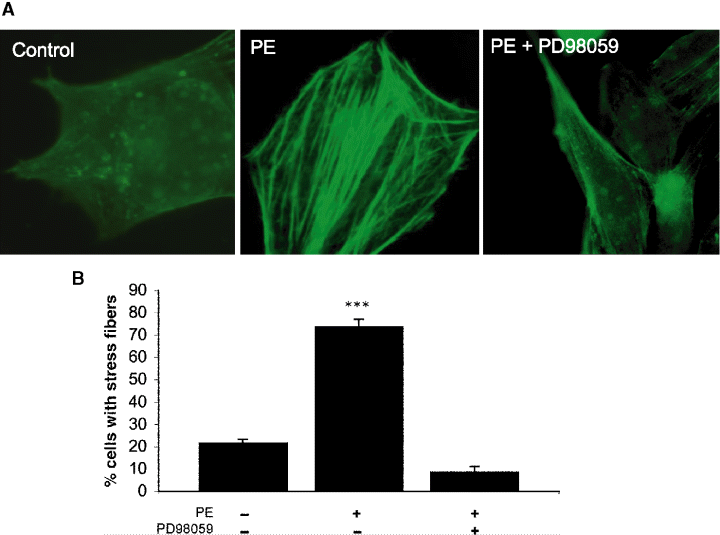
Phenylephrine (PE)-stimulated stress fibers are mediated by extracellular signal-regulated kinase (ERK). Cells grown to 40–50% confluency on glass coverslips were cultured in 0.5% serum overnight and deprived of serum for 1 h in high glucose Dulbecco's modified Eagle's medium media prior to agonist addition. Agonist (50-μm PE) was added and cells were incubated at room temperature for 30 min. Where indicated, cells were pre-incubated for 15 min with PD98015 (10.0 μm) prior to PE stimulation. (A) Images were obtained by using an Olympus IX70 fluorescence microscope equipped with a Hamamatsu Orca 100 12-bit cooled digital camera using simple pci software. (B) Four random fields were counted for each coverslip. The number of cells that displayed stress fiber formation and the total number cells in each field were determined. All data are presented as the mean ± SE (n = 4). Statistical analysis was performed by using anova (p > 0.05) compared with unstimulated control values.
Because thrombin-stimulated formation of stress fibers coincides with increased cell migration in CCL39 cells (19), we next investigated if α1-adrenergic stimulation in CCL39 fibroblasts would also increase cell motility. Wound healing is a classic measure of the ability of cells to migrate (28). CCL39 cells were grown to confluency and serum-starved overnight. The monolayer was wounded with a pipette tip and the ability of the cells to close the scratch was determined in the presence or absence of 50-μm PE or a combination of 50-μm PE and 10-μm PD98059 (Figure 3A). Cell migration was measured as the reduction of the wound width as a percent of the total wound at the beginning of the assay. PE increased the cell migration into the wound 2.5 times above unstimulated cells at 24 h (Figure 3B). As shown in Figure 3A, several cells were able to move into the middle of the wound as individual cells and not as a coherent front. Cells typically displayed an elongated morphology, similar to that observed when cells were grown with serum (data not shown). As anticipated, the inhibition of MEK by PD98059 abrogated the migration into the wound stimulated by PE. This demonstrates for the first time in fibroblasts that α1-adrenergic induced cell migration takes place via an ERK-dependent pathway.
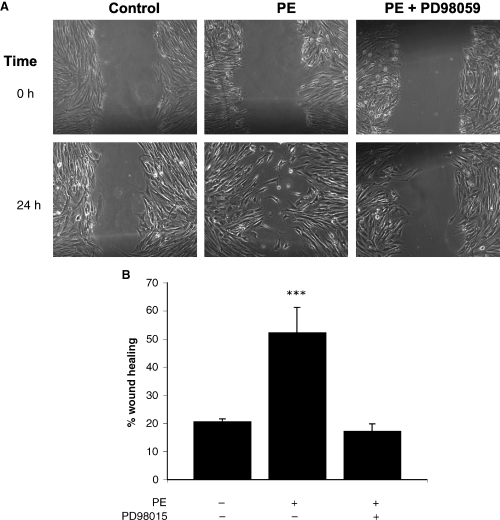
Phenylephrine (PE) stimulates motility of CCL39 fibroblasts. A wound was created in a confluent monolayer of CCL39 fibroblasts. Cell debris was removed by rinsing and the cells were incubated for 24 h without (control) or with 50-μm PE or the combination of 10.0 μm PD98015 and 50-μm PE. Images were taken at the initiation of the experiment (t = 0) and after 24 h (t = 24). Movement of cells into the wounded region resulted in a decrease in the surface area of the wound. The percent change in the surface area of the scarred region was quantified and is displayed graphically. Magnification, 100×. (B). All data are presented as the mean ± SE (n ≥ 4) from a representative experiment. Statistical analysis was performed by using anova (p > 0.05).
Stimulation of the α1-adrenergic receptor leads to increase in phosphatidyl butanol formation
The activation of the Ras-ERK pathway via GPCRs can take place by a number of mechanisms, at least one of which involves PLD (6,9). In lung endothelial cells, ERK activation is regulated by PLD activity (2). Activation of a Gα12/13 pathway in human lung fibroblasts (29) or in Rat1 fibroblasts (30) causes a PLD-dependent activation of stress fiber formation. Generation of the acidic lipid, PA by PLD, is believed to recruit Raf to the membrane by binding directly to a putative PA-binding site of Raf (6). Therefore, it was of interest to see if addition of PE would increase the PLD activity. Serum-starved CCL39 cells prelabeled with [3H] myristate were stimulated with 50-μm PE for 15 min and PLD activity was measured by the formation of [3H]-labeled PtdBut. Addition of PE (50-μm) resulted in a significant increase in PtdBut formation, more than doubling the PLD activity found in resting cells (Figure 4). Consistent with earlier studies by Fee et al. (30), PLD activity was significantly increased in CCL39 cells with the addition of phorbal esters (Figure 4). This establishes that α1-adrenergic receptor activation in fibroblasts can lead to increased PLD activity.
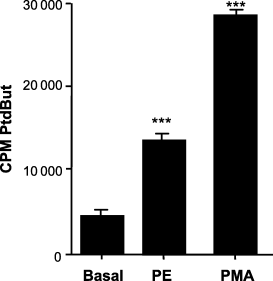
α1-Adrenergic activation of phospholipase D (PLD). Quiescent CCL39 cells were incubated overnight with [H3] myristic acid in media containing 0.5% serum, washed with PBS, incubated for 1 h with serum-free Dulbecco's modified Eagle's medium high-glucose media and incubated with either 50-μm PE or 10-μm phorbal-12-myristate-13-acetate (PMA) for 30 min. PLD assays were terminated with addition of methanol and lipids extracted. Incorporation of radioactivity into the PLD product phosphatidylbutanol (PtdBut) was measured after TLC. All data are presented as the mean ± SE (n ≥ 4) from a representative experiment. Statistical analysis was performed by using anova (p > 0.05).
PLD activity is crucial for the activation of ERK, stress fiber formation and wound healing
Although the preceding results indicate that α1-adrenergic receptor stimulation results in the activation of both PLD and ERK, these data do not provide evidence indicating whether PLD activation is either upstream or downstream of ERK activation. To investigate the role of PLD in cellular signaling events, cells are often treated with either primary or secondary alcohols (8). In these experiments, PLD reacts with primary but not secondary alcohols. Primary alcohols substitute for water in the specific transphosphatidylation reaction catalyzed by PLD and not other lipases (2,8). The transphosphatidylation reaction results in a stable alcohol-derived lipid that blocks the production of PA. In our studies, we used 1-butanol to block the results of PLD activation and 2-butanol as a control to correct for the non-specific result of cronic exposure of living cells to alcohol. As shown in Figure 5, the ability of PE to stimulate ERK phosphorylation was significantly blocked when CCL39 cells were pre-incubated with 1- but not 2-butanol. Addition of PE gave a 5.2 ± 0.08 SEM (n = 4) fold increase in ERK phosphorylation over non-stimulated controls (n = 5). Cells first treated with 2-butanol prior to PE stimulation showed a 4.6 ± 0.03 SEM (n = 4) fold in ERK activation compared with control. However, when fibroblasts were first incubated with 1-butanol, addition of PE resulted in only a 2.3 ± 0.02 SEM (n = 4) fold increase in ERK phosphorlyation over control cells. Thus, addition of 1-butanol resulted in a 67% reduction in ERK activation when compared PE-stimulated cells. The slight reduction of ERK phosphorylation when PE is added in the presence of 2-butanol is <15% of PE-stimulated ERK levels and is likely due to general effects of the alcohol and not PLD activity. The concentration of butanol used in these experiments was well within commonly used ranges (1–4,7,8) and in control experiments with 2-butanol concentrations ranging from 0.1% to 0.54% a minimal inhibition of ERK (<10% of PE-stimulated cells) was observed indicating that at these levels the alcohol was having a minimal non-specific effect (data not shown).
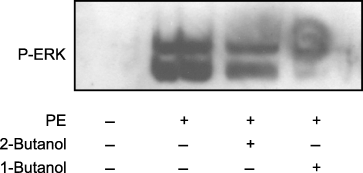
Phospholipase (PLD)-mediated phosphorylation of extracellular signal-regulated kinase (ERK). ERK activation was determined in quiescent, serum-deprived cells. Cells were incubated with or without 0.5% 1- or 2-butanol as indicated for 15 min. Following alcohol treatment, cells were stimulated for 10 min with or without 50-μm PE, rinsed with PBS and lyzed in Laemmli sample buffer. A total of 20 μg of each sample was resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and the phosphorylation state of ERK was determined by immuno-analysis. Data are representative of four separate experiments.
While a significant amount of evidence exists indicating that stress fibers and other cytoskeletal elements are regulated by small G proteins, the data described above adds evidence for a potentially unique mechanism of activating the reorganization of the cytoskeleton that involves PLD (31,32). And, as we have provided evidence that PLD is upstream of ERK, we employed both primary and secondary butanol to investigate if inhibition of PLD signaling blocks the formation of stress fibers. As seen earlier, in the absence of PE stimulation, control cells showed little to no stress fiber formation (Figure 6). Addition of 50-μm PE induced stress fiber formation 2.9-fold when compared with the control cells with over 64.0% of the cells displaying stress fibers. While PE stimulation has been demonstrated as an agonist in CCL39 cells (15,16), it was surprising to see the agonist induces mitogenic responses in fibroblasts. An involvement of PLD was next investigated. In these studies, the pre-incubation of 2-butanol prior to PE stimulation showed no significant effect on stress fiber formation compared with cells treated with only PE (p > 0.05 n = 5). However, 1-butanol did inhibit the ability of PE to induce the stress fiber formation. Under these conditions, only 14.2% of the total cells showed stress fiber formation after PE. This 78% reduction in stress fiber formation was consistent with the loss of ERK phosphorylation with 1-butanol treatment and the result of ERK inhibition on stress fiber formation. Neither 1- nor 2-butanol alone altered the level of stress fiber formation (data not shown) when compared with basal level. These data further substantiate that PLD is an upstream component of a pathway leading from α1-adrenergic receptors.
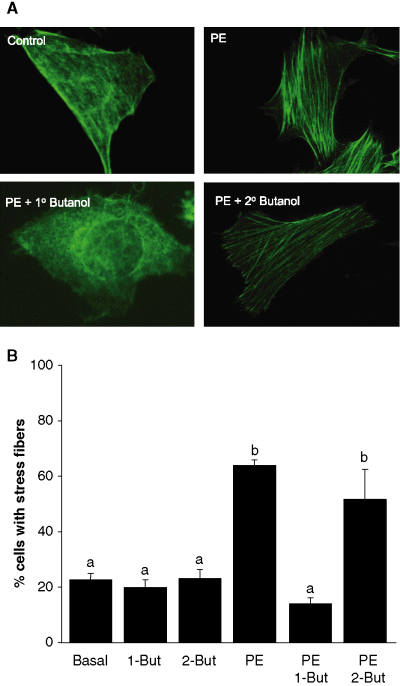
Phenylephrine (PE)-stimulated stress fibers are mediated by phospholipase D (PLD). Cells grown to 40–50% confluency on glass coverslips and cultured overnight in media containing 0.5% serum. The cells were then deprived of serum for 1 h in high-glucose Dulbecco's modified Eagle's medium media. Agonist (50-μm PE) was added and cells were incubated at room temperature for 30 min. (A) Representative images were obtained by using an Olympus IX70 fluorescence microscope equipped with a Hamamatsu Orca 100 12-bit cooled digital camera using simple pci software. (A) Five random fields were counted for each coverslip. The average percent of cells displaying stress fiber formation was determined for each field in each experiment. All data are presented as the mean ± SE. Significance of the difference between means was determined by Student's t-test for non-paired samples (p > 0.001). Data indicated with ‘a’ were not significantly different from the other data marked with the same letter, but were significantly different from the data marked with ‘b’. Data represent eight separate experiments.
To confirm that PLD is involved in α1-adrenergic receptor stimulation of ERK activation and stress fiber formation, the effects of exogenously added PA was observed. Two different scPA were incubated in resting cells. The level of activation of ERK was measured by using a quantitative cell-based ELISA method (Figure 7A,B). Here, ERK activation was measured by using a phospho-specific ERK antibody after fixing and permeabilizing the cells. The total amount of cells per well were measured in the same wells using crystal violet. In these studies, addition of both 6:0 dihexanoyl PA and 12:0 dilauroyl PA resulted in a dose- and time-dependent increase in ERK activation. In each case, the shorter more permeable chain PA gave a stronger activation of ERK. Maximal concentration of either lipid was seen at 40 μm (Figure 7A). As shown in Figure 7B, dilauroyl PA stimulation of ERK was achieved in a time-dependent manner with a peak of ERK activation occurring at 10 min. The longer C-12 lipid also displayed a typical time-dependent activation of ERK, although the decrease in ERK activation was muted at longer times when compared with dilauroyl PA. In parallel studies, the effect of both dilauroyl and dihexanoly PA on stress fiber formation was investigated (Figure 7C). Addition of either scPA significantly increased the number of cells expressing stress fiber formation (dihexanoyl PA, 75.8% ± 3.9% and dilauroyl PA, 72.6% ± 2.41%) when compared with control levels (19.4% ± 3.6%) representing a 3.94-fold increase for cells stimulated with dihexanol PA and a 3.78-fold increase in cells displaying stress fibers when compared with control-unstimulated cells. There was no significant difference in the number of cell with stress fibers in cells stimulated with 50 μm PE or either of the scPA.
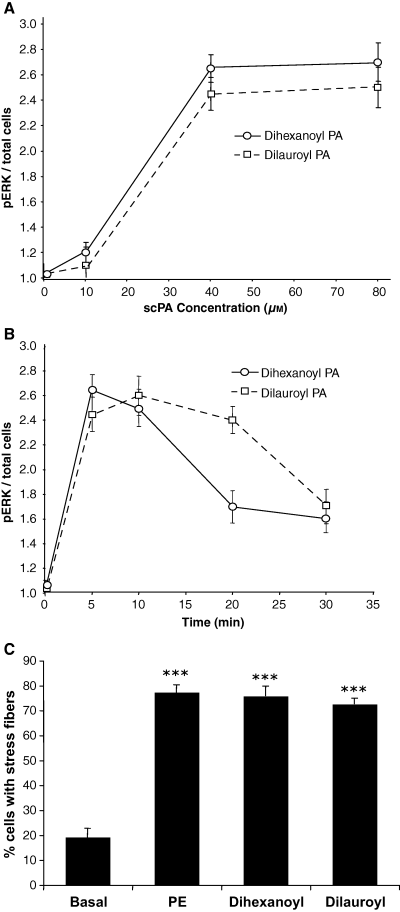
Exogenously added cell-permeable phosphatidic acid (scPA) stimulates extracellular signal-regulated kinase (ERK) activity and stress fiber formation. (A) CCL39 cells were cultured to 70% confluence and serum starved in 96 well plates overnight before being treated with increasing concentrations of scPA for 10 min. The reaction was stopped by addition of ice cold PBS. ERK activation was determined in fixed, permeabilized cells using a phospho-specific ERK antibody detected with a colorimetric ELISA method. Total number of cells was determined in the same wells after the ELISA detection of phosphorylated ERK using crystal violet. Results shown represent the mean ± SE (n = 4) for two separate experiments. (B) Serum-starved cells were incubated with 40-μm of either dihexanoyl PA or dilauroyl PA for the indicated times. ERK activation was determined as described in A. Results shown represent the mean ± SE (n = 4) for two separate experiments. (C) Serum-starved cells were cultured on glass coverslips and treated with vehicle (PBS; basal), 50-μm PE, or 40-μm of scPA (dihexanoyl or dilauroyl) for 15 min. The number of cells with stress fiber formation was determined in five random fields. The results shown represent three different experiments conducted in duplicate presented as the mean ± SE (n = 3). Statistical analysis was performed by using anova (p > 0.001) compared with control values.
We next investigated the possibility that PE-mediated PLD signaling also regulated cell migration. The migration of CCL39 cells was measured in the presence of 10% serum (Figure 8A) or 50 μm PE (Figure 8B). The effects of blocking PLD signaling with 1-butanol was also determined. Measurements of wound width were taken at 0, 12 and 24 h and media replaced with fresh agonist and or alcohol as indicated at 12 h to reduce the loss of evaporated butanol. As seen in Figure 8A, addition of 1-butanol (0.5%) blocked the ability of serum growth factors (10% fetal calf serum) to stimulate cell migration into the wound similar to that found when cells were incubated in serum-free media. This growth indicates a general role for PLD where growth factors (serum) are present. Addition of PE for 24 h decreased the width of the wound by 4.3-fold compared with control. 1-butanol blocked the cell movement into the wounded area to levels below that found in unstimulated cells. In the presence of 2-butanol, the level of cell movement into the wounded area in response to PE at both 12 and 24 h was twice that of the control cells and 43% less than cells stimulated with the agonist alone (data not shown). The combination of 10% serum and 50 μm PE consistently increased the wound healing by 16% at 12 h compared with serum alone, but this enhancement was lost a later times (data not shown). Together these, data suggest that PLD plays a key role in events controlling cell remodeling and migration in lung fibroblasts in a novel regulation involving α1-adrenergic receptors.
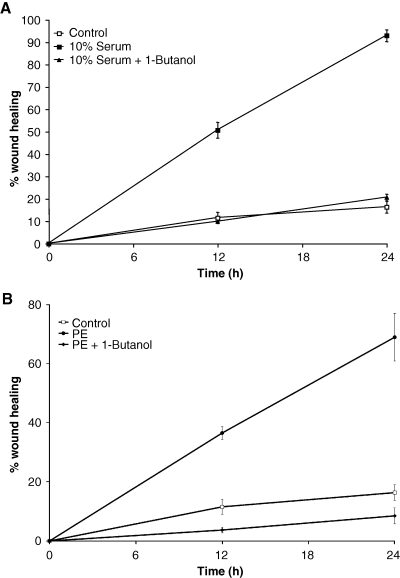
Phenylephrine (PE)-stimulated wound healing requires Phospholipase D (PLD) activity. Cells were grown to 95% confluency in 35-mm dishes and serum starved in low serum (0.5% serum in Dulbecco's modified Eagle's medium) overnight. A reference line was drawn on the bottom of the dish, and wounds were made perpendicular to the line with a 10-μL pipette tip. The cells were rinsed to remove cell debris and then incubated in serum free media. (A) Cells were cultured and allowed to migrate in the presence or absence of 0.5% butanol in media containing 10% serum. (A) Cells were unstimulated or exposed to 50-μm PE in the absence or presence of 0.5% butanol. Media were changed for all dishes at 12 h to correct for potential alcohol evaporation. Images were taken at 0, 12 and 24 h and analyzed for wound closure. Data are presented at the mean ± SE of six separate experiments each measured with an n = 4.
Discussion
These data show that the formation of stress fiber as well as the subsequent increase in cell motility is under the control of α1-adrenergic stimulation in a non-cardiac cell line. Furthermore, we show that PE-stimulated cell motility is under the influence of the choline-specific lipase, PLD. Importantly, this study demonstrates a mechanism for the activation of the ERK pathway as a result of a GPCR, the α1-adrenergic receptor, previously uncharacterized in fibroblast cells. See Figure 9 for structures of agonist and MEK inhibitor.
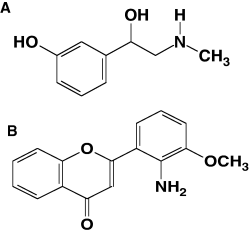
Structure of (A) α1-adrenergic receptor agonist, phenylephrine and (B) MEK inhibitor PD98015.
Phospholipase D was initially found to be involved in stress fiber formation in IIC9 fibroblasts stimulated with thrombin (33). While the lipid hormone, lysophosphatidic acid has been best characterized in its role in PLD mediation of stress fiber formation in aortic endothelial cells (34), in fibroblasts (31), and in lung cells (29), little work has been shown to link α1-adrenergic signaling through PLD to stress fiber formation. While there is a link between PLD activation and cell migration (35–37), none of these findings show a mechanism involving α1-adrenergic GPCRs, PLD, ERK, and cell migration. These effects are thought to be one of the critical factors implicating PLD in cell proliferation in human cancer (4).
Much of the current work on stress fiber formation and cell migration has focused on the GPCR pathways activated by LPA leading to the activation of a family of small G proteins (38). While the data presented here do not rule out the possibility that RhoA or other small G proteins are also involved in PE-mediated cell motility, we find that the PLD-mediated activation of the ERK pathway is important for cellular functions controlling cell motility. The activity of both PLD and ERK was significantly increased by the addition of PE (1, 4). The role for PLD-dependent activation of ERK has been found in several cell types with a variety of agonists (7,39,40). However, PLD has also been reported to be downstream of ERK (41) or independent of ERK (42) signaling pathways. Generation of PA is thought to aid in recruiting Raf1 to the membrane, where it acts to modulate the signaling scaffold of the Ras-ERK signaling pathway (6). Our findings where exogenous PA is added to the cell cultures are consistent with these studies, suggesting that ERK is downstream of PLD, and that both signaling intermediates are crucial for cell migration.
In CCL39 cells, the formation of stress fibers is concurrent with cell migration in a RhoA-dependent pathway (19). While there is a negative relationship between tumor migration and stress fibers in some cells, the increase in both events under either serum or PE stimulation found in this study is consistent with those found by the Barber group in the same cell line where they used thrombin as an agonist. At this time, there is little known about PE-mediated cell migration in non-vascular cell types. Stimulation of α1-adrenergic receptors gave rise to both a significant increase in stress fiber formation and wound healing indicating that the increase in contractile actin filaments is a key for cell motility. Moreover, inhibition of ERK and PLD blocked the cytoskeletal remodeling and cell migration into the vacated wound space. Use of primary butanol to block PLD product formation has been successfully employed between concentrations of 0.1% to 1% in a number of experiments (8). The data presented here demonstrated that blocking PLD signaling with 1-butanol resulted in cells with a wound healing potential similar to unstimulated cells or ERK inhibited cells. These data were mirrored in the ability of PE to stimulate stress fibers in a PLD-dependent manner. In both cases, PLD specificity was confirmed by the addition of 2-butanol, which did little to block the formation of stress fibers by PE (Figure 6A,B). Constant with the effects of butanol on blocking ERK and stress fiber activation, the use of scPA to activate both pathways further indicates that PLD is involved in this pathway. Addition of exogenous PA mimicked the activation and the down-regulation time course of that shown by PE (8). The concentrations necessary to stimulate both ERK and stress fibers are consistent with that found in fibrosarcoma cells where PLD was identified as the mediator in MMP-9 release and activation (43,44). The combination of butanol and scPA studies strongly suggest that PLD is involved in the adrenergic signaling to ERK and stress fiber formation.
While these studies point out the involvement of PLD in cell migration events, they do not indicate which PLD isoform is involved, nor the precise mechanism by which PLD is activated. RhoA is typically thought to be involved stress fiber formation by the activation of myosin light chain kinase and RhoA kinases. In CCL39 cells stimulated with either lysophosphatidic acid or growth factors, the process is in part mediated by directed phosphorylation of the sodium hydrogen exchanger (18). Because RhoA is often involved in cytoskeletal events, it is likely that PLD1 is activated in these cells. PLD1 is specifically involved in enhancing this pathway via ERK activation of a kinase that phosphorylates the exchanger (42). However, PLD2 is more commonly involved in signaling and is known to be activated by various agonists and is therefore also a potential intermediate in α1-adrenergic regulation of cell migration. Clearly, determining which isoform is activated and involved in these cellular events needs to be clarified; however, this study does help clarify that PLD is involved in stress fiber formation, a question raised by recent studies conducted with PLD1 (45).
As these data demonstrate, PLD is an important mediator in cell motility events. While PLD has long been considered to be involved in cell invasion and migration, there has been little evidence pointing to the mechanism. As a result, there is no specific inhibitor of PLD activity. Generation of a PLD isoform-specific inhibitor would have great interest in both basic and clinical application as an anti-cancer drug. Specifically, such a compound would have potential use in blocking α1-adrenergic receptor-mediated cell migration events.
In conclusion, the present study demonstrates for the first time that α1-adrenergic receptors signal through PLD to stimulate cellular events involved in cell migration via the ERK signaling pathway in CCL39 fibroblasts. The involvement of a Gq-mediated cell migration suggests the existence of a novel mechanism of cell proliferation and tumor progression in cells not typically associated with α1-adrenergic signaling and points to a potential target for drug discovery.
Acknowledgments
This work was supported by an NIH-1-R15-HL074924-01A1, MSU Moorhead Faculty Grant, and NSF-MCB0080243. We would like to thank MSUM for supporting undergraduate research, the honor's apprentice scholarship program and the Barry Goldwater Scholarship program. Our kind thanks go to Jadean Anderson and Brad Moran for their initial work on PLD and ERK.




