Molecular Basis of Branched Peptides Resistance to Enzyme Proteolysis
Abstract
We found that synthetic peptides in the form of dendrimers become resistant to proteolysis. To determine the molecular basis of this resistance, different bioactive peptides were synthesized in monomeric, two-branched and tetra-branched form and incubated with human plasma and serum. Proteolytic resistance of branched multimeric sequences was compared to that of the same peptides synthesized as multimeric linear molecules. Unmodified peptides and cleaved sequences were detected by high pressure liquid chromatography and mass spectrometry. An increase in peptide copies did not increase peptide resistance in linear multimeric sequences, whereas multimericity progressively enhanced proteolytic stability of branched multimeric peptides. A structure-based hypothesis of branched peptide resistance to proteolysis by metallopeptidases is presented.
Multimeric peptides are often preferred to monomeric ones for their ability to perform polyvalent interactions by simultaneous binding to biological ligands. This enables polyvalent peptides to bind collectively much more strongly than their monomeric analogues. This general aspect of multimeric molecules has been studied for natural and synthetic molecules (1).
In previous studies (2–5), we demonstrated that multiple copies of peptides assembled together in branched molecules may be used as drugs, by virtue of their effect on the affinity and stability of bioactive peptides to proteolytic degradation.
We previously found that bioactive peptides, including endogenous peptides with different sequences and lengths, generally retain their biological activity and also become more resistant to the proteolytic activity of plasma and serum enzymes (2–5), when synthesized in a tetra-branched Multiple Antigen Peptide (MAP) form. Peptide biological activity can even be enhanced by multimeric binding.
Endogenous peptides and neuropeptides are cleaved very rapidly by peptidases that cut them into inactive peptides (6) (Figure 1). This is the mechanism by which they are inactivated after they have exerted their biological activity, by stimulating their membrane receptor.
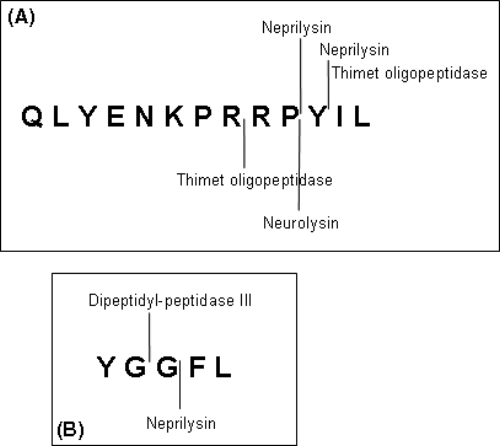
Neurotensin (A) and Leu-enkephalin (B) are inactivated by specific cleavage enzymes.
Peptidases acting on small endogenous peptides are mainly Zn metallopeptidases like neurolysin, the catalytic centre of which is located in a deep channel, to which only small peptides have access (7–8). The steric hindrance of branched peptides may limit their access to the cleavage site of these peptidases, lengthening the peptide half-life, with obvious advantages for their use as drugs.
In addition, parallel peptide backbones of MAPs can generate hydrophobic clusters, and some level of dynamic aggregation may occur, which contribute to the resistance of the final products.
In the present study, we used three different bioactive peptides, Leu-enkephalin (L-enk), neurotensin (NT) and its short active fragment NT(8–13), to discover the molecular basis of dendrimeric peptide resistance to proteolysis. We chose enkephalin and neurotensin because of their interesting pharmacological properties and short half-life in vivo. Moreover, NT is very different in amino acid composition and more than double the length of L-enk, whereas neurotensin short analogue NT(8–13) is similar in length but has a different amino acid sequence to L-enk.
L-enk (YGGFL) is a 5-mer endogenous ligand of opioid receptors (9). We previously synthesized the natural peptide sequence of L-enk in its monomeric and tetra-branched form and tested them for bioactivity and stability. The tetra-branched form of L-enk completely retained its ability to bind opioid receptors and showed increased stability to incubation in human plasma and serum, like all the tetra-branched peptides tested in the same study (4).
Neurotensin (QLYENKPRRPYIL) is a 13-mer peptide (10) with the dual function of neurotransmitter and neuromodulator in the central nervous system and local hormone in the periphery. NT receptors are overexpressed in a number of human tumors (11).
We previously found that the tetra-branched forms of NT and its short 6-mer active fragment NT(8–13) (RRPYIL) can still be detected after 24 h incubation in plasma and serum, while the linear forms have completely disappeared (4).
In order to test the possible influence of peptide length, number of peptide copies, and steric hindrance on branched peptide stability to peptidases, we synthesized the monomeric, two-branched and tetra-branched forms of L-enk, NT and NT(8–13), and compared their stability to incubation with human plasma and serum.
Materials and Methods
Peptide synthesis
Solid-phase synthesis was carried out with a MultiSynTech Syro automated multiple peptide synthesizer (Witten, Germany), employing 9-fluorenylmethoxycarbonyl (Fmoc) chemistry with 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate/N,N-diisopropylethylamine activation. Side chain protecting groups were trityl for His, 2,2,4,6,7-pentamethyldihydro-benzofuran-5-sulfonyl for Arg, tert-butyl ether for Ser and Tyr, tert-butyl ester for Asp and Glu, and tert-butyloxycarbonyl for Trp. Monomeric peptides YGGFL (L-enk), ELYENKPRRPYIL (NT) and RRPYIL (NT8–13) were synthesized on a 4-(2′,4′-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxyacetamido-norleucyl 4-methylbenzhydrylamine (RINK Amide MBHA) resin. Two-branched peptides were synthesized on RINK Amide MBHA resin using Fmoc-Lys(Fmoc)-OH as first amino acid. Tetra-branched analogues were synthesized on Fmoc4-Lys2-Lys-β-Ala Wang resin. Peptides were cleaved from the resins, deprotected by treatment with trifluoroacetic acid (TFA) containing water and triisopropylsilane (95/2.5/2.5), and precipitated with diethyl ether.
High performance liquid chromatography (HPLC)
RP–HPLC was performed in gradient form, for 30 min, on C18 Vydac columns at flow rates of 0.8 and 4 mL/min for analytical and preparatory procedures, respectively, using 0.1% TFA/H2O as eluant A and MeOH as eluant B.
Mass spectrometry (MS)
All compounds were characterized with an Ettan MALDI-TOF (matrix-assisted laser desorption ionization/time-of-flight) mass spectrometer (Amersham Biosciences).
YGGFL: HPLC 5–95% eluant B tr = 21.78 min. MS (M-H+) 555.63 (calcd 554.64). YGGFL)2K HPLC 1–95% eluant B tr = 24.91 min, 1–80% eluant B tr = 21.94 min. MS (M-H+) 1221.40 (calcd 1220.41). YGGFL)4K2K-βAla HPLC 5–80% eluant B tr = 25.25 min. MS (M-H+) 2622.07 (calcd 2622.36). QLYENKPRRPYIL HPLC 1–80% eluant B tr = 16.80 min. MS (M-H+) 1688.87 (calcd 1688.93). QLYENKPRRPYIL)2K HPLC 5–80% eluant B tr = 14.10 min, 1–80% eluant B tr = 19.20 min. MS (M-H+) 3490.19 (calcd 3489.08). QLYENKPRRPYIL)4K2K-βAla HPLC 1–80% eluant B tr = 20.51 min. MS (M-H+) 7160.34 (calcd 7161.37). RRPYIL HPLC 1–80% eluant B tr = 15.35 min, 5–95% eluant B tr = 19.83 min. MS (M-H+) 816.56 (calcd 816.00). RRPYIL)2K HPLC 1–80% eluant B tr = 18.78 min. MS (M-H+) 1742.97 (calcd 1742.09). RRPYIL)4K2K-βAla HPLC 1–80% eluant B tr = 20.59 min. MS (M-H+) 3668.85 (calcd 3668.52). RRPYILGGRRPYIL HPLC 1–80% eluant B tr = 18.72 min. MS (M-H+) 1728.13 (calcd 1729.02). RRPYILGGRRPYILGGRRPYILGGRRPYIL HPLC 1–80% eluant B tr = 20.78 min. MS (M-H+) 3557.92 (calcd 3556.22).
Peptide processing in serum and plasma
About 8 μl of a 1 mg/ml solution of peptides was incubated at 37° C with 20 μl human serum and plasma. Samples withdrawn after 2, 5 or 24 h were precipitated with 200 μl methanol, centrifuged for 1 min at 10,000 × g and diluted with 800 μl 0.1%TFA in water. These solutions were analyzed by HPLC using a C18 column. Controls for peptide retention time in the crude mixture were obtained by adding the same concentration of peptides to supernatants of plasma or serum treated with methanol and centrifuged as above, running the mixture immediately. MS analysis of supernatant of crude solutions was performed with an ETTAN MALDI TOF mass spectrometer.
Structural analysis
Representative structures of proteolytic enzymes were obtained from Protein Data Bank (PDB) (12). PDB structures with code 1O86 (ACE), 1Y8J (NEP), 1S4B (Thimet oligopeptidase), 1I1I (neurolysin) were used. Binding pocket topographies were derived from CASTp database (13). Analysis and measurements were carried out by molmol software (14).
Results and Discussion
Our aim was to test for possible correlations between protease resistance and multimericity. We synthesized three peptides differing in amino acid sequence and length (L-enk, NT(8–13) and NT, 5, 6 and 13 amino acids, respectively) in their monomeric, two-branched and tetra-branched forms and compared their resistances. NT(8–13) was also synthesized as a linear dimer and tetramer. The peptides were incubated in human plasma or serum, and after appropriate time intervals, the crude solutions were analyzed by HPLC and MS as described in (4).
Peptide stability was tested using both plasma and serum, because their proteolytic activity is sensibly different: in plasma, the activated coagulation enzymes, which are mainly serine proteases, are inactivated by heparin, whereas in serum they are active. As a result, proteolysis is higher in serum than in plasma, the latter reproducing more closely blood proteolytic activity in live animals.
Stability of Leu-enkephalin
L-enk (YGGFL) was rapidly degraded in human plasma and serum, whereas its two- and tetra-branched forms were much more stable. The two-branched peptide was not degraded after 24 h incubation in plasma or 2 h incubation in serum. The tetra-branched peptide resisted in serum for 5 h (Table 1).
| Plasma | Serum | |||||
|---|---|---|---|---|---|---|
| 2 h | 5 h | 24 h | 2 h | 5 h | 24 h | |
| YGGFL | + | − | − | − | − | − |
| YGGFL)2K | + | + | + | + | − | − |
| YGGFL)4K2K | + | + | + | + | + | − |
Stability of neurotensin and neurotensin short analogue
The form of neurotensin and neurotensin short analogue NT(8–13) most stable to the action of blood proteases was the tetra-branched form, which was stable in human plasma and also in serum after 24 h incubation (Tables 2 and 3). A correlation was made between the number of peptide copies in branched neurotensin and NT(8–13) and stability in plasma and serum. Two-branched NT and NT(8–13) were more stable than the monomeric forms, being not degraded after 5 h in plasma and serum, whereas monomeric analogues were degraded. The tetra-branched forms were more stable than the corresponding two-branched peptides, being stable in plasma and serum after 24 h incubation.
| Plasma | Serum | |||||
|---|---|---|---|---|---|---|
| 2 h | 5 h | 24 h | 2 h | 5 h | 24 h | |
| QLYENKPRRPYIL | + | − | − | + | − | − |
| QLYENKPRRPYIL)2K | + | + | − | + | + | − |
| QLYENKPRRPYIL)4K2K | + | + | + | + | + | + |
| Plasma | Serum | |||||
|---|---|---|---|---|---|---|
| 2 h | 5 h | 24 h | 2 h | 5 h | 24 h | |
| RRPYIL | + | − | − | + | − | − |
| RRPYIL)2K | + | + | − | + | + | − |
| RRPYIL)4K2K | + | + | + | + | + | + |
The NT and short NT(8–13) had similar resistance to plasma and serum proteases. Because most proteolytic enzymes that inactivate NT have their cleavage sites in the short NT(8–13) sequence (Figure 1) (11), the longer sequence of NT does not introduce new sites.
In order to determine whether multimericity or branched structure contributes to peptide stability, we compared linear and branched multimeric peptides. NT(8–13) was also synthesized as linear dimer (RRPYILGGRRPYIL) and tetramer (RRPYILGGRRPYILRRPYILGGRRPYIL). Repeated sequences were spaced by the addition of two glycine residues. The linear dimer of NT(8–13) showed the same stability in plasma and serum as the two-branched form (5 h in plasma or serum).
Native NT as well as the linear and branched dimers and linear tetramer of the NT(8–13) sequence are cleaved by the same enzymes; evidences were the cleavage fragments identified by HPLC and MS analysis (an example is reported in Figure 2).
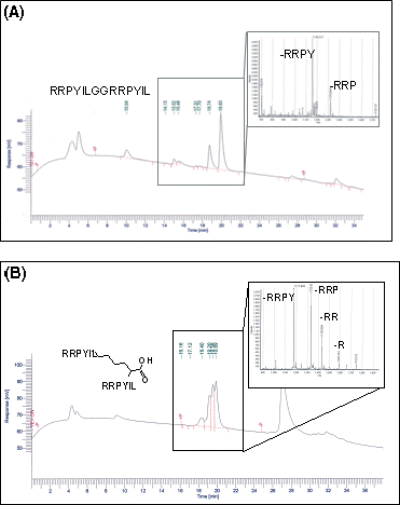
HPLC and MS analysis of linear (A) and branched (B) dimer of NT(8–13) after 5 h incubation in plasma. The peptide fragments indicate that Pro-Tyr and Tyr-Ile are cleavage sites in both peptides.
Tetrameric peptides showed a dramatic difference in proteolytic resistance. The linear tetramer was very labile being cleaved by blood proteases like the linear monomer (Table 4), whereas the branched tetramer was stable up to 24 h in plasma and serum.
| Plasma | Serum | |||||
|---|---|---|---|---|---|---|
| 2 h | 5 h | 24 h | 2 h | 5 h | 24 h | |
| RRPYIL | + | − | − | + | − | − |
| RRPYILGGRRPYIL | + | + | − | + | + | − |
| RRPYILGGRRPYILGG-RRPYILGGRRPYIL | + | − | − | + | − | − |
This established that, as a general rule, multimeric branched forms are more stable than the corresponding monomeric forms and that multimericity progressively enhances the stability of peptides. This was evident both for L-enk and NT derivatives.
We tried to rationalize this evidence with some structural considerations. NT(8–13) presents cleavage sites for major plasma proteolytic enzymes: ACE (EC 3.4.15.1), NEP (EC 3.4.24.11), thimet oligopeptidase (EC 3.4.24.15) and neurolysin (EC 3.4.24.16). Plasma endopeptidases have a long narrow active site like a deep well or crevice open at one or both ends, measuring approximately 40–50 Å in length and 12–15 Å in depth and width, with the catalytic zinc ion usually deeply inside.
In the branched form of NT, the lysine core has greater steric hindrance than the linear peptide backbone, and it is further increased by increasing the number of peptide chains. This might limit the cleavage rates of plasma peptidases, because only one peptide arm at a time can be positioned in the narrow active site. Interestingly, cleavage sites are located on the C-term half of the peptide (11). Although binding of the peptide in the catalytic pocket may still be possible, the cleavage site may be geometrically unreachable by catalytic residues, especially in tetrabranched peptides that, unlike two-branched ones, cannot achieve an extended conformation. The branched core can be thought of as acting like a ‘‘knot’’ in the structure, preventing the peptide from sliding the length of the pocket freely. This would explain the increase in resistance to proteolysis and the effect would increase with the number of copies.
Figure 3 shows neurolysin (EC 3.4.24.16) with the groove containing the catalytically active zinc site. The tetrabranched NT(8–13), which is illustrated in the correct scale, appears as too cumbersome to fit in the cavity.
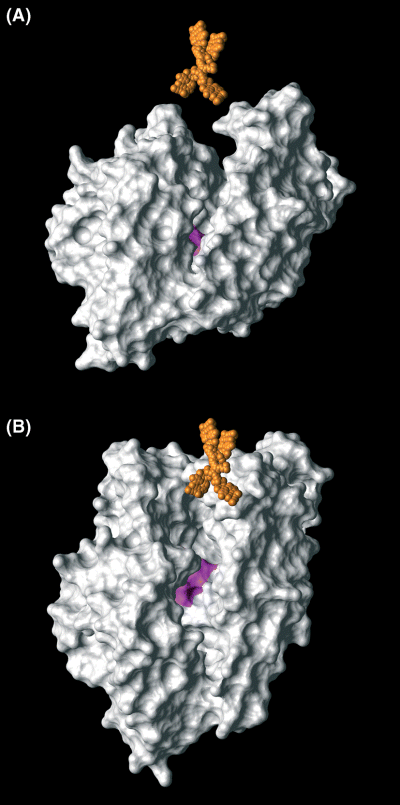
Scale representation of neurolysin (EC 3.4.24.16) (white) and tetrabranched NT (orange) as seen parallel (A) and perpendicular (B) to the major axis of the groove containing the catalytically active zinc site of the enzyme (pink).
There might be another feature of branched peptides accounting for their increased stability, i.e. the possibility to form supramolecular aggregates.
Tetrabranched peptides may arrange their four arms in parallel structures stabilized by intramolecular H-bonding of the backbone. This structure would resemble that of self-assembling β-sheet peptides. This supramolecular phenomenon is very well described, being implicated in a number of diseases caused by fibril formation (15) and is also studied for some applications in nanotechnology (16).
We analyzed the possible presence of aggregates in branched peptide solutions using gel filtration. It is known that increasing ionic strength favours aggregation of self-assembling molecules by screening of electrostatic repulsion (17). Tetrabranched NT(8–13) (3668 KDa, 0.5 mg/ml) was dissolved in PBS at different molarity and loaded on a size exclusion FPLC column (Superdex 200, 10/300 GL, Amersham Biosciences) (Figure 4). The chromatogram at physiological ionic strength (Figure 4B) showed two peaks with molecular weight lower than 20 000 KDa. The larger species increased with the ionic strength, whereas the smaller ones disappeared in 750 mm PBS.
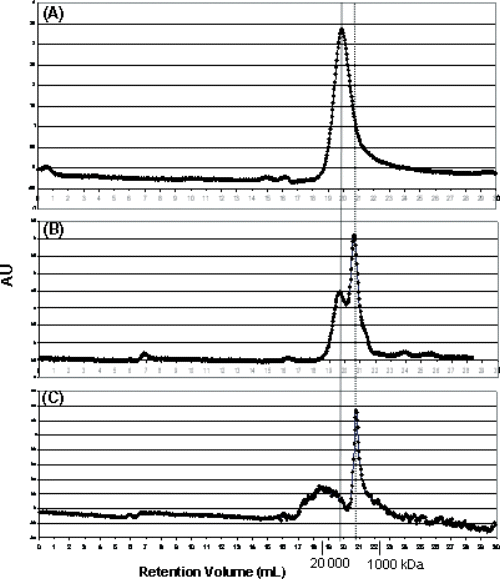
FPLC chromatograms of tetrabranched NT(8–13) Obtained in: (A) PBS 750 mm, (B) PBS 150 mm, (C) PBS 15 mm. The column was calibrated with standard molecular weights (Sigma).
We could then infer that the tetrabranched NT(8–13) monomer might be in equilibrium with a small aggregate of four or five units. The formation of small aggregates might decrease the rate of proteolysis and has to be taken into account for the increased half-life of branched peptides.
Acknowledgments
This work was supported by grants from Italian Association for Cancer Research, AIRC (Regional grant 2004–2006 to LB); Italian Ministry of University and Research (PRIN 2005 to LB); Italian Consortium for Biotechnology, CIB to LB and the University of Siena (PAR project 2005 to AP, LB and LL).




