Fingerprinting Analysis of Rhizoma Chuanxiong of Commercial Types using 1H Nuclear Magnetic Resonance Spectroscopy and High Performance Liquid Chromatography Method
Supported by the National Natural Science Foundation of China (30630073) and the Science Foundation of the State Administration of Traditional Chinese Medicine, China (02-03ZP09).
Abstract
The 1H nuclear magnetic resonance (1H NMR) fingerprints of fractionated non-polar extracts (control substance for a plant drug (CSPD) A) from Rhizoma chuanxiong, the rhizomes of Ligusticum chuanxiong Hort., of seven specimens from different sources were measured on Fourier Transform (FT)-NMR spectrometer and assigned by comparing them with the 1H NMR spectra of the isolated pure compounds. The 1H NMR fingerprints showed exclusively characteristic resonance signals of the major special constituents of the plant. Although the differences in the relative intensity of the 1H NMR signals due to a discrepancy in the ratio of the major constituents among these samples could be confirmed by high performance liquid chromatography analysis, the general features of the 1H NMR fingerprint established for an authentic sample of the rhizomes of L. chuanxiong exhibited exclusive data from those special compounds and can be used for authenticating L. Chuanxiong species.
Nuclear magnetic resonance (NMR) spectroscopy was first described and measured in molecular beams by Isidor Rabi in 1938 (Martin and Zekter 1988). It is now one of the principal techniques used to obtain chemical structural information about organic molecules in solution and the solid state due to the chemical shift and Zeeman effect on the resonant frequencies of the nuclei. Recently, NMR has been used to meta-bolomically profile several types of plants and plant functional genomes, as well as in a fingerprinting tool for authenticating and assessing the quality of natural products (Rycroft 1996; Choi et al. 2004; Shin et al. 2007; Su et al. 2008).
High performance liquid chromatography (HPLC) is a method now used to separate and analyze the chemical constituents of plants, which combines column efficiency with speed of analysis. It provides both quantitative and qualitative data on plant substances, since measurements of the area under the peaks shown on the HPLC trace are directly related to the concentration of different components in the original mixture. Like NMR, HPLC has also been used as a fingerprinting tool for authenticating and assessing the quality of natural products (Qin et al. 2004; Xie et al. 2006; Jin et al. 2006).
Methodological studies on the establishment of the system of the exclusive control substance for a plant drug (CSPD, including CSPD A, B, and C, etc. according to the different metabolites from fractionation of plant drug) and on authenticating plant species by 1H NMR and HPLC fingerprinting analysis of CSPD have been reported previously by the authors (Qin et al. 2004, 2005; Su et al. 2008). It was concluded that the 1H NMR and HPLC fingerprinting techniques are rapid, reproducible and stable with time for the authentication of medicinal plant species. But, it should be pointed out that fingerprinting analysis of medicinal plant using 1H NMR spectroscopy and the HPLC method must be based on the detailed investigation of chemical constituents of the corresponding CSPD for the purpose of clearly exhibiting the existence of their characteristic constituents. In the present paper, we report on the application of 1H NMR and HPLC fingerprinting analysis of specific metabolites for the identification of Ligusticum chuanxiong Hort. species.
L. chuanxiong belongs to the family Umbelliferae and is mainly distributed in southwest China, especially in Sichuan Province. In traditional Chinese medicine (TCM), the commercial soaked, sliced, and dried form of Rhizoma chuanxiong, the rhizomes of L. chuanxiong, has long been used for activating blood circulation and acesodyne, as well as for the treatment of cerebro- and cardio-vascular diseases. Previous investigations on chemical constituents of the plant led to the isolation of aromatic compounds, terpenoids, organic acids, sugars, alkaloids, and phthalides (Cao et al. 1983; Xiao et al. 2002). The rhizomes of L. chuanxiong contain up to 1.2% volatile oil in which alkyl phthalides are the major components with up to 32%–76% (Zhong et al. 1996). In the present paper, the non-polar and neutral CSPD A from Rhizoma chuanxiong of seven commercial types collected from different regions in China were obtained by the procedure reported previously (Qin et al. 2005; Su et al. 2008). The 1H NMR spectra and HPLC profiles of these CSPD A were recorded on an Fourier transform-nuclear magnetic resonance (FT-NMR) spectrometer and on a Shimadzu 10A HPLC instrument, respectively, for the purpose of analyzing and comparing the characteristics and the differences in the major constituents. The signals of the 1H NMR and HPLC fingerprints of the samples were also analyzed and assigned mainly by comparing them with each other and with the spectra and profiles of the isolated pure compounds.
Results
The investigation of the chemical constituents showed that the neutral compounds, phthalide derivatives, were the main and characteristic constituents of CSPD A from the rhizomes of L. chuanxiong. Repeated silica gel column chromatographic processes on the CSPD A allowed the isolation of six phthalides, (Z)-ligustilide (1) (Beck and Stermitz 1995), senkyunolide A (2) (Naito et al. 1992), Z-Levistolide A (3) (Su et al. 2005), (Z)-6,8′,7,3′-diligustilide (6) (Kaouadji et al. 1986), senkyunolide H (7) (Naito et al. 1992), Senkyunolide I (8) (Naito et al. 1992), along with pregnenolone (4) (Terada et al. 1978), 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol (5) (Wang et al. 2006), and eicosanoic acid (9) (Liang et al. 2005) (Figure 1). These structures were identified by comparison of their signals with published data, and compound 5 was isolated from L. chuanxiong for the first time.
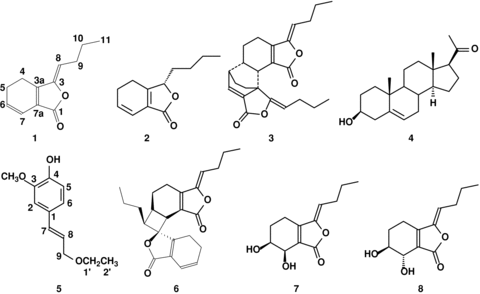
Chemical structures of eight compounds isolated from the control substance for a plant drug (CSPD) A of the rhizomes of Ligusticum chuanxiong (Compounds: (Z)-ligustilide (1), senkyunolide A (2), Z-Levistolide A (3), pregnenolone (4), 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol (5), (Z)-6,8′,7,3′-diligustilide (6), senkyunolide H (7), Senkyunolide I (8)).
The samples of L. chuanxiong used in this work showed good identity to each other in respect to the analysis of their characteristics. The 1H NMR spectra of their CSPD A exhibited exclusive characteristic features in the composition of signals (Table 1, Figure 2). By comparing the 1H NMR spectra with those of the pure compounds 1–9 isolated from CSPD A, it was found that many peaks of aliphatic, alkoxyl and aromatic regions could be detected without the need for chromatographic separation.
| Data of fingerprint | Dujiangyan City Sichun Province | Chongzhou City Sichun Province | Xi'an City Shaanxi Province | Huhehaote City Inner Mongolia | Taiyuan City Shanxi Province | Shijiazhuang City Hebei Province | Beijing City | Patterns of signal |
|---|---|---|---|---|---|---|---|---|
| 7.33–7.90 | + | + | + | + | + | + | + | ND m |
| 6.93 | + | + | + | + | + | + | + | s |
| 6.88 | + | + | + | + | + | + | + | d, J= 8.1 Hz |
| 6.85 | + | + | + | + | + | + | + | d, J= 8.1 Hz |
| 6.62 | + | + | + | + | + | + | + | d, J= 16.2 Hz |
| 6.25 | + | + | + | + | + | + | + | dt, J= 9.5, 1.5 Hz |
| 6.21 | + | + | + | + | + | + | + | d, J= 9.3 Hz |
| 5.97 | + | + | + | + | + | + | + | dt, J= 9.5, 4 Hz |
| 5.91 | + | + | + | + | + | + | + | m |
| 5.20 | + | + | + | + | + | + | + | t, J= 8 Hz |
| 4.93 | + | + | + | + | + | + | +† | dd, J= 6.5, 4 Hz |
| 4.82 | + | + | + | + | + | + | + | d, J= 6.6 Hz |
| 4.12 | − | − | + | + | + | + | + | d, J= 6 Hz |
| 3.91 | + | + | + | + | + | + | + | s |
| 3.89 | + | + | + | + | + | + | + | s |
| 3.54 | − | − | + | + | + | + | + | q, J= 6.6 Hz |
| 2.57 | + | + | + | + | + | + | + | ND m |
| 2.47 | + | + | + | + | + | + | + | ND m |
| 2.43–2.48 | + | + | + | + | + | + | + | ND m |
| 2.37 | + | + | + | + | + | + | + | q, J= 7.5 Hz |
| 1.83–1.91 | + | + | + | + | + | + | + | m |
| 1.50–1.57 | + | + | + | + | + | + | + | m |
| 1.48 | + | + | + | + | + | + | + | sex, J= 7.2 Hz |
| 1.30–1.43 | + | + | + | + | + | + | + | ND m |
| 0.95 | + | + | + | + | + | + | + | T, J= 6.9 Hz |
| 0.90 | + | + | + | + | + | + | + | T, J= 6.6 Hz |
- +, exhibited the preceding signal; ND, not differentiated. †Sometimes gives br s when there is a poor resolution capacity.
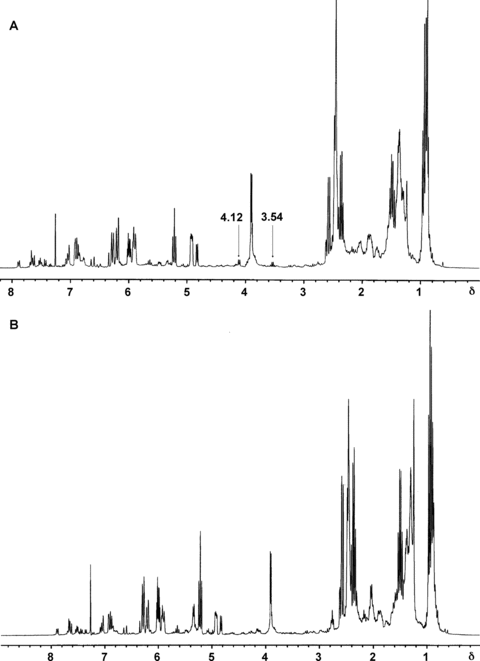
1H nuclear magnetic resonance imaging (NMR) profiles of the control substance for a plant drug (CSPD) A of the rhizomes of Ligusticum chuanxiong (these spectra were recorded on a Varian Mercury-300 NMR spectrometer in CDCl3. Axis X represents chemical shifts (δ), given in ppm using tetramethylsilane (TMS) as an internal standard (δ 0.00). The seven samples of L. chuanxiong used in the present study showed good identity to each other in respect to the composition of signals (axis X). Two typical spectra, A from Xi'an City, Shaanxi Province, and B from Dujiangyan City, Sichun Province, are given in this figure. Differences in the relative intensity of the 1H NMR signals are due to the discrepancy in the ratio of the major constituents among these samples (axis Y). For example, the signals at δ 4.12 (d, J= 6 Hz) and 3.54 (q, J= 6.6 Hz) from CH2-9 and CH2-1′ of compound 5 could be observed on Figure 2A, but not on Figure 2B. This was because the relative intensity of these signals was lower than the signal at δ 3.89 from CH3O of compound 5 due to the difference in the amounts of protons and splitting patterns in the pure compound. This did not mean the absence of compound 5 in the sample from Dujiangyan City, since the other major signals of compound 5 could be observed in Figure 2B.
High performance liquid chromatography was applied to analyze and compare the differences in the major constituents of the CSPD A from these different samples of L. chuanxiong. As shown in Figure 3 and Table 2, the HPLC profiles mainly displayed 15 identical peaks under present chromatographic conditions. Compounds isolated in the present work were measured as reference substances with the same HPLC condition and their retention times were compared with those of the peaks in the HPLC profiles. As a result, some of the peaks were ascribed to their corresponding counterparts, that is, peaks at 5.27, 13.92, 16.90, 18.49 and 29.30 min corresponded to compounds 5, 3, 2, 6, 1, respectively, with (Z)-ligustilide (1) and senkyunolide A (2) predominated, and this analysis was identical to the 1H NMR experiment. The compound corresponding to retention time at 25.03 min showed a considerable amount in all of the samples used. However, it hadn't been isolated as a monomer in the present work.
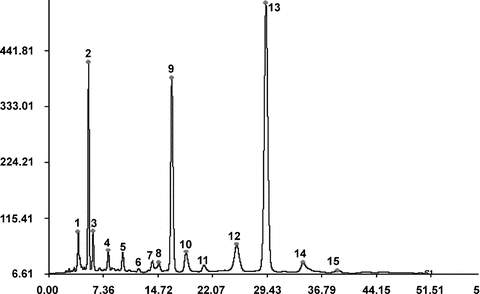
High performance liquid chromatography (HPLC) profile of control substance for a plant drug (CSPD) A from the rhizomes of Ligusticum chuanxiong (the compounds isolated in the present study were measured as control substances in the same chromatographic condition and the retention times of the major ones were as follows: 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol (peak 2), 5.27 min; Z-Levistolide A (peak 7), 13.92 min; senkyunolide A (peak 9), 16.90 min; (Z)-6,8′,7,3′-diligustilide (peak 10), 18.49 min; (Z)-ligustilide (peak 13), 29.30 min. Other peaks could not be detected in the present study.
| Peak No. | Beijing City | Chongzhou City Sichuan Province | Dujiangyan City Sichuan Province | Huhehaote City Inner Mongolia | Xi'an City Shaanxi Province | Shijiazhuang City Hebei Province | Taiyuan City Shanxi Province | Average values |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.021 | 0.069 | 0.156 | 0.016 | 0.266 | 0.009 | 0.011 | 0.078 |
| 2 | 0.818 | 0.078 | 0.205 | 0.318 | 0.737 | 0.242 | 0.202 | 0.371 |
| 3 | 0.151 | 0.018 | 0.053 | 0.061 | 0.154 | 0.048 | 0.041 | 0.075 |
| 4 | 0.094 | 0.019 | 0.032 | 0.029 | 0.074 | 0.037 | 0.037 | 0.046 |
| 5 | 0.157 | 0.001 | 0.003 | 0.044 | 0.021 | 0.046 | 0.038 | 0.044 |
| 6 | 0.017 | 0.003 | 0.008 | 0.005 | 0.023 | 0.009 | 0.011 | 0.011 |
| 7 | 0.034 | 0.012 | 0.040 | 0.020 | 0.140 | 0.041 | 0.008 | 0.042 |
| 8 | 0.066 | 0.010 | 0.027 | 0.088 | 0.088 | 0.027 | 0.018 | 0.046 |
| 9 | 0.962 | 0.196 | 0.414 | 1.337 | 1.337 | 0.424 | 0.538 | 0.744 |
| 10 | 0.188 | 0.027 | 0.084 | 0.359 | 0.359 | 0.065 | 0.058 | 0.163 |
| 11 | 0.086 | 0.003 | 0.007 | 0.089 | 0.089 | 0.027 | 0.045 | 0.049 |
| 12 | 0.117 | 0.07 | 0.142 | 0.263 | 0.263 | 0.145 | 0.088 | 0.155 |
| 13(R) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 14 | 0.458 | 0.013 | 0.035 | 0.096 | 0.096 | 0.034 | 0.025 | 0.108 |
| 15 | 0.056 | 0.005 | 0.014 | 0.040 | 0.040 | 0.017 | 0.017 | 0.027 |
Discussion
The 1H NMR spectra of CSPD A of the rhizomes of L. chuanxiong showed good identity in respect to the specificity for the authentication of L. Chuanxiong species to each other and were mainly formed by overlapping of the signals of phthalide derivatives, fatty compounds and 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol, in which the characteristic signals of (Z)-ligustilide (1) and senkyunolide A (2) predominated, and 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol (5) took third place. The spectra could be simply divided into three sections, that is, an aliphatic region with δ 0.8–2.8, an alkoxyl region with about δ 3.5–4.93 and an aromatic/alkenic region with δ 5.1–7.9, which were identical to the structural characteristics of the phthalide derivatives, fatty compounds and 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol. The first region included the overlapped signals of saturated aliphatic protons of phthalide derivatives. Apart from the well-defined signals from (Z)-ligustilide (1), that is, the signal of methyl group (CH3-11) at δ 0.95 (t, J= 6.9 Hz) and the signals of two methylene groups (CH2-9,10) on the butylidene side chain at δ 1.48 (sex, J= 7.2 Hz), 2.37 (q, J= 7.5 Hz), and those of the two methylene groups (CH2-4,5) at δ 2.47 (m) and 2.60 (m), the remaining signals come mainly from senkyunolide A (2), including δ 0.90 (t, J= 6.6 Hz), 1.30–1.43 (m), 1.50–1.57 (m), 1.83–1.91 (m), and 2.43–2.48 (m) due to CH3-11, CH2-9 and CH2-10, H-8a, H-8b, and CH2-4 and CH2-5, respectively. The signal of Me-2′ of 5 was also overlapped in the aliphatic region at δ–1.25 but could not be differentiated. The second region mainly included the signals at δ 4.12 (d, J= 6 Hz), 3.89 (s), and 3.54 (q, J= 6.6 Hz) from three alkoxyl groups of 5, CH2-9, CH3O, and CH2-1′; and at δ 4.93 (dd, J= 6.5, 4 Hz) from CH-3 of senkyunolide A (2), and the other two strong signals at δ 3.91 (s) and 4.82 (d, J= 6.6 Hz) could not be assigned due to the failure of isolating corresponding compounds. Because of the relatively less content of 5 in some samples, the signals of CH2-9 and CH2-2′ couldn't be detected in their spectroscopy. The aromatic/alkenic region included the overlapped signals of unsaturated protons of phthalide derivatives and phenylpropenoids, in which the well-differentiated signals also come mainly from (Z)-ligustilide (1), senkyunolide A (2) and 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol (5), that is, δ 6.25 (dt, J= 9.5, 1.5 Hz), and 5.97 (dt, J= 9.5, 4 Hz) and 5.20 (t, J= 8 Hz) assigned to those of the olefinic methine groups of CH-7,6 of cyclohexene and the olefinic proton of CH-8 on the butylidene side chain of (Z)-ligustilide (1), δ 6.21 (d, J= 9.3 Hz) and δ 5.91 (m) could be recognized as the signals of H-7 and H-6 of senkyunolide A (2), and δ 6.93 (s), 6.88 (d, J= 8.1 Hz), 6.85 (d, J= 8.1 Hz), 6.62 (d, J= 16.2 Hz) to H-2, H-6, H-5 and H-7 of compound 5.
A few weak signals at δ 7.33–7.90 were not assigned because the separation of the corresponding compounds failed, and compounds 1, 2, and 5 predominated to the extent that the other compound's signals were suppressed among the chemical constituents of CSPD A of the rhizomes of L. chuanxiong. By comparison of the spectral data with published values, the unknown peaks were preliminarily ascribed to those of butylphthalide and butylidene phthalide, which has been isolated and identified from the rhizomes of L. chuanxiong previously (Wang and Gao 1985).
Although the relative area of each peak in the HPLC profiles of CSPD A of the rhizomes of L. chuanxiong showed distinct differences among seven samples (Table 2), the 15 peaks mentioned in the section of results were determined to be common in CSPD A of the rhizomes of L. chuanxiong. On the basis of careful comparison of the HPLC profiles of the seven samples, as well as the assignment of the major peaks with comparison of control substances, a conclusion could be obtained that the HPLC profiles could also be used easily for original identification of L. chuanxiong. The differences from relative content of the major similar constituents of their CSPDs explained the differences in the relative signal intensity of the corresponding 1H NMR spectroscopy, and this property belongs to the natural character of the specific metabolites L. chuanxiong.
Materials and Methods
General experimental procedures
Nuclear magnetic resonance spectra were recorded on a Varian Mercury-300 NMR spectrometer in CDCl3 using VMR 6.2C NMR software package and Z-axis gradients. Chemical shifts (δ) were given in ppm using tetramethylsilane (TMS) as internal standard (δ 0.00). HPLC were recorded at Shimadzu 10A instrument with UV detector, and the G. R. methanol was produced by the Institute for Fine Chemical Engineering of Huaian Plastic Product Factory, Jiangsu province, China; silica gel (Qingdao Marine Chemical Factory) was used for column chromatography (CC). Solvents were of analytical grade and were purchased from Beijing Chemical Company, Beijing, China.
Plant material
The rhizomes of Ligusticum chuanxiong Hort. of the commercial types used were purchased from seven different cities in China (Table 1). All samples were authenticated by authors by comparison of the 1H NMR fingerprints of their CSPD A with that of an authentic sample, as well as by comparing with a voucher specimen at the Herbarium of the Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College. In terms of morphology, all of these samples showed the same features as the description for Rhizoma chuanxiong in the state Pharmacopoeia of China (The State Pharmacopoeia Commission of China 2005). Voucher specimens were deposited in the Department of Natural Medicinal Chemistry, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Experimental procedures for 1H NMR fingerprints of CSPD A
The air-dried and powdered rhizome (50 g) of L. chuanxiong was extracted with 95% aq. EtOH (2 × 100 mL, 1 h, 0.5 h) under reflux. The combined EtOH extracts were evaporated in vacuum to yield a brown residue, which was suspended in 80% aq. EtOH (100 mL). The resulting suspension was extracted with petroleum ether (60–90 °C) (3 × 100 mL). Evaporation of the aq. layer in vacuum yielded a brown residue, which was dissolved in water (100 mL), and then extracted with EtOAc (2 × 100 mL). On finishing the extraction, 20 g of NaCl was added to the aq. phase, which was re-extracted with EtOAc (100 mL). The combined EtOAc phases were washed with 5% aq. NaHCO3 solution (3 × 100 mL) and H2O (2 × 100 mL, to pH 7), respectively, and then dried over Na2SO4. Complete removal of EtOAc in vacuo and boiling water led to CSPD A[0]. The 1H NMR spectrum was recorded directly. A variety of sample replicates have previously been prepared to assess the validity of this procedure (Qin et al. 2005).
Isolation of chemical constituents of CSPD A
The treatment of the air-dried and powdered rhizomes (6 kg) of L. chuanxiong by the procedure mentioned above gave CSPD A (58.5 g). In the extraction process, many sucrose crystals were isolated when cooling the concentrated 95% EtOH solution. Chromatographic separation of the CSPD A on silica gel (200–300 mesh) using petroleum ether (60–90 °C)/EtOAc as eluent with increasing polarity (petroleum ether/EtOAc = 50:1 → 5:5) led to 260 fractions (250 mL for each). The fractions were combined according to TLC monitoring, which afford 1 (22 g), 2 (7 g) with the eluent ratio of 20:1; 3 (85 mg), 5 (2 g), 6 (13 mg) and 9 (16 mg) with the ratio of 10:1, and 4 (9 mg) with the ratio of 5:1. Fraction with the eluent ratio of 4:1 (800 mg) was finally purified by RP-HPLC, eluted with 18% CH3CN in water, to give compound 7 (9 mg) and 8 (23 mg).
Experimental procedures for HPLC profiles of CSPD A
Approximately 8 mg of each CSPD A was dissolved in methanol to give a total volume of 10.0 mL and then filtered with a 0.45 μm polytetrafluoroethylene (PTFE) syringe filter. The solutions were injected into the HPLC system, and after about 60 min running time, the profiles of CSPD A were recorded. Approximately 3 mg of reference compounds 1, 2, 3, 5, and 6 isolated and identified by the authors in our laboratory were dissolved in methanol to give a total volume of 2.0 mL and then injected into HPLC after filtration with a 0.45 μm syringe filter (PTFE).
The analysis was carried out with a Kromasil C18 column (250 mm × 4.6 mm, I.D. 5 μm) at column temperature of 25 °C. The mobile phase consisted of methanol, water and phosphoric acid using an invariable elution at 600:400:5. The flow rate was 1.0 mL/min and the wave length of the UV detector was set at 280 nm. The volume of injection was 10 μL.
(Handling editor: Katie Dehesh)
Acknowledgements
We thank the Department of Instrumental Analysis, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, for the measurements of NMR spectra.




