Localization and Dynamic Change of Saponin in Vegetative Organs of Polygala tenuifolia
Supported by Funded Projects of Independent Innovation of Northwest University Postgraduates (07YZZ25) and the Shaanxi Provincial Natural Science Foundation (SJ08-ZT02).
Abstract
Anatomical, histochemical and phytochemical methods were used to investigate the structure, localization and dynamic changes of total saponin and senegenin of vegetative organs in Polygala tenuifolia Willd. Histochemical localization results showed that saponin accumulated mainly in parenchyma cells of vegetative organs. The phytochemical results also showed that the saponin accumulated in the vegetative organs of P. tenuifolia, with higher content in roots and lower content in the aerial parts that included stems and leaves. The saponin content and dry weight of the vegetative organs of P. tenuifolia had dynamic variance at the developmental stages and all reached the highest level in the post-fruit period. Hence, the roots and aerial parts should be gathered in August to make full use of the plant. As the root is the main medicinal organ of P. tenuifolia, the content of total saponin and senegenin of different aged and different parts of the root were determined. The content of total saponin and senegenin exhibited a sustained decreasing trend with increasing root age; therefore, the annual roots had high quality. The content of total saponin and senegenin in different parts of the root showed obvious variation. The content in the “skin areas” was much higher than that of xylem. The results offer a theoretical basis for determining the appropriate harvesting stage and a reasonable harvest of P. tenuifolia.
Polygala tenuifolia Willd (Polygalaceae) is a perennial herbaceous plant widely distributed in the Shanxi, Shaanxi and Henan provinces, China. It is a well-known traditional Chinese herb and its root has many medicinal functions, such as eliminating phlegm, detumescence, smoothing the nerves and enhancing intelligence (Chinese Pharmacopoeia Committee 2005). Extensive phytochemical and pharmacological studies on this plant proved the saponin to be the main bioactive ingredient (Fu and Zhang 2006). In recent years, research has indicated that saponin can also decrease high blood sugar, enhance the immune system, and reduce high blood pressure (Nagai and Suzuki 2001; Sun 2005). As far as medicinal parts are concerned, Chinese Pharmacopoeia stipulates the root of P. tenuifolia to be a crude drug (2005). At present, there have been many studies made on P. tenuifolia. Most of them have focused on the chemical structure and identification of saponin (Sakuma and Shoji 1981a,b), pharmacology (Chen et al. 2004), clinical application (Jung et al. 2007), content determination (Liu et al. 2001; Yang and Sun 2007) as well as the root development and anatomy (Teng and Hu 2008). However, studies on the localization and accumulation of saponin in vegetative organs of P. tenuifolia have not been reported. Originally, P. tenuifolia mainly grows wild in nature, but in recent years, the wild drugs of P. tenuifolia were nearly extinct because of over-harvesting. At the same time, cultivated crude drugs in Shanxi and Shaanxi are unable to meet market demand because of limited amounts. The aim of this study was to analyze the structural characteristics of vegetative organs, and ultimately investigate the localization and dynamic change of saponin in P. tenuifolia at different developmental stages, so as to offer a theoretical basis for rational application and development of P. tenuifolia resources.
Results
The secondary structure of root and histochemical localization of saponin
P. tenuifolia roots at different ages were collected from the middle part of the taproot (the primary structure occurs at the root apex). The root secondary structure of P. tenuifolia consists of periderm and secondary vascular tissue crosswise (Figure 1A), and the structures of different aged roots were similar. Periderm consisted of phellem, phellogen and phelloderm. With a suberized wall, the seven to nine cell layers of phellem were closely arranged. Phellogen had one layer of cells, and phelloderm had two to three layers of parenchyma cells (Figure 1B). Secondary vascular tissue consisted of secondary phloem, vascular cambium and secondary xylem. The root cross-section was mainly composed of secondary phloem, the thickness of which made up about three-fifths to two-thirds of the root diameter. Phloem parenchyma cells were round or nearly round in shape, large or small in size, and formed the majority of secondary phloem (Figure 1A,B). There were rare sieve vessels and companion cells among phloem parenchyma cells and phloem fiber was not found. In comparison with secondary phloem, secondary xylem made up two-fifths to one-third of the whole diameter of the taproot and was composed of woody fibers, vessels, a few xylem parenchyma cells and xylem rays (Figure 1A,C). Vessels had different diameters and were scattered with higher frequency. Woody fibers were distributed in a clumped manner. Most xylem rays comprised a row of cells, and few comprised two rows of cells (Figure 1A,C).
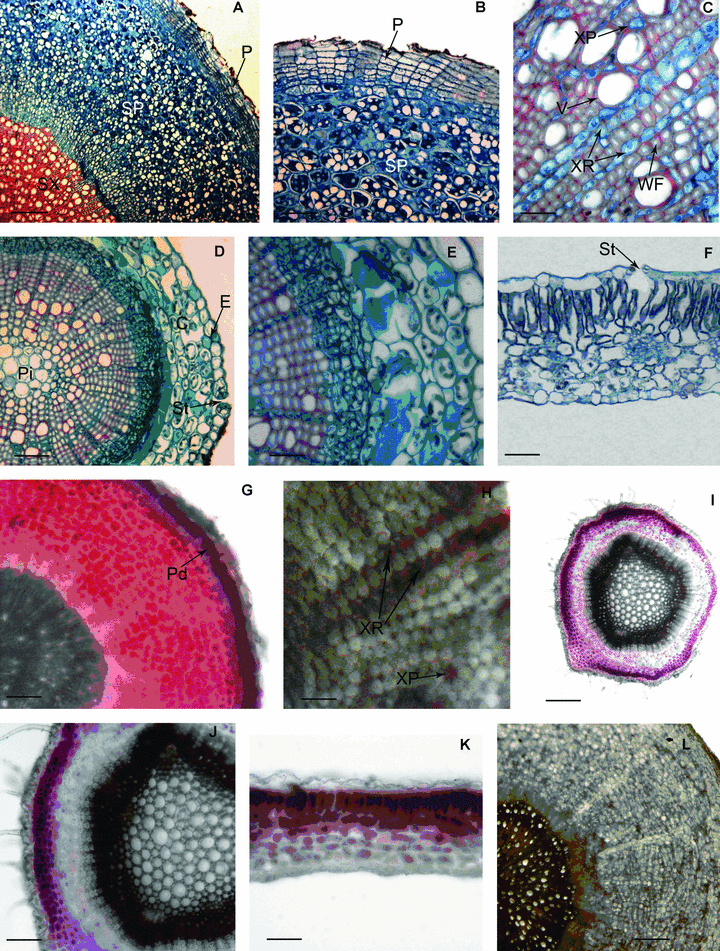
(A–L) Structure of the vegetative organs of Polygala tenuifolia and the localization of saponin in them. (A) Portion of cross-section of biennial taproot, showing each part of secondary structure. P, periderm; SP, secondary phloem; SX, secondary xylem (scale bars, 200 μm). (B) Portion of cross-section of biennial taproot, showing the secondary phloem and periderm. P, periderm; SP, secondary phloem (scale bars, 100 μm). (C) Portion of cross section of biennial taproot, showing the vessels, wood fibers and xylem rays of secondary xylem. V, vessel; XR, sylem ray; XP, sylem parenchyma cell; WF, wood fiber (scale bars, 25 μm). (D) Portion of cross-section of stem, showing the epiderm, cortex, vascular cylinder and stoma. C, cortex; E, epiderm; Pi, pith; St, stoma (scale bars, 50 μm). (E) Portion of cross-section of stem, showing the characteristics of cells in each part (scale bars, 25 μm). (F) Portion of cross-section of leaf, showing the portion of every part, the epiderm, mesophyll tissue, stoma and substomatal chamber. St, stoma (scale bars, 50 μm). (G) Cross-section of root having secondary structure, showing secondary phloem displayed red and phelloderm displayed purplish red. Pd, phelloderm (scale bars, 100 μm). (H) Cross-section of root having secondary structure, showing xylem rays and xylem parenchyma cells displayed pale red. XR, xylem ray; XP, xylem parenchyma cell (scale bars, 25 μm). (I) Cross-section of stem, showing cortex displayed purplish red, epidermis and secondary phloem displayed pale red (scale bars, 200 μm). (J) Showing the partial magnification of the Figure 1I (scale bars, 100 μm). (K) Cross-section of leaf, showing palisade tissue displayed red and epidermis as well as spongy tissue displayed pale red (scale bars, 50 μm). (L) Cross-section of root having secondary structure, showing the root sections could not produce characteristic color in the control experiment (scale bars, 100 μm).
Histochemical localization results in roots with secondary structures showed that phelloderm displayed a purplish red color, secondary phloem red, xylem ray and xylem parenchyma were pale red, while other cells displayed no color (Figure 1G,H).
Structure of stem and histochemical localization of saponin
The stem of P. tenuifolia was composed of epidermis, cortex and vascular bundle from outside to inside (Figure 1D,E). The epidermis cells were square or rectangular in shape, small or big in size, and arranged closely. There were stomatal apparatus among epidermis cells (Figure 1D). In the cortex, there were four to five layers of big parenchyma cells that were nearly round and arranged loosely. There were chloroplasts in the outer two to three layers of parenchyma cells, and the inner one to two layers of parenchyma cells were big and dark-dyed (Figure 1D,E). The central cylinder consisted of vascular bundle and pith, and the vascular bundle connected into a circle. In the vascular bundle, the phloem consisted of sieve tubes, companion cells and phloem parenchyma cells. The cells of phloem were lined together closely. Vascular cambium that lied between phloem and xylem was not obvious. Xylem consisted of a large number of xylem fibers, vessels and few xylem parenchyma cells. Xylem fibers lined radially, and vessels had wider diameter and were arranged irregularly. Xylem rays were obvious, most of them comprised one row of cells; few comprised two rows (Figure 1E). There were well-developed pith in the center of the stem, and the pith was composed of big parenchyma cells, which were nearly round (Figure 1D).
Histochemical localization results showed that in the stem, the cortex displayed a purplish red color, epidermis and secondary phloem were pale red, while the other tissues had no color (Figure 1I,J).
Structure of leaf and histochemical localization of saponin
The leaf of P. tenuifolia was typically bifacial. The lamina of P. tenuifolia consisted of epidermis, mesophyll and leaf vein. Two sides of leaf superficies comprised a layer of epidermic cells, and the cells were mainly rectangular or square, arranged irregularly and closely. There were stomatal apparatus among the epidermic cells of the upper and lower epidermis. Stomatal apparatus had a bigger substomatal chamber. The mesophyll was composed of distinguished palisade tissue and spongy tissue. The palisade tissue cells were arranged orderly. The spongy tissue was composed of three to four layers of cells that arranged loosely and had obvious interspace (Figure 1F).
Histochemical localization results showed that palisade tissue displayed a red color, and epidermis and spongy tissue was pale red (Figure 1K).
In the control experiment, the sections of different vegetative organs that had been treated with FAA (formalin-aceticacid-70% alcohol) could not react with 5% vanillin-glacial acetic acid-perchloric acid solution to produce characteristic color (Figure 1L). The above results indicate that saponin accumulates in root, stem and leaf of P. tenuifolia, and mainly in parenchymal cells of vegetative organs.
Content change of total saponin and dry weight of vegetative organs of P. tenuifolia at different developmental stages
The results of histochemical localization had preliminarily proved that the saponin was distributed in vegetative organs of P. tenuifolia. We determined the content of total saponin at different developmental stages in vegetative organs of annual plants of P. tenuifolia to support the above results. The results showed that the saponin accumulated in the vegetative organs of P. tenuifolia, with higher content in roots and lower content in the aerial parts, which include stems and leaves (Figure 2). The content in the aerial parts was on average about one-fifth as much as that in roots at all developmental stages, and was about a quarter of those in roots in the post-fruit period. The statistical analysis demonstrated that the total saponin content of vegetative organs at the four stages showed significant differences (P < 0.05). At the same time, the total saponin content of vegetative organs showed a dynamic variance trend during the whole growth period from April to October. At the pre-blossom period, the saponin content in the root was high. With the efflorescence and fruits forming, accumulation of saponins decreased. After the blossom-fruit period, the content increased comparatively quickly, reaching the highest level of 5.176% at the post-fruit period. With the withering period coming, the content decreased again, and came to the lowest level during the whole growth period. On the other hand, the saponin content in aerial parts increased with the plant development before the post-fruit period, and also reached the highest level of 1.296% at the post-fruit period. Afterward, the content decreased and was close to the level of the pre-blossom period.
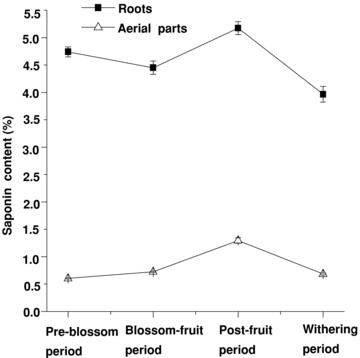
The dynamic variation of saponin content in annual roots and aerial parts at different stages (bars are mean ± standard error (SE)).
The dry weight of roots and aerial parts increased from the blossom-fruit period to the post-fruit period, and reached the crescendo at the post-fruit period. After the post-fruit period the dry weight began to decrease (Figure 3). The saponin content and dry weight in annual roots and aerial parts all were at their highest-level during the post-fruit period (2, 3).
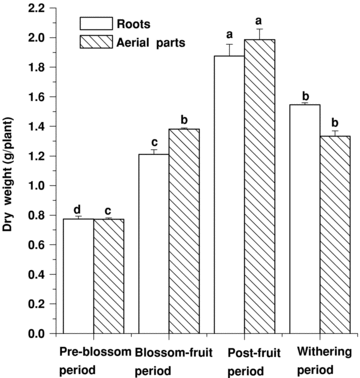
The dynamic variation of dry weight in annual roots and aerial parts at different stages (bars are mean ± standard error (SE)). Different letters among the different materials of the same item means significant difference at 0.05 level.
Content change of total saponin and senegenin in different aged roots of P. tenuifolia
Practically, the crude drugs of roots of P. tenuifolia are usually gathered at autumn of the second or third growth year. We determined the content of total saponin and senegenin in different aged roots of P. tenuifolia. The result shows that the content of total saponin and senegenin of annual roots was higher than that of biennial roots, and that the content of biennial roots was higher than that of triennial roots (Figure 4). The content of total saponin and senegenin of different aged roots showed a significant difference (P < 0.05).
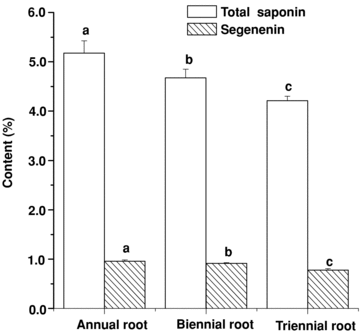
The content change of total saponin and senegenin in different year old roots of Polygala tenuifolia (bars are mean ± standard error (SE)). Different letters among the different materials of the same item means significant difference at the 0.05 level.
Content change of total saponin and senegenin in different parts of roots of P. tenuifolia
The results of histochemical localization had preliminarily proved that the saponin was mainly distributed in “skin areas” of the root of P. tenuifolia, and less distributed in the xylem. The roots of P. tenuifolia collected in August of the first, second, and third growth year were manually separated into two parts: the xylem and the “skin areas”, which included periderm and phloem, both for determination of total saponin and senegenin content. The results indicated that the content of total saponin and senegenin in different parts of root differed noticeably. On average, the content of total saponins and senegenin of the “skin areas” of different aged roots were about 13.51 and 14.25 times that in the xylem at the different developmental stages, respectively, indicating that the “skin areas” were the main storage locus. At the same time, the content of total saponin and senegenin of the “skin areas” decreased with increasing growth year, the content of total saponin and senegenin of the xylem of different aged roots were lower and mutually approximate. It could be found that the quality of annual root was better (Figure 5).
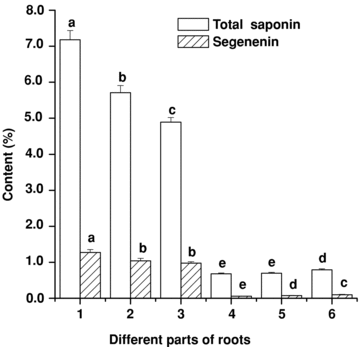
The content change of total saponin and senegenin in different parts of roots of Polygala tenuifolia (bars are mean ± standard error (SE)). 1, “skin areas” of annual roots; 2, “skin areas” of biennial roots; 3, “skin areas” of triennial roots; 4, xylem of annual roots; 5, xylem of biennial roots; 6, xylem of triennial roots. Different letters among the different materials of the same item means significant difference at the 0.05 level.
Discussion
The results from the study showed that the structure of vegetative organs of P. tenuifolia resembled those of usual dicots. However, in the root, the secondary phloem took up a great part of secondary vascular tissue, and mainly contained parenchyma. This characteristic is consistent with the storing function of the root of P. tenuifolia.
The histochemical localization of saponin had been carried out on many plants, including Panax, Gynostemma pentaphyllum, and Bupleurum chinense (Kubo 1980; Liu et al. 2005; Tan et al. 2007). These studies indicate that saponin can react with vanillin-glacial acetic acid-perchloric acid solution to produce characteristic colors, from pale red to red to purplish red, and the accumulation of saponin is positively related to different shades of red. The saponin in P. tenuifolia was pentacyclic triterpenoid, which belongs to the oleanane type, so we evaluated the localization and accumulation of saponin by the above-mentioned method. Through histochemical experiments, saponin in P. tenuifolia was found to be accumulating in root, stem and leaf. In the root, phelloderm and secondary phloem showed purplish red and red color, whereas xylem ray cells and xylem parenchyma cells showed pale red color. Consequently saponin accumulated mainly in phelloderm, secondary phloem, rarely in xylem ray cells and xylem parenchyma cells. In stem, saponin was distributed mainly in cortex, less in epidermis and secondary phloem. While in leaf, saponin was mainly distributed in palisade tissue, and less in epidermis and spongy tissue. Thus, it can be hypothesized that saponin accumulates mainly in parenchyma of vegetative organs. The result was consistent with the study made on G. pentaphyllum in which the distribution of ginsenoside was concluded to be located mainly in the parenchyma cells of the vegetative organ (Liu et al. 2005).
Through ultraviolet spectrophotometer determination, we found that there was saponin accumulating in roots and aerial parts of P. tenuifolia; this result was consistent with the findings obtained through the histochemistry experiment. At the same time, the total saponin content in roots was higher than that in aerial parts at whole developmental stages. The content in the aerial parts was about one-fifth as much as that in roots on average during the developmental stages, and was about a quarter of that in roots at the post-fruit period. Based on these results, we advocate the use of aerial parts of P. tenuifolia facing the shortage of P. tenuifolia resources today.
The total saponin content in vegetative organs of P. tenuifolia at whole developmental stages showed dynamic variance trends. The trends displayed that the total saponin content was low before June. Because vegetative growth and reproductive growth were exuberant in this period, a great amount of photosynthesis products were consumed, and the total saponin accumulation was low. After the middle 10 d of June, accompanied with the reproductive growth nearly finishing, the nutrient consumption decreased, so the content of saponin increased comparatively quickly from the blossom-fruit period, and reached a crescendo in the post-fruit period. Afterward, with the withering of stems and leaves, the saponin content in roots and aerial parts decreased because of the decreasing amount of photosynthesis product.
The trend of dynamic variance of dry weight of vegetative organs showed that the dry weight both in roots and aerial parts reached the crescendo at the post-fruit period; this trend was consistent with that of the saponin contents. Therefore, P. tenuifolia should be harvested in the post-fruit period for obtaining more saponin. We should collect the stems and leaves before they wither in order to make full use of P. tenuifolia.
Judging from the content of total saponin and senegenin, we found that the content of annual roots was higher than that of the biennial roots and triennial roots, so the annual roots had better quality. We guessed that triterpenoid in P. tenuifolia may be chemical defenses based on the fact that P. tenuifolia grows in barren and drought land, and is not susceptible to plant diseases and insect pests. From the former research, some triterpenoids had been confirmed to be chemical defenses (Govindachari et al. 2000). For example, the triterpenoids of plant in Meliaceae have repellent and poisonous effects to some insects (Zhang and Zhao 1983; Zhao et al. 1984; Champagne et al. 1992), and have broad-spectrum insecticidal and bactericidal effects (Locke 1995; Govindachari et al. 1999). The annual plants were small, and the resistance was low, so they produce more saponin to resist diseases, insects and environmental stress. As the plant grows, the resistance of the plant enhances and the synthesis of saponin decreases. Therefore, the content of total saponin and senegenin of annual roots was higher than that of the biennial roots and triennial roots.
In general, we took the dynamic accumulation of active components and developmental stages of plants as two criteria to determine the best collecting period (Han et al. 2003). Teng and Hu observed the status of different aged roots; they found the diameters of the annual roots, biennial roots and triennial roots were 2.5–3.0 mm, 4.0–5.0 mm and 7.0–8.0 mm, respectively (Teng and Hu 2008). Therefore, the annual roots and the biennial roots have lower biological yield, and the triennial roots have the higher one. Judging from the total yield of saponin, we think it is appropriate to harvest at the third planting year.
Analyzing the content of total saponin and senegenin of “skin areas” and xylem of three different aged roots, the content of total saponin and senegenin in the “skin areas” was about 13.51 and 14.25 times, respectively, as much as that in the xylem on average at the various developmental stages, showing a significant difference. Thus, the secondary phloem is the main storage locus for saponin. The result is consistent with the histochemical localization. Accordingly, the crude drug that had thick “skin areas” and thin xylems was of top grade.
Materials and Methods
Plant material
Polygala tenuifolia Willd. was collected from the planting base at Zhengbeizhuang village near Yuncheng City, Shanxi Province, China, from April to October 2007. All materials were identified by Professor Zheng-Hai Hu, Northwest University.
Anatomical method
Materials of vegetative organs of P. tenuifolia at different developmental stages were separately taken from the plants in the pre-blossom period (20–30 April 2007), blossom-fruit period (10–20 June 2007), post-fruit period (10–20 August 2007) and withering period (10–20 October 2007). The fresh materials were taken randomly as 10 samples for anatomical and histochemical study, and 30 samples for phytochemical study. Afterward the samples for phytochemical study were dried to constant weight in an oven at 60 °C. The fresh materials were cut into small blocks and fixed in formalin-acetic acid-alcohol (FAA) at room temperature, then dehydrated in a graded alcohol series and embedded in paraffin. The thickness of sections was 8–10 μm, cutting with a Leica (Nussloch, Germany) microtome (RM 2135), and subsequently sections were doubly stained with Safranin O and Fast Green FeF, observed and photographed under a Leica-DMLB light microscope.
Histochemical method
Sections of fresh roots at different developmental stages were cut by hand or by using a Leica-CM 1850 cryostat microtome at −19 °C, and stained with 5% vanillin-glacial acetic acid-perchloric acid solution. Then sections were observed and photographed under the Leica-DMLB light microscope.
Determination of saponin by ultraviolet-visible spectrophotometry
Instruments and standards
Instruments: KS-80D supersonic extractor, DR-HW-1 thermostat-controlled water-bath, MA 200 electric balance, 752C ultraviolet-visible spectrophotometer.
Standard: Authentic senegenin standards were obtained from China National Institute for the Control of Pharmaceutical and Biological Products (No. 111572-200301).
Standard curve
The senegenin standard (2 mg) was put into a 10 mL volumetric flask and dissolved in methanol. The methanol was then added carefully and the volume was fixed to 10 mL. We moved separately 100, 200, 300, 400, 500 μL of the sample solution and put them into five vials and evaporated them in a thermostat-controlled water-bath. After which 200 μL of 5% vanillin-glacial acetic acid solution and 800 μL perchloric acid was added, the five vials were heated for 15 min in a thermostat-controlled water-bath at 70 °C, and finally taken out and cooled with cool water. Afterward, 5 mL of glacial acetic acid was added. Finally, the saponins content of the above-mentioned standard solutions was determined by 752C ultraviolet-visible spectrophotometer. The detective wavelength was 570 nm. The regression equation between absorbance (Y) and the quantity of senegenin (X) was: Y= 0.136 3X+ 0.006 9, r= 0.999 2.
Determination of the total content of saponins
Dried powder (1.0 g) material of vegetative organs was put into a 50 mL conical flask. After 25 mL of methanol was added, it was extracted with ultrasonic for 0.5 h at room temperature, and then kept for a night. Then 5 mL of the upper clear liquid was moved and 15 mL ether was added. After completely settling, the upper clear liquid was moved. Then the sediment was dissolved in methanol at a 25 mL volumetric flask and made homogenous by shaking after adding methanol to its scale. Finally, according to the standard curve method, 300 μL of the sample solution was used for reaction and then the content of saponins was determined.
Determination of senegenin content by HPLC
Instruments and conditions
Instruments: AS3120A supersonic extractor, DR-HW-1 thermostat-controlled water-bath, FA 1140 electric balance, LC-10Atvp High performance liquid chromatography (HPLC).
Conditions: Shimadzu VP-ODS Column (150 mm × 4.6 mm, 5.0 μm); mobile phase: acetonitrile-0.05% phosphoric acid solution (40:60); flow rate: 1 mL/min; injection volume: 20 μL; detection wavelength: 205 nm. Column temperature: 25 °C.
Standard curve
The senegenin standard was diluted into 0.01 mg/mL, 0.05 mg/mL, 0.1 mg/mL, 0.2 mg/mL, 0.3 mg/mL, 0.4 mg/mL, and 0.5 mg/mL solutions with methanol. The standard curve for senegenin was constructed by separate injection of 20 μL of the above-mentioned standard solutions. The regression equation between the peak area (Y) and the quantity of senegenin (X) was: Y = 7 2320 06X + 15 124, r = 0.999 0.
Determination of senegenin content
Dried root powder (1.0 g) was put into a 25 mL conical flask. Then 10 mL of methanol was added for 30 min ultrasonic extraction at room temperature, and left to stand overnight. After 2 mL of the clear supernatant fluid was removed, three times that volume of ether was added. When precipitation appeared, the supernatant fluid was decanted. The precipitate was re-dissolved with 10 mL of 10% hydrochloric acid solution, then transferred little by little to a round flask and was refluxed for 2 h in a thermostat-controlled water-bath at 100 °C, cooled to room temperature and filtered. The brown disposition on the filter paper was re-dissolved with methanol and transferred to a 10-mL volumetric flask and shaken vigorously after adding methanol to its scale. The sample solutions were filtered through 0.45 μm filters prior to HPLC analysis.
(Handling editor: Jinxing Lin)




