A New Acylated Anthocyanin from the Red Flowers of Camellia hongkongensis and Characterization of Anthocyanins in the Section Camellia Species
Abstract
Twelve anthocyanins (1–12) were isolated from the red flowers of Camellia hongkongensis Seem. by chromatography using open columns. Their structures were elucidated on the basis of spectroscopic analyses, that is, proton-nuclear magnetic resonance, carbon 13-nuclear magnetic resonance, heteronuclear multiple quantum correlation, heteronuclear multiple bond correlation, high resolution electrospray ionization mass and ultraviolet visible spectroscopies. Out of these anthocyanins, a novel acylated anthocyanin, cyanidin 3-O-(6-O-(Z)-p-coumaroyl)-β-galactopyranoside (6), two known acylated anthocyanins, cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactopyranoside (7) and cyanidin 3-O-(6-O-(E)-caffeoyl)-β-galactopyranoside (8), and three known delphinidin glycosides (10–12) were for the first time isolated from the genus Camellia. Furthermore, pigment components in C. japonica L., C. chekiangoleosa Hu and C. semiserrata Chi were studied. The results indicated that the distribution of anthocyanins was differed among these species. Delphinidin glycoside was only detected in the flowers of C. hongkongensis, which is a special and important species in the section Camellia. Based on the characterization of anthocyanins in the section Camellia species, there is a close relationship among these species, and C. hongkongensis might be an important parent for creating new cultivars with bluish flower color.
Genus Camellia, the largest in the family Theaceae, is endemic to southeastern Asia. It is estimated that more than 400 Camellia species have been named and published. Sealy (1958) monographed the genus comprehensively and grouped the species into a cluster of similar species, known as a “section”. Chang (1981) worked with much more material at his disposal and modified by adding several sections to Sealy's system. Ming (2000) reduced the number of species by combination and reduction, but mostly followed the sectional structure set out by Sealy.
Section Camellia (L.) Dyer is the largest section in the genus Camellia with about 60 species, subspecies and varieties. They are native to China, except for C. japonica L. (Chang 1981; Ming 2000). It is one of the most famous ornamental plants in the world with red-series or white-series flowers. In fact, most cultivated Camellia cultivars are C. japonica L., C. reticulata Lindl., C. saluenensis Stapf ex Bean, or hybrids among them. Thus, it is very important to study characterization of anthocyanins in the section Camellia.
C. hongkongensis Seem., one species of the section Camellia, has rose-red flowers with a purplish hint on the petals. The species is commonly named ‘Xianggang Hong Shan-Cha’ in Chinese and ‘Hongkong Tsubaki’ in Japanese (Ohwi 1965). It is mainly distributed in Hong Kong, Guangdong Province, and offshore islands, China. C. semiserrata Chi, C. chekiangoleosa Hu and C. japonica are also distributed in the areas from southern China to eastern China, and to Japan (Chang 1981).
In the genus Camellia, Hayashi and Abe (1953) first reported the isolation of petal anthocyanins. The constitution of anthocyanins in the petals of C. hongkongensis has been discussed, where the main pigment, cyanidin 3-glucoside (1), and the existence of delphinidin derivatives were determined, but the detailed chemical structures of these anthocyanins were not reported (Sakata and Arisumi 1985). Recently, delphinidin 3-O-β-glucoside (9), delphinidin 3-O-β-galactoside, and delphinidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactoside were found in the red leaves of Benibana-cha (C. sinensis O. Kuntze.) (Terahara et al. 2001). We reported 18 anthocyanins, cyanidin 3-sambubioside, 3-lathyroside, 3-glucoside (1), and their acylated derivatives, and cyanidin 3-galactoside (5), in red flowers of wild species distributed in the areas of the southwestern China (Li et al. 2007, 2008a) and cultivar “Dalicha” (C. reticulata cv. Queen of Dali) (Li et al. 2008b). Despite these efforts, there is still no information available on the distribution of pigments in C. hongkongensis.
In the present paper, we report the isolation and structural elucidation of 12 anthocyanins from the flowers of C. hongkongensis using various kinds of gels such as MCI-gel CHP-20P, Sephadex LH-20, and ODS gel for reversed phase open column chromatography (CC). Out of them, six anthocyanins were isolated from the genus Camellia for the first time. In addition, the distribution of anthocyanins in the red flowers of C. hongkongensis, C. japonica, C. chekiangoleosa and C. semiserrata was analyzed with high performance liquid chromatography (HPLC).
C. hongkongensis is the ancestor of three other wild species, based on their geographical distribution and morphological characteristics (Ye and Chang 1997; Ming 1998). Since floral anthocyanins are considered to be the key products that can provide information on the color of flowers, and also provide the basics for plant chemotaxonomy based on principle component and cluster analyses (Wang et al. 2004). It is very important to clarify the specific anthocyanins from C. hongkongensis for chemotaxonomy, genetic, breeding or other aspects of the section Camellia.
Results
Determination of chemical structures of anthocyanins in the flowers of C. hongkongensis
High performance liquid chromatography analysis indicated the anthocyanins in C. hongkongensis flowers have at least 12 peaks. Those major pigments (1–12) were isolated as reddish amorphous powders of acetic acid salts (Figure 1). In order to determine the chemical structures of these anthocyanins, proton-nuclear magnetic resonance (1H-NMR) (Table 1) and carbon 13-nuclear magnetic resonance (13C-NMR) spectra (Table 2), and high resolution electrospray ionization mass spectrometry (HR ESI-MS) were measured, and hydrolyses were carried out. The 12 anthocyanins were as follows: cyanidin 3-O-β-glucopyranoside (1) (Saito et al. 1987), cyanidin 3-O-(6-O-(Z)-p-coumaroyl)-β-glucopyranoside (2) (Boido et al. 2006; Li et al. 2007), cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-glucopyranoside (3) (Saito et al. 1987), cyanidin 3-O-(6-O-(E)-caffeoyl)-β-glucopyranoside (4) (Wu et al. 2004; Boido et al. 2006; Li et al. 2007), cyanidin 3-O-β-galactopyranoside (5) (Sakata et al. 1986), cyanidin 3-O-(6-O-(Z)-p-coumaroyl)-β-galactopyranoside (6), cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactopyranoside (7) (Yamamoto et al. 2007), cyanidin 3-O-(6-O-(E)-caffeoyl)-β-galactopyranoside (8) (Sparrow et al. 2005), delphinidin 3-O-β-glucopyranoside (9) (Mas et al. 2000), delphinidin 3-O-(6-O-(Z)-p-coumaroyl)-β-glucopyranoside (10) (Boido et al. 2006), delphinidin 3-O-(6-O-(E)-p-coumaroyl)-β-glucopyranoside (11) (Lamikanra 1989; Monagas et al. 2006), and delphinidin 3-O-(6-O-(E)-caffeoyl)-β-glucopyranoside (12) (Fujiwara et al. 1998).
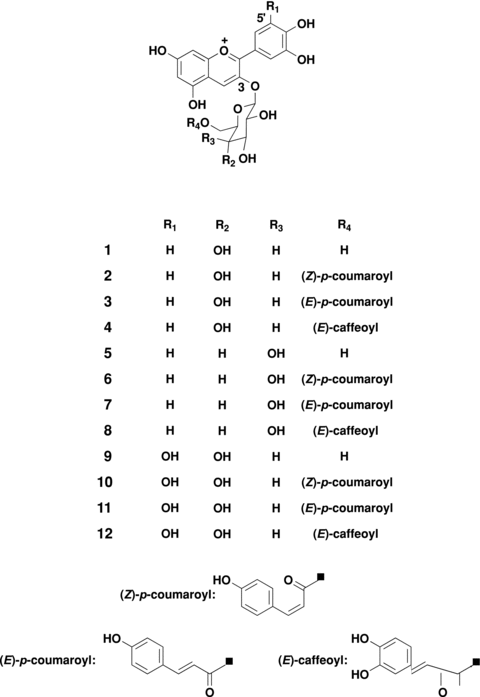
Structure of anthocyanins isolated from the red flowers of Camellia hongkongensis.
| No. | 6 | 7 | 8 | 10 | 11 |
|---|---|---|---|---|---|
| Aglycone | |||||
| Cyanidin | Delphinidin | ||||
| 4 | 8.88 s | 8.90 s | 8.94 s | 8.71 s | 8.83 s |
| 6 | 6.51 d 1.7 | 6.51 d 1.8 | 6.55 br.s | 6.62 s | 6.52 d 1.8 |
| 8 | 6.76 d 1.7 | 6.79 d 1.8 | 6.83 br.s | 6.69 s | 6.76 d 1.8 |
| 2′ | 7.99 d 2.4 | 8.01 d 2.2 | 8.07 br.s | 7.71 s | 7.70 s |
| 5′ | 6.98 d 8.7 | 6.98 d 8.7 | 7.05 d 8.5 | ||
| 6′ | 8.20 dd 8.7,2.4 | 8.20 dd 8.7,2.2 | 8.23 br.d 8.5 | 7.71 s | 7.70 s |
| Sugars | |||||
| Galactose | Glucose | ||||
| 1″ | 5.30 d 7.7 | 5.28 d 7.7 | 5.27 d 7.7 | 5.34 d 7.5 | 5.36 d 7.7 |
| 2″ | 4.05 dd 9.6,7.7 | 4.05 dd 9.4,7.7 | 4.05 dd 9.4,7.7 | 3.77 dd 8.8,7.5 | 3.78 dd 8.9,7.7 |
| 3″ | 3.76 dd 9.6,3.2 | 3.76 dd 9.4,3.2 | 3.75 dd 9.4,3.2 | 3.60 dd 9.0,8.8 | 3.64 dd 9.1,8.9 |
| 4″ | 4.01 br.d 3.2 | 4.00 br.d 3.0 | 4.01 br.d 3.1 | 3.45 dd 9.5,9.0 | 3.51 dd 9.6,9.1 |
| 5″ | 3.85 br.dd 8.7,3.5 | 4.12 br.dd 8.9,3.6 | 4.13 br.dd 8.9,3.6 | 3.87 ddd 9.5,7.6,2.8 | 3.88 ddd 9.6,7.5,2.2 |
| 6″a | 4.57 dd 11.6,8.7 | 4.58 dd 11.6,8.9 | 4.59 dd 11.6,8.9 | 4.48 dd 12.0,2.8 | 4.53 dd 12.0,2.2 |
| 6″b | 4.32 dd 11.6,3.5 | 4.32 dd 11.6,3.6 | 4.32 dd 11.6,3.7 | 4.46 dd 12.0,7.6 | 4.39 dd 12.0,7.5 |
| Acyl groups | |||||
| (Z)-p-coumaroyl | (E)-p-coumaroyl | (E)-caffeoyl | (Z)-p-coumaroyl | (E)-p-coumaroyl | |
| 2 | 7.35 d 8.7 | 7.26 d 8.5 | 6.92 br.s | 7.35 d 8.7 | 7.26 d 8.5 |
| 3 | 6.53 d 8.7 | 6.78 d 8.5 | 6.52 d 8.7 | 6.77 d 8.5 | |
| 5 | 6.53 d 8.7 | 6.78 d 8.5 | 6.78 m | 6.52 d 8.7 | 6.77 d 8.5 |
| 6 | 7.35 d 8.7 | 7.26 d 8.5 | 6.78 m | 7.35 d 8.7 | 7.26 d 8.5 |
| a | 5.75 d 12.8 | 6.24 d 15.9 | 6.21 d 15.8 | 5.74 d 12.9 | 6.22 d 15.8 |
| b | 6.58 d 12.8 | 7.41 d 15.9 | 7.36 d 15.8 | 6.59 d 12.9 | 7.41 d 15.8 |
- br., broad; d, doublet; dd, double doublet; m, multiplet; s, singlet; t, triplet.
| No. | 6 | 7 | 8 | 10 | 11 |
|---|---|---|---|---|---|
| Aglycone | |||||
| Cyanidin | Delphinidin | ||||
| 2 | 164.2 | 164.4 | 164.5 | 163.8 | 164.1 |
| 3 | 145.2 | 145.3 | 145.1 | 145.5 | 145.3 |
| 4 | 135.8 | 136.9 | 137.1 | 135.8 | 135.9 |
| 5 | 159.2 | 159.3 | 159.1 | 159.2 | 159.2 |
| 6 | 103.6 | 103.7 | 103.8 | 103.4 | 103.7 |
| 7 | 170.5 | 170.6 | 170.8 | 170.3 | 170.5 |
| 8 | 95.3 | 95.3 | 95.3 | 95.1 | 95.2 |
| 9 | 157.5 | 157.7 | 157.8 | 157.4 | 157.6 |
| 10 | 113.2 | 113.3 | 113.3 | 113.3 | 113.2 |
| 1′ | 121.2 | 121.2 | 121.3 | 120.1 | 120.1 |
| 2′ | 118.4 | 118.5 | 118.6 | 112.7 | 112.7 |
| 3′ | 147.4 | 147.5 | 147.5 | 147.6 | 147.6 |
| 4′ | 155.8 | 155.9 | 155.9 | 144.6 | 144.8 |
| 5′ | 117.5 | 117.5 | 117.4 | 147.6 | 147.6 |
| 6′ | 128.3 | 128.3 | 128.3 | 112.7 | 112.7 |
| Sugars | |||||
| Galactose | Glucose | ||||
| 1″ | 103.6 | 103.7 | 103.8 | 102.4 | 102.8 |
| 2″ | 71.9 | 71.9 | 72.0 | 74.6 | 74.7 |
| 3″ | 74.7 | 74.8 | 74.8 | 77.9 | 77.9 |
| 4″ | 70.2 | 70.2 | 70.2 | 71.9 | 71.9 |
| 5″ | 75.2 | 75.2 | 75.2 | 76.1 | 76.1 |
| 6″ | 64.6 | 64.6 | 64.6 | 64.4 | 64.6 |
| Acyl groups | |||||
| (Z) C | (E) C | (E) Caf | (Z) C | (E) C | |
| 1 | 127.3 | 127.1 | 127.7 | 127.3 | 127.1 |
| 2 | 133.6 | 131.3 | 115.6 | 133.6 | 131.3 |
| 3 | 115.8 | 116.9 | 149.7 | 115.7 | 116.9 |
| 4 | 159.9 | 161.4 | 146.8 | 159.9 | 161.4 |
| 5 | 115.8 | 116.9 | 116.6 | 115.7 | 116.9 |
| 6 | 133.6 | 131.3 | 123.2 | 133.6 | 131.3 |
| a | 115.9 | 114.7 | 114.8 | 115.8 | 114.6 |
| b | 144.5 | 146.9 | 147.3 | 144.6 | 147.0 |
| C=O | 168.4 | 169.1 | 169.1 | 168.4 | 169.2 |
- (E) C, (E)-p-coumaroyl; (E) Caf, (E)-caffeoyl; (Z) C, (Z)-p-coumaroyl.
The six anthocyanins (6–8 and 10–12) are reported to be isolated from the red flowers of the genus Camellia. Of these six anthocyanins, compound 6 is a new acylated anthocyanin, while the spectroscopic data of 7, 8 and 10–12 are reported for the first time.
The 1H- and 13C-NMR spectra of (6) showed proton signals similar to those of cyanidin 3-O-β-galactopyranoside (5) (Tables 1, 2). In addition, the signals appeared at δ 7.35 (2H, d, J= 8.7 Hz) and 6.53 (2H, d, J= 8.7 Hz), as well as at the two olefinic protons, δ 5.75 (1H, d, J= 12.8 Hz) and 6.58 (1H, d, J= 12.8 Hz) which are ascribable to a cis-p-coumaroyl group (Inami et al. 1996). The configuration of the double bond can be easily distinguished by the coupling constant of the olefinic protons of the typical AB-type spectrum, that is, J= approximately 13 Hz for the cis-configuration and approximately 16 Hz for the trans-configuration (Nakatani et al. 1995; Silverstein and Webster 1998). The anomeric proton at δ 5.30 (1H, d, J= 7.7 Hz) of the galactose was found to have a β-configuration according to the coupling constant. Since the 6-position of the galactose moiety at δ 4.32 (1H, dd, J= 11.6, 3.5 Hz) and 4.57 (1H, dd, J= 11.6, 8.7 Hz) in 1H-NMR and δ 64.6 in 13C-NMR was observed as lower field shifted, the cis-p-coumaroyl group was concluded to attach to this position. HR ESI-MS of (6) showed a molecular ion peak at m/z 595.4984 [M]+, which supports this assignment. The hydrolysis of (6) gave cyanidin, galactose, and methyl (Z)-p-coumaroate. Thus, pigment (6) was identified as cyanidin 3-O-(6-O-(Z)-p-coumaroyl)-β-galactopyranoside (6).
The 1H-NMR spectrum of (7) showed signals analogous to those in pigment (6), the only difference being the trans-configuration for the p-coumaroyl group due to the doublet signals at δ 6.24 and 7.41 (each 1H, d, J= 15.9 Hz). HR ESI-MS showed a molecular ion peak at m/z 595.4894 [M]+, which supports this assignment. The hydrolysis of (7) gave cyanidin, galactose, and methyl (E)-p-coumaroate. Thus, pigment (7) was identified as cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactopyranoside (7).
The 1H-NMR spectrum of (8) again showed signals analogous to those in (6), the only difference being the trans-configuration for the caffeoyl group and due to the ABX-type signals at δ 6.92 (1H, br. s), 6.78 (2H, m), 6.21 (1H, d, J= 15.8 Hz), and 7.36 (1H, d, J= 15.8 Hz) (Otsuki et al. 2002). A lower field shift of the protons was found at the 6-position of the galactose moiety at δ 4.32 and 4.59 (each 1H, dd) and a lower field shift of 13-C was observed at δ 64.6; thus, the attachment of the trans-caffeoyl group was at the 6-position of the galactose. A molecular ion peak at m/z 611.4463 [M]+ in the HR ESI-MS was observed. The hydrolysis of (8) gave cyanidin, galactose, and methyl (E)-caffeoate. Based on these spectral data, (8) was identified as cyanidin 3-O-(6-O-(E)-caffeoyl)-β-galactopyranoside (8).
Qualitative and quantitative HPLC analyses of anthocyanins in the flowers of C. hongkongensis, C. japonica, C. semiserrata and C. chekiangoleosa
The amounts of pigments (1–12) were compared in the HPLC chromatograms among the red flowers of C. hongkongensis, C. japonica, C. semiserrata and C. chekiangoleosa (Table 3). The constitution of anthocyanins was the same as in the fresh and dried petals of C. hongkongensis and C. japonica based on the HPLC analysis. Delphinidin glycosides (9–12) were only contained in the flowers of C. hongkongensis. The quantities (μg/100 mg dried petals) and the constitution (%) of acylated anthocyanins were reduced from 437.8 to 145.2 to 78.6, and to 3.3, and from 90.4 to 53.9 to 15.1, and to 1.6 in the species from C. hongkongensis to C. japonica to C. semiserrata, and to C. chekiangoleosa, respectively. The quantities of major acylated anthocyanins, cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-glucopyranoside (3) and cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactopyranoside (7) were reduced from 95.0 to 42.4, and to 2.0, and from 33.2 to 21.8, and to 1.3 μg/100 mg dried petals in the species from C. semiserrata to C. japonica, and to C. chekiangoleosa, respectively. Furthermore, the quantities of cyanidin 3-O-(6-O-(E)-caffeoyl)-β-glucopyranoside (4) and cyanidin 3-O-(6-O-(E)-caffeoyl)-β-galactopyranoside (8) were also reduced from 9.8 to 9.5 to 2.3, and to 0.0, and from 2.6 to 2.4 to 1.1, and to the undetected levels in dried petals of the species from C. hongkongensis to C. semiserrata to C. japonica, and to C. chekiangoleosa, respectively. The red flowers of C. chekiangoleosa were found to lack the pigments (2), (4), (6) and (8). Furthermore, less acylated anthocyanins such as (3) and (7) were found when compared with the other three species, C. hongkongensis, C. japonica and C. semiserrata. However, the constitution (%) of anthocyanins (1) and (5) were increased from 7.3 to 40.2 to 58.7, and to 70.4, and from 0.3 to 5.9 to 25.9, and to 28.0 in the species from C. hongkongensis to C. semiserrata to C. japonica, and to C. chekiangoleosa, respectively. The results indicated that C. hongkongensis is an important special species, and it showed a close relationship among the four species.
| Species | Anthocyanins | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| Concentration (μg/100 mg petals)a | ||||||||||||||
| C. hongkongensis | FP | 4.2 ± 0.1 | 3.4 ± 0.1 | 29.8 ± 1.0 | 1.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 1.7 ± 0.1 | 0.3 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.1 | 12.9 ± 0.2 | 0.4 ± 0.1 | 56.8 ± 1.6 |
| C. japonica | FP | 32.8 ± 1.4 | 0.8 ± 0.1 | 4.7 ± 0.1 | 0.3 ± 0.1 | 14.3 ± 0.5 | 0.5 ± 0.1 | 2.3 ± 0.1 | 0.3 ± 0.1 | 56.0 ± 2.2 | ||||
| C. hongkongensis | DP | 35.2 ± 2.3 | 24.5 ± 1.2 | 247.9 ± 13.6 | 9.8 ± 0.7 | 1.3 ± 0.2 | 0.7 ± 0.1 | 13.2 ± 1.9 | 2.6 ± 0.1 | 11.1 ± 0.5 | 12.7 ± 2.1 | 123.0 ± 4.7 | 3.4 ± 0.2 | 485.5 ± 24.2 |
| C. semiserrata | DP | 108.3 ± 4.1 | 1.9 ± 0.2 | 95.0 ± 2.6 | 9.5 ± 0.5 | 15.8 ± 0.7 | 3.2 ± 0.1 | 33.2 ± 0.8 | 2.4 ± 0.2 | 269.3 ± 6.0 | ||||
| C. japonica | DP | 300.5 ± 17.7 | 6.8 ± 0.3 | 42.4 ± 2.0 | 2.3 ± 0.2 | 132.5 ± 7.8 | 4.6 ± 0.3 | 21.8 ± 0.6 | 1.1 ± 0.1 | 512.0 ± 27.3 | ||||
| C. chekiangoleosa | DP | 144.7 ± 2.0 | 2.0 ± 0.1 | 57.4 ± 0.6 | 1.3 ± 0.1 | 205.4 ± 1.5 | ||||||||
| Constitution (%)b | ||||||||||||||
| C. hongkongensis | FP | 7.5 ± 0.1 | 6.0 ± 0.1 | 52.5 ± 0.4 | 2.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 2.9 ± 0.1 | 0.6 ± 0.1 | 2.0 ± 0.1 | 2.5 ± 0.1 | 22.6 ± 0.4 | 0.7 ± 0.1 | 100.0 |
| C. japonica | FP | 58.6 ± 0.2 | 1.4 ± 0.1 | 8.5 ± 0.1 | 0.5 ± 0.1 | 25.5 ± 0.1 | 0.9 ± 0.1 | 4.2 ± 0.1 | 0.5 ± 0.1 | 100.0 | ||||
| C. hongkongensis | DP | 7.3 ± 0.1 | 5.0 ± 0.1 | 51.1 ± 0.4 | 2.0 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 2.7 ± 0.1 | 0.5 ± 0.1 | 2.3 ± 0.1 | 2.6 ± 0.1 | 25.3 ± 0.5 | 0.7 ± 0.1 | 100.0 |
| C. semiserrata | DP | 40.2 ± 0.2 | 0.7 ± 0.1 | 35.3 ± 2.0 | 3.5 ± 0.2 | 5.9 ± 0.1 | 1.2 ± 0.1 | 12.3 ± 0.1 | 0.9 ± 0.1 | 100.0 | ||||
| C. japonica | DP | 58.7 ± 0.2 | 1.3 ± 0.1 | 8.3 ± 0.1 | 0.4 ± 0.1 | 25.9 ± 0.1 | 0.9 ± 0.1 | 4.3 ± 0.1 | 0.2 ± 0.1 | 100.0 | ||||
| C. chekiangoleosa | DP | 70.4 ± 0.1 | 1.0 ± 0.1 | 28.0 ± 0.2 | 0.6 ± 0.1 | 100.0 | ||||||||
- aConcentration (μg/100 mg petals): the pigment quantities (μg) in 100 mg petals. bConstitution (%): concentration of pigment in total pigments. FP, fresh petals; DP, dried petals.
Discussion
C. saluenensis and C. reticulata, distributed in southwestern China, are rare and wonderful ornamental plants with radiant and charming flowers during winter and spring (Xia 1986). The blooming season of the other varieties in the Camellia species distributed in southern China, for example, C. hongkongensis, C. japonica and C. chekiangoleosa, is later than that of C. saluenensis and C. reticulata (Chang 1981). The different new flower colors are the most important breeding target for Camellia flowers. Since the anthocyanins in the flowers of Camellia species and cultivars are considered to be the key products that can provide information on the color of flowers (Endo 1958; Sakata and Arisumi 1985), it is necessary to completely clarify the floral anthocyanins in the flowers of Camellia species.
Our recent research results indicated that a characteristic feature of the pigmentation in the four original species can thus be described as the biogenesis of anthocyanins. It should be noted that the variation of glycosides and the attached position of glycosides to the anthocyanidin core structure seemed likely to be preserved among the closely related species in the section Camellia, that is, 3-monoglycosylation for C. hongkongensis, C. japonica, C. semiserrata and C. chekiangoleosa; 3-(2-xylosyl)-monoglycosylation for C. reticulata and C. pitardii var. yunnanica Sealy (Li et al. 2007), and cultivar ‘Dalicha’ (Li et al. 2008b); and 3,5-diglycosylation for C. saluenensis and C. pitardii Sealy (Sakata and Arisumi 1992; Li et al. 2008a). Since it completely shows the different pigment compositions in the flowers of C. hongkongensis, C. japonica, C. reticulata and C. saluenensis, these species became very rich plant resources to create famous ornamental cultivars with beautiful flower colors and different flower forms. Furthermore, delphinidin is the key product that can provide important information for the blue color of flowers (Hashimoto et al. 2002), but delphinidins were found only in C. hongkongensis in the section Camellia, so, C. hongkongensis might be the best valuable resource that would be excellent parents for creating new cultivars with bluish flower colors.
C. hongkongensis was one species of the most primitive characteristics in the genus Camellia (Ye and Chang 1997; Ming 1998), and a major differentiation scheme was as from C. hongkongensis to C. semiserrata to C. japonica or to C. chekiangoleosa among the section Camellia distributed in southern China (Sakata 1988; Ming 1998). According to our recent examinations, floral flavonoids could also provide the basis for the plant chemotaxonomy based on principle component and cluster analyses (Wang et al. 2004). The results indicated that particular information can be easily obtained on evolutionary development and phylogenetic relationships, as well as the origin of the inter-specific hybrids or others of tree peony species, based on PCA and cluster analyses. So, it is very important to clarify the anthocyanin components of C. hongkongensis, C. semiserrata, C. japonica and C. chekiangoleosa for the chemotaxonomy, genetic and phylogenetic relationship of the section Camellia. The 3-glucopyranoside and 3-galactopyranoside of cyanidin and their acylated derivatives were detected in the petals of C. hongkongensis, C. semiserrata, C. japonica and C. chekiangoleosa, but delphinidin 3-glucopyranoside-series were only found in C. hongkongensis. Furthermore, the HPLC analyses in the present study showed that the quantities and constitution as the percentages of the acylated anthocyanins were reduced in species from C. hongkongensis to C. semiserrata to C. japonica or to C. chekiangoleosa. Thus, it might be suggested that these species differed morphologically and physiologically from their ancestors as a result of natural selection along with the deficiency of acylation of anthocyanins.
Materials and Methods
General procedures
Details of the instruments and chromatographic conditions used in this study were essentially the same as described in the previous report (Li et al. 2007). The nuclear magnetic resonance (NMR) experiments were obtained at 600.17 and 150.92 MHz for 1H and 13C, respectively, on a JNM-ECA600 NMR spectrometer (JEOL Ltd, Tokyo, Japan; Venture Business Lab., Kagoshima University, Kagoshima, Japan), and chemical shifts are expressed on a δ (ppm) scale with tetramethylsilane (TMS) as an internal standard (Tables 1, 2). The solvents used for NMR measurements were a combination of methanol-d4+ 0.03% TMS (CD3OD) and trifluoroacetic acid-d at a ratio of 9:1. The 2D field gradient (FG)-heteronuclear multiple quantum correlation (FG-HMQC) and heteronuclear multiple bond correlation (HMBC) spectra were measured to investigate the linkages among the aglycone, sugar, and acyl units (data not shown). HR ESI-MS was measured on a MAT900XL (Finnigan Inc., Tokyo Japan; The United Graduate School of Agriculture, Kagoshima University) in 10% AcOH-MeOH without a matrix in a positive mode. Ultraviolet visible (UV-vis) spectra were recorded on a MPS-2000 spectrophotometer (Shimadzu Co., Ltd, Kyoto, Japan) in 0.01% HCl-MeOH.
To check the purity of each anthocyanin and analyze the distribution of pigments in wild flowers, HPLC (JASCO Gulliver series, JASCO, Tokyo Japan) was conducted at 525 nm with a TSK gel ODS-80Ts QA column (4.5 mm i.d. × 150 mm, Tosoh) at 40°C. The flow rate was 0.8 mL/min with a linear flow gradient elution for 35 min. where solvent B (1.5% H3PO4–20% HCOOH-25% CH3CN-5% tetrahydrofuran [THF] in H2O, v/v) was increased linearly from 18% to 70% in solvent A (1.5% H3PO4 in H2O, v/v). Thin layer chromatography (TLC) was carried out on precoated Kiesel gel 60 F245 aluminum plates (0.2 mm thick, Merck Ltd, Tokyo, Japan) with a benzene-HCOOEt-HCOOH-H2O system as described in the former report (Hashimoto et al. 2002).
Plant materials
Fresh petals of wild C. hongkongensis were collected at the experimental farm of Kagoshima University, Kagoshima, Japan, every year in February and March from 1999 to 2006. For HPLC, red flowers of C. hongkongensis and C. japonica were collected at the same experimental farm in February 2002. The petals of C. chekiangoleosa and C. semiserrata were collected at Fuyang City, Zhejiang Province, China, in February 2004. Furthermore, fresh petals were treated with boiling water for about 3–4 s to kill polyphenol oxidase enzymes and dried at room temperature, and were kept in a dryer with silica gel before analysis (Sakata 1988).
Extraction and isolation
The fresh petals (approximately 12 kg) of C. hongkongensis were immersed in 50% AcOH-MeOH overnight (24 h) and the extracted solution was filtered. The extraction was repeated twice. The filtered extract was concentrated under reduced pressure, and was kept in a freezer before purification. The crude extract contained 12 anthocyanins whose retention times (in min) in the HPLC chromatogram were as follows: pigment (9), 10.9; pigment (5), 11.8; pigment (1), 12.9; pigment (8), 23.5; pigment (12), 23.7; pigment (6), 24.1; pigment (10), 24.9; pigment (4), 25.2; pigment (2), 26.4; pigment (7), 27.8; pigment (11), 28.3; pigment (3), 31.1 (Figure 1). The extract was subjected to MCI gel CHP 20P CC with 5% AcOH-H2O and an increasing amount of 5% AcOH-MeOH (10, 20, 30, 40, 50, 60, 70% and 100% of 5% AcOH-MeOH) to afford fractions. The fractions were further subjected to Sephadex LH-20 CC and ODS-gel CC repeatedly, to furnish 12 anthocyanins: (1), 30 mg; (2), 10 mg; (3), 32 mg; (4), 20 mg; (5), 15 mg; (6), 12 mg; (7), 26 mg; (8), 15 mg; (9), 14 mg; (10), 11 mg; (11), 32 mg; (12), 15 mg.
Identification of anthocyanins
The 12 pigments were identified based on the spectroscopic data, that is, 1H-NMR, 13C-NMR, HMQC, HMBC, HR ESI-MS, and UV-Vis spectra.
UV-Vis spectral data
Pigment 6: UV-vis λmax 0.01% HCl-MeOH (nm) (log ɛ): 284 (4.28), 310 (4.15), 530 (4.28); AlCl3-MeOH: 289 (4.25), 313 (4.27), 405 (3.66), 571 (4.39).
Pigment 7: UV-vis λmax 0.01% HCl-MeOH (nm) (log ɛ): 284 (4.31), 312 (4.19), 530 (4.32); AlCl3-MeOH: 289 (4.25), 313 (4.26), 401 (3.54), 558 (4.32).
Pigment 8: UV-vis λmax 0.01% HCl-MeOH (nm) (log ɛ): 284 (4.30), 330 (4.18), 531 (4.29); AlCl3-MeOH: 287 (4.13), 312 (4.13), 351 (4.16), 569 (4.38).
Pigment 10: UV-vis λmax 0.01% HCl-MeOH (nm) (log ɛ): 285 (4.32), 300 (4.25), 545 (4.16); AlCl3-MeOH: 288 (4.32), 315 (4.27), 586 (4.25).
Pigment 11: UV-vis λmax 0.01% HCl-MeOH (nm) (log ɛ): 283 (4.31), 300 (4.26), 542 (4.36); AlCl3-MeOH: 288 (4.27), 315 (4.27), 584 (4.43).
Hydrolyses of pigments
Each anthocyanin (approximately 2 mg) was dissolved in 2N HCl (2 mL) and heated at 95°C for 2 h. The sugars that were liberated by acid hydrolysis were directly compared by TLC with the following authentic sugars: glucose, galactose, mannose, rhamnose, fructose, xylose, arabinose and fucose. The solvent systems for TLC used for distinguishing sugars were n-BuOH-AcOH-H2O (2:1:1) and CHCl3-MeOH-H2O (7:3:0.5), and the detection of sugars was achieved by spraying with 5% H2SO4, followed by heating.
Pigments (6–8) (approximately 1.5 mg) were treated with NaOMe in MeOH (0.3 mL) at room temperature. The hydrolysate was neutralized with 2N HCl and the methyl esters, methyl (Z)-p-coumaroate, methyl (E)-p-coumaroate, and methyl (E)-caffeoate were detected by TLC with a benzene-EtOAc (9:1) solvent system. The Rf values of each methyl ester were 0.266, 0.241, and 0.216, respectively (Li et al. 2008b).
Qualitative and quantitative HPLC analyses
The qualitative and quantitative analyses were carried out in the four wild species with these pigments (1–12) by HPLC analyses under similar conditions (Table 3). For this, approximately 1 mg each of authentic anthocyanins (1–12) was dissolved in 100 mL of an acidic solution (MeOH-HCOOH-CF3COOH-H2O, 70:2:1:27, v/v%) (Hashimoto et al. 2002). Dried petals of C. hongkongensis (60.2 mg), C. japonica (35.0 mg), C. chekiangoleosa (82.0 mg), and C. semiserrata (70.3 mg) were extracted with 5 mL of this acidic solution. HPLC analyses were repeated three times. It should be noted that the constitution of anthocyanins in the dried petals was the same with fresh petals (Sakata 1988).
(Handling Editor: Ninghua Tan)
Acknowledgements
The authors are grateful to Professor Ji-Ying Gao of the Research Institute of Subtropical Forestry at the Chinese Academy of Forestry, China, for materials of C. chekiangoleosa and C. semiserrata.




