Identification of Quantitative Trait Loci for Rice Quality in a Population of Chromosome Segment Substitution Lines
Supported by the Ministry of Science and Technology of China (2006AA10Z1F7 and 2007AA10Z187), the Chinese Academy of Sciences (KSCX1-YW-03), and the National Natural Science Foundation of China (30821004).
Abstract
The demand for high quality rice represents a major issue in rice production. The primary components of rice grain quality include appearance, eating, cooking, physico-chemical, milling and nutritional qualities. Most of these traits are complex and controlled by quantitative trait loci (QTLs), so the genetic characterization of these traits is more difficult than that of traits controlled by a single gene. The detection and genetic identification of QTLs can provide insights into the genetic mechanisms underlying quality traits. Chromosome segment substitution lines (CSSLs) are effective tools used in mapping QTLs. In this study, we constructed 154 CSSLs from backcross progeny (BC3F2) derived from a cross between ‘Koshihikari’ (an Oryza sativa L. ssp. japonica variety) as the recurrent parent and ‘Nona Bokra’ (an O. sativa L. ssp. indica variety) as the donor parent. In this process, we carried out marker-assisted selection by using 102 cleaved amplified polymorphic sequence and simple sequence repeat markers covering most of the rice genome. Finally, this set of CSSLs was used to identify QTLs for rice quality traits. Ten QTLs for rice appearance quality traits were detected and eight QTLs concerned physico-chemical traits. These results supply the foundation for further genetic studies and breeding for the improvement of grain quality.
Rice is one of the most important staple cereal grains. Along with improvements in the standard of living, the demand for high quality rice is increasingly becoming a priority issue in many rice-producing areas of the world. The primary rice grain qualities include appearance, eating, cooking, physico-chemical, milling and nutritional qualities.
Most traits of rice show continuous genetic variation in natural populations and among inbred strains. These traits are quantitative traits controlled by quantitative trait loci (QTLs). The genetic identification of rice grain qualities is difficult because not only are most of these characters quantitative qualities controlled by polygenes, but the hereditary features of these characters are also quite complicated. Genetic identification of QTLs for rice quality is very important because this achievement can undoubtedly provide insights into the molecular mechanisms of the development of complex quality traits. Rice cultivars can also be improved by our endeavor to identify QTLs of grain qualities.
During past years, many genetic studies using QTL analysis have been carried out to identify genes controlling genetically complex traits in rice (Xiao et al. 1998; Lin et al. 2000, 2002, 2003) and other plant species (Howell et al. 1996; Bernacchi et al. 1998; Burns et al. 2003). Studies identifying QTLs for rice grain quality, such as cooking and eating quality (Tan et al. 1999), milling quality (Tan et al. 2001) and other qualities (Septiningsih et al. 2003; Aluko et al. 2004) have been carried out.
Quantitative trait loci with relatively major effects can be identified by the genetic analysis of primary mapping populations, such as F2 (Price and Tomos 1997), advanced backcross population (BC2F2) (Septiningsih et al. 2003) and recombinant inbred lines (RILs) (Price et al. 2002). However, some QTLs with minor effects and those with an epistatic interaction with other loci may not be detected with these methods. Such QTLs can be identified by advanced backcross progenies (Yamamoto et al. 2000; Thomson et al. 2003). Furthermore, construction of nearly isogenic lines (NILs) is necessary for further fine mapping and cloning of QTLs (Lin et al. 2002). These studies remain tedious and time consuming (Glazier et al. 2002) and as a result the detection of QTLs and their molecular identification have proved to be serious bottlenecks in studies of quantitative traits.
Recently, to identify the QTLs, researchers have developed novel mapping populations such as chromosome segment substitution lines (CSSLs), introgression lines (ILs) or recombinant chromosome substitution lines (RCSLs) in rice (Doi et al. 1997; Sobrizal et al. 1999; Kubo et al. 2002; Ebitani et al. 2005; Li et al. 2005; Hao et al. 2006; Tian et al. 2006; Xi et al. 2006; Tan et al. 2007), Brassica napus (Howell et al. 1996; Burns et al. 2003; Sharpe and Lydiate 2003), tomato (Eshed and Zamir 1995, 1996), Arabidopsis (Koumproglou et al. 2002), wheat (Pestsova et al. 2001), and barley (Matus et al. 2003; Korff et al. 2004).
In CSSL lines, a particular chromosome segment in the genetic background of the recurrent line is substituted by the corresponding chromosome segment from the donor line. The substituted segments of all substitution lines cover the entire genome of the recurrent line. Therefore, the advantage of these substitution lines is the complete integration of the substituted segments with the stability of a certain character. Thus, these substitution lines can be used not only for the primary identification of QTLs, but also to finely map QTLs, to investigate the functions of each QTL, and to study the effects of QTLs on each other. Using these substitution lines, researchers can carry out detailed subsequent QTL analyses (Eshed and Zamir 1996; Koumproglou et al. 2002; Ebitani et al. 2005). Wan et al. (2004) localized 25 QTLs for rice grain physico-chemical qualities by using rice CSSLs. Hao et al. (2006) identified 15 QTLs of rice grain qualities, including nine QTLs controlling appearance and milling qualities and six QTLs controlling physico-chemical qualities. It will be necessary to develop more substitution lines with a wide range of cross combinations to improve the genetic mapping and map-based cloning of QTLs. Additional constructions of rice CSSLs can also help us breed better cultivars to improve rice quality.
The japonica rice cultivar “Koshihikari” is a well-known cultivar in Japan. Koshihikari has often been used as a parental line to study functions of QTLs, because it has several characteristics of economic value, such as good eating quality, cool temperature tolerance at the booting stage, and high resistance to pre-harvest germination (Ebitani et al. 2005). The indica variety ‘Nona Bokra’ is greatly different from ‘Koshihikari’. For example, Nona Bokra is a highly salt-tolerant variety (Lin et al. 2004; Ren et al. 2005), but it has poor eating quality. Thus, these two varieties are appropriate parental lines to construct mapping populations for further investigations. Using an F2 population derived from a cross between Nona Bokra and Koshihikari, eight QTLs responsible for variations in K+ and Na+ content were mapped (Lin et al. 2004). Among these QTLs, a QTL (SKC1) that encoded a Na+ -selective transporter regulating K+/Na+ homeostasis was isolated by map-based cloning (Ren et al. 2005).
In this study, we constructed a series of 154 overlapping, homozygous CSSLs from backcross progeny (BC3F2) derived from a cross between Koshihikari as the recurrent parent and Nona Bokra as the donor parent. In this process, we carried out marker-assisted selection (MAS) by using 102 cleaved amplified polymorphic sequence (CAPS) and simple sequence repeat (SSR) markers covering most of the rice genome. Using these CSSLs, we identified 18 QTLs for eight rice quality traits, including appearance quality, physico-chemical properties, and milling traits. Furthermore, we calculated the additive effect of every QTL on its corresponding trait.
Results
Development of CSSLs
The process of CSSLs selection is shown in Figure 1. To construct the CSSLs, rice cultivar Koshihikari was crossed with Nona Bokra, and the F1 plants were backcrossed three times with Koshihikari to produce the BC3F1. A total of 680 BC3F1 individuals were obtained. In the process of selecting BC3F1 individuals, we applied MAS by using 102 CAPS (Harushima et al. 1998) and SSR (McCouch et al. 2002) markers with polymorphisms covering the whole rice genome. We selected 71 individuals from all 680 BC3F1 individuals. Most of these 71 individuals have a single, relatively long heterozygous chromosome segment. All 71 individuals were self-pollinated to produce BC3F2 populations (a total of ∼3 266 plants). Based on the MAS, appropriate BC3F2 plants were selected. The principle for selection was as follows: a single, relatively long chromosome segment of Koshihikari was substituted by a corresponding segment of Nona Bokra, the genetic background regions kept a high level of homozygosity of the Koshihikari genotype, and substituted segments partially overlapped. The selected lines covered the whole rice genome. Ultimately, we selected 154 BC3F2 plants as the CSSLs for the further mapping of rice quality trait QTLs. Figure 2 shows 55 of the 154 lines.
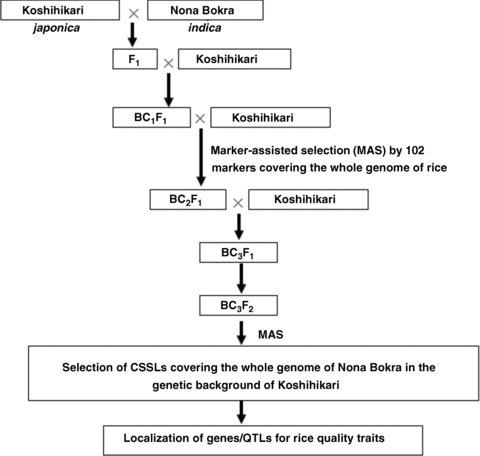
The strategy for constructing chromosome segment substitution lines (CSSLs) covering the whole rice genome. QTLs, quantitative trait loci.
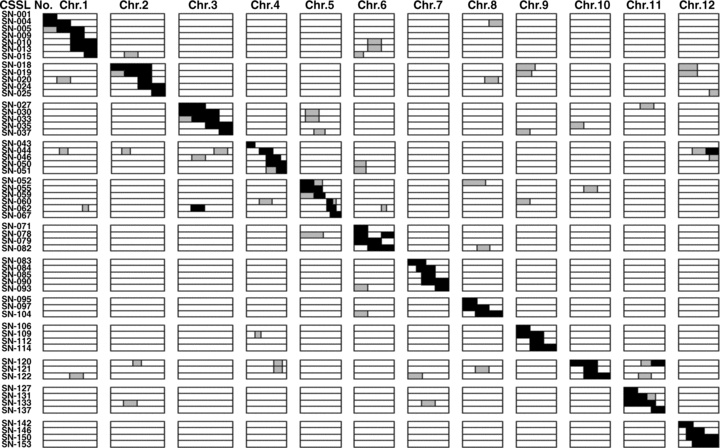
Graphical genotypes of the chromosome segment substitution lines (CSSLs) developed in this study. Chromosome segment substitution lines having the same substitution segment are represented by one row. The horizontal axis indicates one substitution line. The serial number at the left end of each row is the number of a line, and the number on the top of the chart is the chromosome number. The white regions represent the segments homozygous for Koshihikari alleles; the black regions represent donor segments homozygous for Nona Bokra alleles; and the gray regions indicate heterozygous regions.
Characteristics of the CSSLs
Based on the polymorphism of the 102 CAPS and SSR markers, we selected 154 substituted lines. The substituted chromosome segments in the CSSLs covered most of the rice genome, except for two small regions at the position of 18.7–52 cM of chromosome 4 and 53.5–74.2 cM of chromosome 5. The total substitution ratio of all 12 chromosomes is 96.2%. The average distance between two neighboring markers for all 102 markers was 14.0 cM.
A total of 308 substituted segments were detected in this set of CSSLs. Theoretically, it is preferred that every CSSL line has only one substituted segment from the donor parent. However, many substituted lines in our CSSLs had two or even more substituted segments because of the restricted number of generations for back-crossing and self-pollination. Sixty-two (40.3%) lines had only one substituted segment from Nona Bokra. Forty-nine (31.8%) lines had two substituted segments, and 43 (27.9%) lines had more than two substituted segments (2, 3). The average length of all 308 substituted segments was 38.9 cM, and they ranged from 3.4 cM to 105.5 cM (Figure 4). 70.5% of the substituted segments had a length between 10 cM and 50 cM. Seven substituted segments were longer than 100 cM. In each CSSL line the substitution ratio of the length of substituted segments, accounting for the total length of 12 chromosomes, ranged from 0.64% to 15.11%, with an average of 5.40% (Figure 5). The substitution ratio of most CSSL lines was below 10.0%.
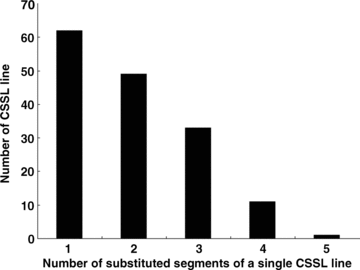
Frequency distribution of the number of substituted segments in single chromosome segment substitution lines (CSSLs).
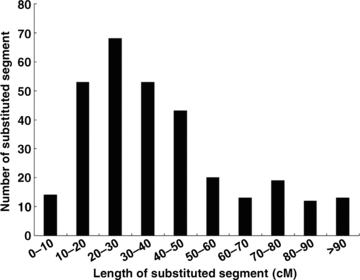
Frequency distribution of the genetic length of substituted segments (homozygous, heterozygous) in all chromosome segment substitution lines (CSSLs).
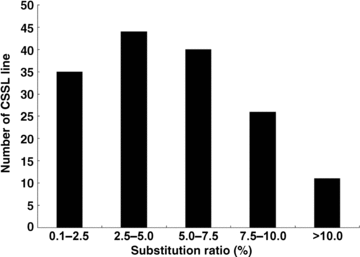
Frequency distribution of the substitution ratio of the length of substituted segments accounting for total genome length in each chromosome segment substitution lines (CSSLs).
Identification of QTLs for rice quality traits
We planted the above described set of CSSLs in Shanghai, China. QTLs were detected based on a t-test assessing the difference between the mean of each CSSL and the recurrent parent Koshihikari. A probability level of 0.01 was used as the threshold for the detection of a putative QTL. To increase accuracy, we identified the given QTL if at least two lines containing the same substituted segment satisfied the above requirement. In total we identified 18 QTLs for eight traits, including red kernel rice (RKR), percentage of chalky rice grains (PCRG), degree of chalkiness (DC), head rice percentage (HRP), transparency (TP), gelatinization temperature (GT), gel consistency (GC) and amylose content (AC) (Figure 6). Furthermore, four QTLs for TP and GC, three QTLs for HRP and AC, one QTL for RKR, PCRG, DC and GT were detected (Table 1, Figure 6). The details are as follows.
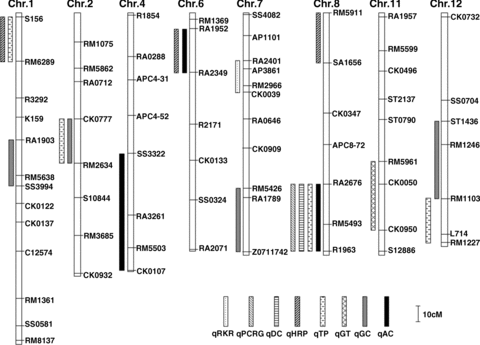
Distribution of 18 quantitative trait loci (QTLs) for rice quality traits on chromosomes. Long bare stripes indicate chromosomes. Because no QTL was detected on four chromosomes, chr.3, chr.5, chr.9, and chr.10, they are not shown. DNA markers are shown on the right side of each chromosome and indicate the respective relative genetic locations. Short stripes filled with different hatched regions on left side of chromosomes represent the locations of different QTLs.
| Line no. | Chromosome no. | Position (cM) | Substituted region | Trait | Additive effect |
|---|---|---|---|---|---|
| RKR | |||||
| SN-84 | 7 | 0.8–49.0 | SS4082-CK0039 | Red | –a |
| SN-85 | 7 | 35.1–69.8 | RA2401-CK0909 | Red | – |
| SN-86 | 7 | 35.1–69.8 | RA2401-CK0909 | Red | – |
| SN-89 | 7 | 69.8–117.4 | CK0909-Z0711742 | White | – |
| Koshihikari | White | ||||
| PCRG (%) | |||||
| SN-97 | 8 | 32.8–88.6 | SA1656-RA2676 | 5.33 ± 0.33 | −5.33 |
| SN-98 | 8 | 32.8–88.6 | SA1656-RA2676 | 7.67 ± 1.20 | −4.17 |
| SN-102 | 8 | 88.6–118.0 | RA2676-R1963 | 36.67 ± 2.19** | 10.33 |
| SN-104 | 8 | 88.6–118.0 | RA2676-R1963 | 35.33 ± 1.20** | 9.67 |
| Koshihikari | 16.00 ± 3.22 | ||||
| DC (%) | |||||
| SN-97 | 8 | 32.8–88.6 | SA1656-RA2676 | 1.92 ± 0.29 | −1.96 |
| SN-98 | 8 | 32.8–88.6 | SA1656-RA2676 | 2.82 ± 0.79 | −1.51 |
| SN-102 | 8 | 88.6–118.0 | RA2676-R1963 | 14.57 ± 1.10** | 4.37 |
| SN-104 | 8 | 88.6–118.0 | RA2676-R1963 | 13.32 ± 0.86** | 3.74 |
| Koshihikari | 5.83 ± 1.28 | ||||
| HRP (%) | |||||
| SN-1 | 1 | 5.6–29.7 | S156-RM6289 | 69.33 ± 0.27*** | −3.79 |
| SN-2 | 1 | 5.6–29.7 | S156-RM6289 | 71.72 ± 0.34*** | −2.60 |
| SN-5 | 1 | 5.6–93.6 | S156-SS3994 | 70.44 ± 0.17*** | −3.24 |
| SN-6 | 1 | 29.7–45.4 | RM6289-R3292 | 73.86 ± 0.68** | −1.53 |
| SN-9 | 1 | 71.3–93.6 | RA1903-SS3994 | 76.48 ± 0.16 | −0.21 |
| SN-11 | 1 | 93.6–181.8 | SS3994-RM8137 | 77.29 ± 0.21 | 0.19 |
| SN-71 | 6 | 9.9–32.1 | RA1952-RA2349 | 72.76 ± 0.45*** | −2.08 |
| SN-75 | 6 | 9.9–32.1 | RA1952-RA2349 | 73.72 ± 0.17*** | −1.60 |
| SN-76 | 6 | 9.9–32.1 | RA1952-RA2349 | 71.33 ± 0.52*** | −2.79 |
| SN-79 | 6 | 32.1–51.0 | RA2349-R2171 | 74.40 ± 0.24 | −1.26 |
| SN-82 | 6 | 51.0–85.9 | R2171-SS0324 | 75.79 ± 0.26 | −0.56 |
| SN-95 | 8 | 0.0–32.8 | RM5911-SA1656 | 73.47 ± 0.27*** | −1.72 |
| SN-96 | 8 | 0.0–32.8 | RM5911-SA1656 | 69.23 ± 0.08*** | −3.84 |
| SN-98 | 8 | 32.8–88.6 | SA1656-RA2676 | 77.23 ± 0.16 | 0.16 |
| SN-102 | 8 | 88.6–118 | RA2676-R1963 | 77.16 ± 0.06 | 0.13 |
| Koshihikari | 76.91 ± 0.08 | ||||
| TP | |||||
| SN-1 | 1 | 5.6–29.7 | S156-RM6289 | 0.47 ± 0.02** | −0.09 |
| SN-2 | 1 | 5.6–29.7 | S156-RM6289 | 0.50 ± 0.01** | −0.08 |
| SN-4 | 1 | 5.6–93.6 | S156-SS3994 | 0.41 ± 0.02** | −0.12 |
| SN-5 | 1 | 5.6–93.6 | S156-SS3994 | 0.38 ± 0.01*** | −0.14 |
| SN-6 | 1 | 29.7–45.4 | RM6289-R3292 | 0.38 ± 0.01*** | −0.14 |
| SN-9 | 1 | 71.3–93.6 | RA1903-SS3994 | 0.55 ± 0.02 | −0.05 |
| SN-10 | 1 | 93.6–169.7 | SS3994-SS0581 | 0.67 ± 0.02 | 0.01 |
| SN-11 | 1 | 93.6–181.8 | SS3994-RM8137 | 0.67 ± 0.02 | 0.01 |
| SN-16 | 2 | 17.6–81.0 | RM1075-RM2634 | 0.46 ± 0.01*** | −0.10 |
| SN-18 | 2 | 17.6–81.0 | RM1075-RM2634 | 0.48 ± 0.02** | −0.09 |
| SN-20 | 2 | 57.3–98.2 | CK0777-S10844 | 0.42 ± 0.00*** | −0.11 |
| SN-22 | 2 | 57.3–98.2 | CK0777-S10844 | 0.46 ± 0.01*** | −0.10 |
| SN-24 | 2 | 98.2–134.2 | S10844-CK0932 | 0.63 ± 0.01 | −0.01 |
| SN-25 | 2 | 116.7–134.2 | RM3685-CK0932 | 0.64 ± 0.02 | −0.01 |
| SN-95 | 8 | 0.0–32.8 | RM5911-SA1656 | 0.55 ± 0.02 | −0.05 |
| SN-97 | 8 | 32.8–88.6 | SA1656-RA2676 | 0.63 ± 0.01 | −0.01 |
| SN-98 | 8 | 32.8–88.6 | SA1656-RA2676 | 0.66 ± 0.01 | 0.01 |
| SN-102 | 8 | 88.6–118.0 | RA2676-R1963 | 0.54 ± 0.00** | −0.06 |
| SN-104 | 8 | 88.6–118.0 | RA2676-R1963 | 0.52 ± 0.01** | −0.06 |
| SN-141 | 12 | 7.9–57.4 | CK0732-ST1436 | 0.69 ± 0.03 | 0.02 |
| SN-147 | 12 | 48.6–91.4 | SS0704-RM1103 | 0.67 ± 0.03 | 0.01 |
| SN-149 | 12 | 48.6–107.0 | SS0704-RM1227 | 0.54 ± 0.01** | −0.06 |
| SN-150 | 12 | 48.6–107.0 | SS0704-RM1227 | 0.49 ± 0.01** | −0.08 |
| SN-153 | 12 | 57.4–107.0 | ST1436-RM1227 | 0.54 ± 0.02 | −0.05 |
| Koshihikari | 0.65 ± 0.02 | ||||
| GT | |||||
| SN-127 | 11 | 6.7–30.3 | RA1957-CK0496 | 7.00 ± 0.00 | 0 |
| SN-131 | 11 | 6.7–45.0 | RA1957-ST2137 | 6.83 ± 0.01 | −0.09 |
| SN-135 | 11 | 19.8–79.9 | RM5599-RM5961 | 5.50 ± 0.17 | −0.75 |
| SN-137 | 11 | 79.9–109.3 | RM5961-CK0950 | 5.75 ± 0.25** | −0.63 |
| SN-138 | 11 | 79.9–109.3 | RM5961-CK0950 | 5.92 ± 0.09** | −0.54 |
| Koshihikari | 7.00 ± 0.00 | ||||
| GC (mm) | |||||
| SN-1 | 1 | 5.6–29.7 | S156-RM6289 | 65.00 ± 3.00 | −9.25 |
| SN-4 | 1 | 5.6–93.6 | S156-SS3994 | 55.50 ± 0.50** | −14.00 |
| SN-5 | 1 | 5.6–93.6 | S156-SS3994 | 55.00 ± 1.00** | −14.25 |
| SN-6 | 1 | 29.7–45.4 | RM6289-R3292 | 84.50 ± 0.50 | 0.50 |
| SN-9 | 1 | 71.3–93.6 | RA1903-SS3994 | 49.00 ± 1.00** | −17.25 |
| SN-10 | 1 | 93.6–169.7 | SS3994-SS0581 | 76.50 ± 1.50 | −3.50 |
| SN-11 | 1 | 93.6–181.8 | SS3994-RM8137 | 80.00 ± 2.00 | −1.75 |
| SN-16 | 2 | 17.6–81.0 | RM1075-RM2634 | 38.50 ± 1.50** | −22.50 |
| SN-18 | 2 | 17.6–81.0 | RM1075-RM2634 | 48.50 ± 1.50** | −17.50 |
| SN-19 | 2 | 31.2–81.0 | RM5862-RM2634 | 41.00 ± 1.00** | −21.25 |
| SN-20 | 2 | 57.3–98.2 | CK0777-S10844 | 49.00 ± 1.00** | −17.25 |
| SN-22 | 2 | 57.3–98.2 | CK0777-S10844 | 51.00 ± 1.00** | −16.25 |
| SN-24 | 2 | 98.2–134.2 | S10844-CK0932 | 80.00 ± 2.00 | −1.75 |
| SN-84 | 7 | 0.8–49.0 | SS4082-CK0039 | 73.00 ± 3.00 | −5.25 |
| SN-85 | 7 | 35.1–69.8 | RA2401-CK0909 | 74.00 ± 2.00 | −4.75 |
| SN-89 | 7 | 69.8–117.4 | CK0909-Z0711742 | 53.50 ± 1.50** | −15.00 |
| SN-90 | 7 | 69.8–117.4 | CK0909-Z0711742 | 60.00 ± 2.00 | −11.75 |
| SN-92 | 7 | 95.8–117.4 | RM5426-Z0711742 | 50.00 ± 0.00** | −16.75 |
| SN-93 | 7 | 95.8–117.4 | RM5426-Z0711742 | 40.00 ± 0.00** | −21.75 |
| SN-142 | 12 | 7.9–57.4 | CK0732-ST1436 | 81.00 ± 1.00 | −1.25 |
| SN-146 | 12 | 48.6–91.4 | SS0704-RM1103 | 39.00 ± 3.00** | −22.25 |
| SN-147 | 12 | 48.6–91.4 | SS0704-RM1103 | 56.00 ± 2.00** | −13.75 |
| SN-150 | 12 | 48.6–107.0 | SS0704-RM1227 | 49.00 ± 1.00** | −17.25 |
| SN-153 | 12 | 57.4–107.0 | ST1436-RM1227 | 39.00 ± 3.00** | −22.25 |
| SN-154 | 12 | 57.4–107.0 | ST1436-RM1227 | 52.50 ± 2.50** | −15.50 |
| Koshihikari | 83.50 ± 1.50 | ||||
| AC (%) | |||||
| SN-43 | 4 | 4.8–18.7 | R1854-RA0288 | 12.52 ± 0.32 | −0.02 |
| SN-46 | 4 | 52.0–72.6 | APC452-SS3322 | 13.91 ± 0.07 | 0.67 |
| SN-49 | 4 | 52.0–128.9 | APC452-CK0107 | 18.80 ± 0.14** | 3.12 |
| SN-51 | 4 | 72.6–128.9 | SS3322-CK0107 | 19.46 ± 0.01** | 3.45 |
| SN-71 | 6 | 9.9–32.1 | RA1952-RA2349 | 22.94 ± 0.14** | 5.19 |
| SN-75 | 6 | 9.9–32.1 | RA1952-RA2349 | 22.45 ± 0.00*** | 4.94 |
| SN-76 | 6 | 9.9–32.1 | RA1952-RA2349 | 17.63 ± 0.05** | 2.53 |
| SN-79 | 6 | 32.1–51.0 | RA2349-R2171 | 12.59 ± 0.03 | 0.01 |
| SN-82 | 6 | 51.0–85.9 | R2171-SS0324 | 13.41 ± 0.15 | 0.42 |
| SN-95 | 8 | 0.0–32.8 | RM5911-SA1656 | 12.47 ± 0.20 | −0.05 |
| SN-97 | 8 | 32.8–88.6 | SA1656-RA2676 | 14.13 ± 0.11 | 0.78 |
| SN-102 | 8 | 88.6–118.0 | RA2676-R1963 | 19.24 ± 0.24** | 3.34 |
| SN-104 | 8 | 88.6–118.0 | RA2676-R1963 | 19.92 ± 0.43** | 3.68 |
| Koshihikari | 12.57 ± 0.21 |
- **, ***indicate significant differences at the level of 0.01 and 0.001, respectively. aRed kernel rice could not be quantified and corresponding additive effects could not be calculated. AC, amylose content; DC, degree of chalkiness; GC, gel consistency; GT, gelatinization temperature; HRP, head rice percentage; PCRG, percentage of chalky rice grains; RKR, red kernel rice; TP, transparancy.
QTL analysis of RKR
We detected one QTL, qRKR-7, controlling the color of rice kernels. It was located in the 35.1−49 cM region of chromosome 7 The corresponding segment from Nona Bokra can change the color of Koshihikari rice kernel from white to red.
QTL analysis of PCRG
We detected one QTL, qPCRG-8, for the percentage of chalky rice grains, which was located in the 88.6−118 cM region of chromosome 8. The corresponding segment from Nona Bokra can increase the percent of chalky rice grains of Koshihikari.
QTL analysis of DC
We detected one QTL, qDC-8, for degree of chalkiness in the 88.6−118 cM region of chromosome 8, which was the same region harboring a QTL for the percentage of chalky rice grains. According to the values of phenotypes and additive effects, the QTL from Nona Bokra can increase degree of chalkiness of milled rice of Koshihikari.
QTL analysis of HRP
In total we identified three QTLs, qHRP-1, qHRP-6 and qHRP-8, for head rice percentage. They were located in the 5.6−29.7 cM region of chromosome 1, the 9.9−32.1 cM region of chromosome 6 and the 0−32.8 cM region of chromosome 8, respectively. According to the values of phenotypes and additive effects, these three corresponding segments from Nona Bokra can all decrease the head rice percentage of Koshihikari.
QTL analysis of TP
For transparency, we detected a total of four corresponding QTLs, qTP-1, qTP-2, qTP-8 and qTP-12. They were located in the 5.6−29.7 cM region of chromosome 1, the 57.3−81.0 cM region of chromosome 2, the 88.6−118 cM region of chromosome 8 and the 91.4−107 cM region of chromosome 12, respectively. These four QTLs from Nona Bokra can all decrease the transparency of Koshihikari milled rice.
QTL analysis of GT
We detected one QTL, qGT-11, for milled rice gelatinization temperature, located in the 79.9−109.3 cM region of chromosome 11. This QTL from Nona Bokra can decrease the gelatinization temperature of milled rice of Koshihikari.
QTL analysis of GC
We detected four QTLs for gel consistency: qGC-1, qGC-2, qGC-7 and qGC-12. They were located in the 71.3−93.6 cM region of chromosome 1, the 57.3−81.0 cM region of chromosome 2, the 95.8−117.4 cM region of chromosome 7 and the 57.4−91.4 cM region of chromosome 12, respectively. The corresponding four segments from Nona Bokra all can decrease the gel consistency of Koshihikari milled rice.
QTL analysis of AC
For the amylose content of milled rice, we identified a total of three QTLs: qAC-4, qAC-6 and qAC-8. They were located in the 72.6−128.9 cM region of chromosome 4, the 9.9−32.1 cM region of chromosome 6 and the 88.6−118 cM region of chromosome 8, respectively. These three QTLs from Nona Bokra can all increase the amylose content of Koshihikari milled rice.
Discussion
Potential of CSSLs for mapping QTLs for rice quality
Rice is an important agricultural crop and is the staple food for more than half of the world's population. In years past, the sequencing of the entire genome of both indica and japonica rice has been a remarkable achievement (Goff et al. 2002; Yu et al. 2002). Based on this achievement, some researchers have found many molecular markers that can be used in molecular analysis of complex traits (Harushima et al. 1998; McCouch et al. 2002). On the premise of marker-assisted selection, a series of CSSLs have been developed in rice (Doi et al. 1997; Sobrizal et al. 1999; Kurakazu et al. 2001). CSSLs can be used in genetic analyses to identify QTLs from particular chromosome segments in rice (Li et al. 2005; Tian et al. 2006).
Rice grain qualities such as cooking and eating quality and appearance are serious issues in many rice-producing areas of the world. Grain quality is determined by many factors, including grain shape and size, grain appearance, cooking and eating quality, and physico-chemical quality, and most of these factors are determined by QTLs. Recently, many QTLs for rice grain qualities have been identified by using various mapping populations. Septiningsih et al. (2003) constructed 285 advanced backcross population BC2F2 families from an interspecific cross between rice cultivar IR64 and O. rufipogon and they identified 23 independent QTLs for 14 rice grain quality traits. Aluko et al. (2004) identified and characterized QTLs for grain quality using 312 doubled haploid lines derived from the BC3F1 of an interspecific cross of O. sativa×O. glaberrima, and they identified 27 QTLs for nine grain quality traits. Sun et al. (2006) detected 35 QTLs for four rice eating and cooking qualities by using a backcross inbred line (BIL) derived from a cross between Koshihikari (japonica) and Kasalath (indica). At the same time, other researchers detected many QTLs for rice grain quality by using various CSSLs. Wan et al. (2004) identified a total of 25 QTLs for nine traits on nine chromosomes in a population of CSSLs containing 66 lines derived from a cross between Asominori and IR24. Hao et al. (2006) detected 15 QTLs for five appearance and physico-chemical traits on nine chromosomes in a series of CSSLs derived from a cross between a cultivar Teqing and wild rice O. rufipogon.
In this study, a set of CSSLs derived from the cross between Koshihikari (japonica) and Nona Bokra (indica) was used to identify QTLs for eight rice grain qualities. A total of 18 QTLs were identified on eight chromosomes. Of these, eight of the total 18 QTLs had been found in former studies, and the other 10 QTLs were detected for the first time. qRKR-7 was identified and mapped to the same region on chromosome 7 as a red grain QTL detected by Septiningsih et al. (2003). qPCRG-8 and qDC-8 were detected on the same segment of chromosome 8 containing the qPGWC-8 and qDEC-8 QTLs identified by Wan et al. (2005). qHRP-1, qHRP-6 and qHRP-8 were located on the same regions of chromosome 1, 6 and 8, respectively, as hr1, hr6 and hr8 identified by Aluko et al. (2004). qGC-2 was detected in the same region of chromosome 2 as qGC-2 identified by Sun et al. (2006). qAC-6 was located on the same segment of chromosome 6 as amy6, qAC-6 and a QTL for amylose content detected by Aluko et al. (2004), Sun et al. (2006) and Septiningsih et al. (2003), respectively. The other 10 QTLs were identified for the first time.
Some QTLs controlling different traits mapped to the same segment of a chromosome. qPCRG-8 and qDC-8 were located on the same segment of chromosome 8. Because the percentage of chalky rice grains multiplied by area of chalkiness is the degree of chalkiness, the degree of chalkiness partially correlates with the percentage of chalky rice grains. qTP-8 was also mapped to the same segment as the two QTLs just mentioned. This phenomenon indicates that chalkiness could possibly affect transparency, and that the low transparency is induced by a large degree of chalkiness. Besides these three QTLs, qAC-8 was also detected in the same region of chromosome 8. We speculate that the amylose content of rice grain is an importantly basal element affecting chalkiness and transparency. Our study clarifies that a higher content of amylose results in larger chalkiness and lower transparency.
In the 9.9–32.1 cM region of chromosome 6, we identified a QTL, qAC-6, for amylose content. The gene waxy is located precisely in this region (Septiningsih et al. 2003) and encodes the granule-bound starch synthase, which controls the production of amylose. We identified qPCRG-6 and qDC-6 in this segment indicating that amylose content is strongly controlled by the waxy gene.
QTL qRKR-7 controlled the color of rice kernels. Septiningsih et al. (2003) also identified this QTL on chromosome 7. Sweeney et al. (2006) confirmed that the gene Rc is located in the same segment of chromosome 7. Rc encodes a basic helix-loop-helix (bHLH) protein and produces the red pigment proanthocyanidin.
Rice grain quality is strongly restricted by environmental elements such as photoperiod, temperature and soil condition. Therefore, a fine genetic population is very important for the research of rice quality. CSSLs are the appropriate materials for this study. CSSLs lines contain a homozygous genetic background and have stable heredity, which can effectively reduce the fluctuation of rice grain quality.
Potential of CSSLs as experimental material
The current way to identify QTLs that control phenotypic differences between two cultivars involves three steps (Nadeau et al. 2000). The first step is genetic mapping in a cross between donor and recurrent parents and selection of appropriate progeny for the trait of interest. Large crosses are required to provide sufficient power to detect typical QTLs. The second step towards detecting QTLs is the construction of near isogenic lines (NILs). Phenotypic noise can make it difficult to confirm the location of a target QTL but NILs can resolve this uncertainty. Finally, the third step to detect a QTL is fine mapping. Backcrosses between the NILs and the recurrent parent enable the precise location of a QTL to be mapped. However, it may be difficult to find NILs in a particular chromosomal region, and a large number of pairs are necessary to cover the whole genome. Therefore, the overall process of QTL identification remains tedious and time-consuming, requiring 5–10 generations and 3–5 years.
An alternative approach is to construct CSSLs. Besides the CSSLs of rice, many CSSLs of other species have already been constructed, such as wheat (Pestsova et al. 2001), Brassica napus (Howell et al. 1996; Ramsay et al. 1996; Burns et al. 2003; Sharpe and Lydiate 2003), tomato (Eshed et al. 1992; Eshed and Zamir 1995, 1996; Zamir 2001), Arabidopsis (Koumproglou et al. 2002) and barley (Matus et al. 2003; Korff et al. 2004). In addition, similar CSSLs have been produced in mice (Nadeau et al. 2000; Singer et al. 2004).
Chromosome segment substitution lines have several advantages over other methods that are useful for dissecting complex traits. A series of CSSLs can partition the whole genome into many chromosome segments. The pattern of phenotypic differences between CSSLs and the recurrent parent reveals the chromosomal location of QTLs. Due to less phenotypic noise, QTLs having weaker phenotypic effect for a given trait can also be identified more easily than by traditional populations such as an F2 and RILs. CSSLs can also be used to test a QTL for dominance or recessivity by comparing the phenotypes of CSSLs with the two parents (Cowley et al. 2004). Moreover, the cross progeny of two CSSLs containing different substitution segments affecting the same trait are novel and powerful materials for discovering additive or epistatic effects in polygenic traits. In order to precisely map QTLs as single Mendelian factors, the development of NILs for target QTLs is required in a primary mapping population. In contrast, the purity of the genetic background of each CSSL makes it relatively easy to rapidly proceed to the mapping of target QTLs. If the substitution segment is even short enough, the CSSL can be immediately used as the NIL for the target trait.
The use of CSSLs may also have disadvantages for the identification of QTLs. The detection of target QTLs may be disturbed if the genetic background is not very pure. If a QTL is detected in a relatively longer segment, the subsequent fine-mapping may be difficult. Finally, it may be very difficult to observe phenotypic effects generated by the combination of two or more chromosome segments from the donor cultivar. To resolve these problems, CSSLs that carry substituted chromosome segments containing target QTLs can be used as parental lines for the construction of F2 populations.
Finally, breeding becomes easier by using technology of MAS, and the construction of CSSLs offers a new breeding strategy. CSSLs having the appropriate character can be selected and used as breeding materials. Zamir (2001) found several tomato CSSLs showing different fruit color and carotenoid-content from their parents. Tian et al. (2006) developed CSSLs carrying wild rice segments in cultivated rice background and characterized the yield-related traits of these lines. These CSSLs of different species all supplied excellent materials for the corresponding breeding. Thus, the CSSLs constructed in this study could be very useful for improving rice quality traits in future breeding.
Materials and Methods
Construction of CSSLs
We selected Koshihikari (japonica) and Nona Bokra (indica) as recurrent and donor parents, respectively. The multi-step construction of the CSSLs is shown in Figure 1. The F1 plants were derived from the cross of Koshihikari and Nona Bokra. Then the F1 plants were consecutively backcrossed with Koshihikari three times to produce the BC3F1. We selected 71 appropriate individuals from a total of 680 BC3F1 plants by marker-assisted selection (MAS). From BC1F1 to BC3F1, we chose plants according to the following principles by using the MAS technique: (i) appropriate plants contained a single chromosome segment as possible and relatively long heterozygous chromosome segments; (ii) the genetic background regions kept a high level of homozygosity of the Koshihikari genotype; and (iii) substituted segments partially overlapped. The selected BC3F1 plants were self-pollinated to produce BC3F2 plants. We planted 46 offspring of every BC3F1 individual, amounting to a total of 3266 BC3F2 plants for MAS. Ultimately, 154 BC3F2 plants containing specific different substituted segments were selected to construct the CSSLs. We applied the method of MAS during all steps of selection described above and sorted out 102 CAPS (Harushima et al. 1998) and SSR (McCouch et al. 2002) markers covering the whole rice genome by MAS. All of these 102 markers are polymorphic between Koshihikari and Nona Bokra. Genetic maps and length of chromosome segments are determined according to the linkage maps in previous studies (Harushima et al. 1998, McCouch et al. 2002).
DNA extraction and marker-assisted selection
We picked fresh leaves about 2 cm and put them into 1.5 mL tubes. DNA extraction and polymerase chain reaction (PCR) analysis were carried out following the procedure described by Ebitani et al. (2005). We selected suitable plants based on the PCR or restriction enzyme digestion polymorphism. The principles of selection were that the background of Koshihikari and the substituted segments of Nona Bokra were both relatively homozygous, and also that the CSSLs containing only one substitution segment were better than the others.
Phenotypic evaluation of eight traits
All 154 CSSLs and the recurrent parent Koshihikari were planted at Shanghai, China. Eighteen individuals of each line were distributed over three rows and every row contained six individual lines. The intervals between plants and between rows were both 15 cm. When these CSSL lines matured, we harvested the paddies and mixed seeds of 18 individuals of each line. The harvested paddy rice was combined and stored at room temperature for 3 months before testing. Then we analyzed the following eight traits.
QTL mapping
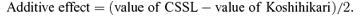
Red kernel rice
The harvested seeds were de-husked and then observed for kernel color. If the color of de-husked rice was red and due to genetic factors, we considered the corresponding segment that resulted in the red color to contain a potential QTL controlling the capsule color.
Head rice percentage
Rough rice was de-hulled and milled in triplicate using a mill (Taizhou Food and Oil Machinery Factory, Zhejiang, China) according to the National Standard NY 147-88 of China. Head rice included the whole milled grains and the broken grains with two-thirds of the whole grain. The head rice percentage was considered to be the proportion of head rice to the rough rice weight.
Percentage of chalky rice grains and degree of chalkiness
The percentage of chalky rice grains was defined as the proportion of grains having an opaque, chalky appearance compared with all of the milled head rice grains. Chalkiness covered a region of the grain. The degree of chalkiness was the result obtained by multiplying the percentage of chalky rice grains by the chalkiness areas.
Transparency
Transparency was denoted by efficiency of light permeation through the whole milled rice grain. We measured the transparency of rice grains using a light permeation instrument (China National Rice Research Institute, Hangzhou, China).
Gelatinization temperature
The GT, GC and AC were all measured according to the methods of Tan et al. (1999). The GT was measured based on the alkali spread value (ASV). Twenty intact milled grains were put into a boat with 15 mL of 1.7% KOH. The grains were incubated at 30°C for 24 h to allow the grains to spread. Grains that were unaffected were given an ASV score of 1, and grains that disappeared completely were given an ASV score of 10. A low score indicates a high GT and a high score corresponds to a low GT.
Gel consistency
In a 10 mm × 110 mm culture tube, we added 0.2 mL of 95% ethanol containing 0.025% thymol blue. One milliliter of 0.2 M KOH was subsequently added. The tubes were covered with glass marbles and boiled in a water bath for 8 min. After being placed on ice for 20 min, they were laid down horizontally onto a table surface. After 1 h, the gel length from the bottom of the tube to the front of the gel was measured. A longer length indicates a softer gel consistency.
Amylose content
Exactly 10 mg of rice grain flour was gelatinized overnight in 3 mL of 2% NaOH in a water bath at 30°C. The solution was boiled in the water bath for 10 min, and was diluted to a volume of 100 mL. Three milliliters of color developing agent containing 0.02% KI and 0.002% I2 was added to 1 mL of the above solution. The absorbance of this solution was then measured at 620 nm. A standard curve was charted by several standard amylose solutions (5%, 10%, 15%, 20%, 25% and 30%).
(Handling editor: Dabing Zhang)




