The Role of Viral Infection in Inducing Variability in Virus-Free Progeny in Tomato
Abstract
The effect of virus-host interactions on subsequent generations is poorly understood. The evaluation of the effects of viral infection on inheritance of quantitative traits in the progeny of infected plants and elucidation of a possible relationship between chiasma frequency in the infected plants and variability of traits in the progeny were investigated. The current study involved genotypes of four intraspecific hybrids of tomato (Solanum lycopersicum L.), their parental forms and two additional cultivars. Used as infection were the tobacco mosaic virus (TMV) and potato virus X (PVX). The consequences of the effect of viral infection were evaluated based on chromosome pairing in diakinesis and/or by examining quantitative and qualitative traits in the progeny of the infected tomato plants. Tomato plants infected with TMV + PVX were found to differ in chiasma frequency per pollen mother cell or per bivalent. Deviations have been observed for genotypes of both F1 hybrids and cultivars. At the same time, differences in mean values of the traits under study have only been found for progeny populations (F2-F4) derived from virus-infected F1 hybrids, but not in the case of progeny of the infected cultivars. The rate of recombinants combining traits of both parents increased significantly (2.22–8.24 times) in progeny populations of hybrids infected with TMV + PVX. The above suggests that the observed effects could be the result of modification of recombination frequencies that can be manifested in heterozygous hybrids and make small contributions to variability in cases of ‘homozygous’ tomato genotypes (i.e. cultivars).
Since the first reports by Kostoff (1933), studies of effects of viral infection on host plant genetic material have advanced considerably. It has been established that viral infection can contribute to chromosome breaks (Nagar et al. 1995, 2002; Bass et al. 2000), activation of transposing elements (Dellaporta et al. 1984; Johns et al. 1985; Kalendar et al. 2000; Ikeda et al. 2001) and bring about modifications in genome regulation and structure in the host plant (Madlung and Comai 2004; Boyko et al. 2007).
The contribution of viral infection to the enhancement of host-plant genetic variability has been recognized. Moreover, a number of studies elucidate the role of viral infection in evolution of organisms, that is, viral infection acting as a factor of selection favoring new genotypes both in plants and animals (Anderson and May 1982; Alexandrov and Golubovsky 1983; Kondrashov 1988; Kelley 1994; Yamauchi 1999; Busch et al. 2004). In the progeny of infected plants some phenomena have been observed, such as aberrant ratio (Nelson 1981) or genetic instability (Sandfaer 1979; Mottinger et al. 1984). Later on, deviations in inheritance of quantitative and qualitative traits as a result of virus infection action have been established in agricultural crops, such as bean (Sarrafi and Ecochard 1985), wheat (Burdun et al. 1984) and sorghum (Mock et al. 1985; Stokes et al. 1988). The above evidence suggests that variability generated in this manner can be used in crop improvement programs.
Various abiotic factors are commonly used for increasing variability in tomato (Singh 1981; Gavazzi et al. 1987), and there is only fragmentary evidence for use of viral infection for inducing variability in tomato (Chiriac and Bujoreanu 1996). Traditionally, combining two or more valuable traits in a genotype is achieved by crossing parental forms possessing the traits to be combined. However, attaining the desired result in tomato takes a long time due to linked inheritance of valuable traits, which tend to be negatively correlated (Tanksley et al. 1992). The opportunity for shortening the breeding time and increasing the frequency of individual plants combining valuable traits can be realized through the use of factors increasing recombination frequency and spectrum (Schuermann et al. 2005). Recently, viral infection has been found to be involved in the enhancement of mitotic (Kovalchuk et al. 2003) and meiotic (Chiriac et al. 2006) recombination, and their effects could persist in the subsequent untreated generation.
In view of the above, the objectives of the present study were as follows:
- 1
Evaluation of the effect of viral infection on the inheritance of quantitative traits (fruit weight, earliness, soluble solids content) in the progeny of plants infected with tobacco mosaic virus (TMV) and potato virus X (PVX);
- 2
Elucidating a possible relationship between chiasma frequency in the infected plants and variability of traits in the progeny; and
- 3
Identifying the genotypic particularities of the effect of viral infection.
Results
Viral infections have been known for the broad spectrum of effects they produce on various processes in the host plant (Dinner 1963; Ajayi 1986). In tomato, some features of the effect of TMV or PVX on plant quantitative traits have been studied (Tanksley et al. 1998; Olusegun et al. 2002). While manifesting a peculiar mode of parasitism consisting of exploiting the host plant's replication system and material, viral infections have also been known for their effects on the host plant's genetic material (Andronic 1997; Bass et al. 2000; Berta et al. 2000; Nagar et al. 2002). In view of the above, it is of interest to examine meiotic chromosome behavior in infected plants. Similarly, an important objective of the present study was ascertaining the consequences of the effect of TMV + PVX on the progeny of the virus-infected plants.
Experiment 1: cytogenetic analysis
Following studies of chromosome behavior at meiotic diakinesis, the number of chiasmata per pollen mother cell (PMC) has been found to increase substantially in 3 F1 hybrid genotypes and in one of two cultivars included in the cytogenetic analysis. This was due to an increase in the number of bivalents with two or more chiasmata per bivalent (Table 1).
| Genotype | Variants (no. PMC) | Rod bivalents | Ring bivalents | Other types | Total chiasmata no. per PMC |
|---|---|---|---|---|---|
| Nw × C | Non-infected (48) | 6.98 ± 0.21 | 3.98 ± 0.12 | 1.04 ± 0.09 | 17.57 ± 0.20 |
| Infected (49) | 5.08 ± 0.12*** | 5.15 ± 0.13*** | 1.77 ± 0.13*** | 19.75 ± 0.29*** | |
| La × V | Non-infected (48) | 7.58 ± 0.16 | 3.56 ± 0.15 | 0.86 ± 0.11 | 16.92 ± 0.24 |
| Infected (43) | 6.12 ± 0.13*** | 4.18 ± 0.12** | 1.70 ± 0.08*** | 18.38 ± 0.22*** | |
| N × K | Non-infected (50) | 7.65 ± 0.19 | 3.68 ± 0.17 | 0.67 ± 0.08 | 16.60 ± 0.25 |
| Infected (50) | 5.88 ± 0.17*** | 4.54 ± 0.18*** | 1.58 ± 0.14*** | 19.02 ± 0.26*** | |
| Prizior | Non-infected (50) | 6.94 ± 0.19 | 4.34 ± 0.18 | 0.66 ± 0.18 | 17.42 ± 0.65 |
| Infected (50) | 6.12 ± 0.16** | 4.50 ± 0.20 ns | 1.32 ± 0.19* | 18.18 ± 0.70 ns | |
| Fakel | Non-infected (50) | 8.22 ± 0.22 | 3.24 ± 0.23 | 0.54 ± 0.09 | 16.26 ± 0.22 |
| Infected (50) | 5.00 ± 0.13*** | 5.38 ± 0.16*** | 1.62 ± 0.15*** | 19.54 ± 0.27*** |
- *, **, *** significant at P≤ 0.05, 0.01 and 0.001, respectively. PMC, pollen mother cell; Rod, one crossing-over (one chiasma); Ring, two crossing-over (two chiasmata); Other types, the bivalent types (more than two chiasmata) that differed from previous mentioned types, as well as the univalent (non crossing-over). Mean value ± standard error.
At diakinesis, the chiasma frequency was 1.38–1.46 per bivalent in the non-infected plants of the F1 hybrids and 1.53–1.65 in infected plants, and 1.36–1.45 chiasmata in non-infected cultivars and 1.52–1.63 for infected one.
Typical meiotic bivalent types were attested in healthy and infected plants (Figure 1). F1 hybrids and cultivars have been found to significantly differ in the bivalents with one chiasma (rod bivalents) (Table 1). In all attested cases the number of rod bivalents have decreased in virus infected plants. On the contrary, the ring bivalents have increased significantly or remained unchanged (cv. Prizior). At the same time the numbers of other types of bivalents (univalent as well as bivalents with three chiasmata) have increased too, in hybrids and cultivars genotypes.
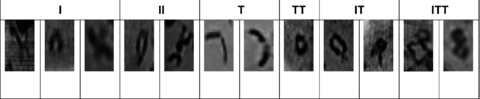
Bivalent types according to chiasma position during diakinesis.
Modified frequencies of types of bivalents classified according to the chiasma number were observable in both hybrid genotypes and cultivars when comparing infected plants with non-infected ones. The number of bivalents with one terminal chiasma (T) decreased from 7.50 down to 4.14 (P < 0.001) upon infection of plants of the Fakel cultivar, and from 6.22 down to 5.34 (P < 0.001) following infection of plants of the Prizior cultivar (Table 2). Concurrently, the number of bivalents with two (IT, P < 0.01) and three (ITT, P < 0.001 and P < 0.05 for Fakel and Prizior, respectively) chiasmata per bivalent increased significantly. In other words, infected plants exhibited a significant increase in the number of bivalents with two and three interstitial chiasmata, whereas the number of bivalents with a single chiasma (T, I) decreased or remained unchanged.
| Bivalent type | Cv. Fakel | Cv. Prizior | ||
|---|---|---|---|---|
| Non-infected (50 PMC) | Infected (51 PMC) | Non-infected (49 PMC) | Infected (53 PMC) | |
| I | 0.72 ± 0.10 | 0.86 ± 0.06 ns | 0.72 ± 0.06 | 0.79 ± 0.08 ns |
| II | 0.36 ± 0.07 | 0.58 ± 0.09* | 0.32 ± 0.07 | 0.80 ± 0.09*** |
| T | 7.50 ± 0.19 | 4.14 ± 0.13*** | 6.22 ± 0.17 | 5.34 ± 0.13*** |
| TT | 1.60 ± 0.15 | 2.40 ± 0.14*** | 2.48 ± 0.13 | 1.30 ± 0.10*** |
| IT | 1.28 ± 0.13 | 2.40 ± 0.11*** | 1.54 ± 0.13 | 2.40 ± 0.16*** |
| 0 | 0.02 ± 0.02 | 0.36 ± 0.07*** | 0.06 ± 0.03 | 0.30 ± 0.06** |
| ITT | 0.44 ± 0.08 | 0.36 ± 0.07*** | 0.48 ± 0.11 | 0.88 ± 0.10* |
| IIT | 0.08 ± 0.04 | 1.26 ± 0.14*** | 0.12 ± 0.05 | 0.02 ± 0.02 ns |
| TTII | 0.02 ± 0.02 | – | 0.00 | 0.12 ± 0.05 |
- *, **, *** significant at P≤ 0.05, 0.01, 0.001, respectively. ns, not significant differences. Type of bivalents: 1 interstitial (I), 2 interstitial (II), 1 terminal (T), 2 terminal (TT), 1 interstitial + 1 terminal (IT), univalents (0), 1 interstitial + 2 terminal (ITT), 2 interstitial + 1 terminal (IIT) and 2 terminal + 2 interstitial (TTII). Mean value ± standard deviation.
Analysis of the correlation between various bivalent types and the total number of chiasmata (S) per PMC revealed differences for infected plants. In conformity with the diagrams presented (Figure 2A–F), the increase in the total number of chiasmata (S) per PMC is dependent on the increase in the number of TT and ITT bivalents, followed by II and IT bivalents, and the degree of correlation varies according to the variant (infected or non-infected) and genotype.
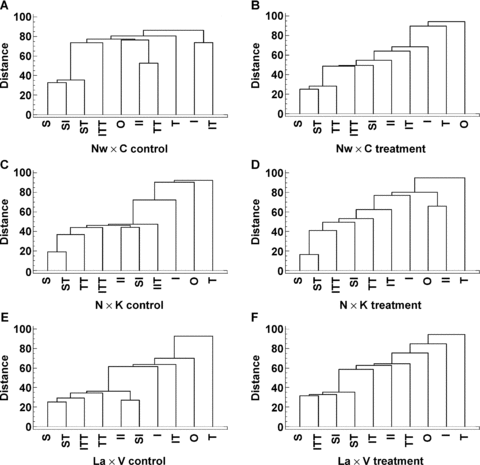
Distribution of bivalent types according to the degree of correlation in tobacco mosaic virus (TMV) and potato virus X (PVX)-infected (treatment) and non-infected (control) variants of F1 hybrid tomato plants. Bivalent types: 1 interstitial (I), 2 interstitial (II), 1 terminal (T), 2 terminal (TT), 1 interstitial + 1 terminal (IT), univalents (0), 1 interstitial + 2 terminal (ITT), 2 interstitial + 1 terminal (IIT) and 2 terminal + 2 interstitial (TTII), S, total chiasmata per pollen mother cell (PMC), SI, total interstitial chiasmata per PMC, ST, total terminal chiasmata per PMC. Distance expressed in Squared Euclidean units.
Recombination frequency
The frequency of recombinants in marked segments of the virus-infected progeny of F1 hybrid Mo × K differed from that of the non-infected progeny.
The exhibition of the traits determined by genes d, aw, c, and m-2 in homozygous genotypes remained unaffected in both infected plants and their progeny. The ratio of dominant to recessive phenotype classes was within the expected range of 3:1 in both the control and treatment (Table 3).
| Genes | Control | Treatment | ||||
|---|---|---|---|---|---|---|
| D/R | χ2 | P | D/R | χ2 | P | |
| d | 3.19 | 1.77 | ≤ 0.05 | 3.45 | 16.22 | ≤ 0.05 |
| aw | 3.24 | 2.72 | ≤ 0.05 | 3.55 | 23.63 | ≤ 0.01 |
| c | 2.84 | 1.95 | ≤ 0.05 | 3.18 | 2.86 | ≤ 0.05 |
| m-2 | 2.72 | 4.91 | ≤ 0.05 | 3.09 | 0.74 | ≤ 0.05 |
An increase in the frequency of recombinants from 10.39 ± 1.11 to 13.75 ± 0.86 (P < 0.001) (%) was noted for the d-aw segment (chromosome 2), as the result of the appearance of different rates of the phenotypes determined by the genotypes D-/awaw and dd/Aw- in the treatment variant of F2 Mo × K compared with control one (Table 4). At the same time, no difference in rf was observed in the c-m-2 segment (chromosome 6) (rf = 26.58 ± 1.12, %) between the control and the treatment variant (rf = 27.49 ± 0.94, %) of the F2 hybrids Mo × K and La × V (data not shown).
| Segment | Variants | Number of plants | χ2 | rf (%) | |||
|---|---|---|---|---|---|---|---|
| D-/Aw- | D-/awaw | dd/Aw- | dd/awaw | ||||
| d-aw | Control | 1 844 | 128 | 121 | 488 | 1 388.68 | 10.39 ± 1.11 |
| Treatment | 3 437 | 305 | 280 | 773 | 20 008.10 | 13.75 ± 0.86*** | |
| C-/M-2- | C-/m-2m-2 | cc/M-2- | cc/m-2m-2 | ||||
| c-m-2 | Control | 1 590 | 297 | 315 | 379 | 396.56 | 26.58 ± 1.12 |
| Treatment | 3 075 | 347 | 372 | 601 | 635.32 | 27.49 ± 0.94 ns | |
- *** significant at P≤ 0.001. ns, not significant differences.
Quantitative traits
Quantitative traits were examined in virus-free populations (F2–F4, G1–G2) descending from infected F1 plants and their parental forms. The results suggested that although viral infection was not present in the progeny, the difference (from the control) was still observable. In F2–F4 populations derived from infected F1 hybrids, a significant difference in mean values were observed for 40.91–90.48% of the traits analyzed (Table 5). Some traits tended to evolve in a similar way, exhibiting mean values higher or lower than those of the control over three generations analyzed (data notshown).
| Genotype | Generation | Number of analyzed traits | Traits with significant difference | |
|---|---|---|---|---|
| (Number) | (%) | |||
| La × V | F2 | 21 | 13 | 61.90 |
| F3 | 22 | 9 | 40.91 | |
| N × K | F2 | 21 | 19 | 90.48 |
| F3 | 22 | 16 | 72.72 | |
| F4 | 14 | 9 | 64.29 | |
| Nw × C | F2 | 21 | 13 | 61.90 |
| F3 | 22 | 13 | 59.09 | |
| F4 | 14 | 9 | 64.29 | |
The substantial differences in fruit weight between forms involved in crossing resulted in a wide range of distribution for this trait in F2 segregating populations (Table 6). In each of the four categories of F2 plants examined for the fruit weight, plant frequency in the population derived from infected F1 hybrids differed from the control. The largest deviations were noted in F2 populations of the La × V and N × K crosses. Thus, the frequency of plants with a fruit weight of 20–50 g was 23.81% in the treatment variant of the F2 hybrid N × K, compared with 7.94% in the control. At the same time, the frequency of plants with a fruit weight of more than 100 g was 2.38% in the treatment variant, compared with 13.49% in the control.
| Genotype | Variant (no. individuals) | The proportion of plants of a particular fruit weight category (%) | Fruit weight (g) | |||
|---|---|---|---|---|---|---|
| <20 g A | 20–50 g B | 50–100 g C | >100 g D | |||
| F2 La × V | Control (350) | 30.15 | 58.29 | 11.56 | 0 | 30.0 ± 1.0 |
| Treatment (424) | 17.45 | 64.63 | 17.45 | 0.47 | 36.4 ± 0.9*** | |
| F2 N × K | Control (279) | 0 | 7.94 | 78.57 | 13.49 | 80.2 ± 2.3 |
| Treatment (268) | 0.79 | 23.81 | 73.02 | 2.38 | 62.8 ± 1.8*** | |
| F2 Nw × C | Control (250) | 1.65 | 24.79 | 67.77 | 5.79 | 66.7 ± 2.2 |
| Treatment (336) | 0.62 | 19.14 | 72.84 | 7.40 | 68.4 ± 1.8 ns | |
| Victorina (V) | Control (30) | – | – | 83.33 | 16.67 | 101.4 ± 3.4 |
| Treatment G1 (32) | – | – | 78.12 | 21.88 | 94.6 ± 5.8 ns | |
| La0651 (La) | Control (20) | 85.00 | 15.00 | – | – | 19.8 ± 0.9 |
| Treatment G1 (20) | 80.00 | 20.00 | – | – | 20.7 ± 1.1 ns | |
| Nota (N) | Control (32) | – | – | 53.13 | 46.87 | 101.2 ± 2.6 |
| Treatment (35) | – | – | 48.57 | 51.43 | 99.7 ± 3.6 ns | |
| Krasnoyarskii rannii (K) | Control G1 (34) | – | 41.18 | 58.82 | – | 54.1 ± 2.9 |
| Treatment G1 (33) | – | 42.42 | 57.58 | – | 60.1 ± 0.2 ns | |
| Colocolchik (C) | Control (35) | – | 45.71 | 54.29 | – | 59.3 ± 2.7 |
| Treatment G1 (29) | 34.48 | 65.52 | 53.1 ± 2.6 ns | |||
| Novichok (Nw) | Control (31) | – | – | 51.61 | 48.39 | 102.3 ± 6.6 |
| Treatment G1 (30) | – | – | 50.00 | 50.00 | 102.8 ± 7.1 ns | |
- *** significant at P≤ 0.001; ns, not significant differences. A, B, C and D represent individual plant groups, seeds of which were cultivated and analyzed separately in the subsequent F3–F4 generations. Treatment G1 represents the first generation descendent from cultivars infected plants. Mean value ± standard error.
In each hybrid combination, one of the parental forms – that with a smaller fruit weight – was characterized by an earliness of ripening while the other parental form – the one with a larger fruit weight – showed a later maturity (Figure 3). It can be assumed, therefore, that the F2–F4 plants with an earlier maturity will also tend to have a smaller fruit weight since, according to the evidence in the literature, these traits are inherited as linked characters.
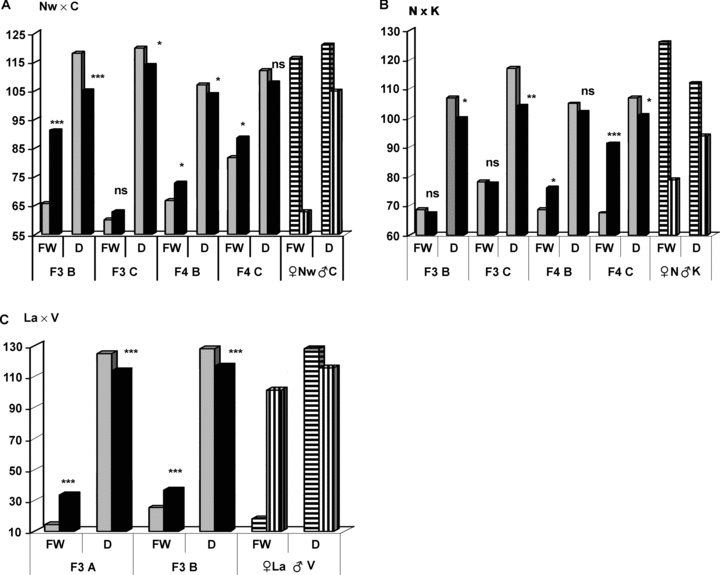
Relationship between fruit weight and the number d before mass ripening of fruit in F3–F4 populations descending from virus-infected and healthy F1 plants as compared with parental forms. Hybrids, grey columns represent control variants. Dark columns represent treatment variants, where FW and D are fruit weight (grams) and number of days to the time when 60% of the plants have ripe fruit, respectively. Parental forms, columns with horizontal lines represent maternal forms, whereas those with vertical lines are paternal forms (as described in Materials and Methods). A, B and C groups represent descendents from F2 plants with < 20, 20–50 and 50–100 g fruit weight, respectively for F3 and F4 generations. The F3 generation was analyzed in 2001, and the F4 generation in 2002. The hybrid La × V was only examined in generations F2 and F3.*, **, *** significant at P≤ 0.05, 0.01, and 0.001, respectively.
Earlier floral initiation and fruit ripening were observed in the treatment variant as compared with the control; at the same time, fruit weight also showed larger values or no significant difference (Figure 3). According to our results, fruit reached maturity later in the C group of plants than in the B group in treatment variants of the F3–F4 populations of the Nw × C cross and the F3 population of the N × K cross, or the differences were negligible (the A group in population F3 of the La × V cross and the C group in population F4 of the N × K cross) (Figure 3A–C).
At the same time, mass ripening of fruit occurred 1–12 d earlier in the treatment variant than in the control. Thus, in the F3 population of the Nw × C cross, the control exhibited delayed maturing (118–120 d), whereas the treatment variant plants in the B and C groups showed medium (112–114 d) and early (105–107 d) maturity, respectively, in line with Brezhnev's (1982) classification of maturity dates.
Concurrently with an increase in mean values of fruit weight and earliness in treatment variants, the proportion of recombinants with higher mean values of these two traits increased. Therefore, it can be stated that in the treatment variants, early fruit ripening occurred more frequently in those plant forms that had larger fruit weights. Approximately 94.4% and 82.0% of the F3 plants of the N × K cross in the treatment variant showed early maturity (105–107 d), whereas in the control they constituted 69.6% and 29.2% for the B and C groups, respectively (Figure 4A).
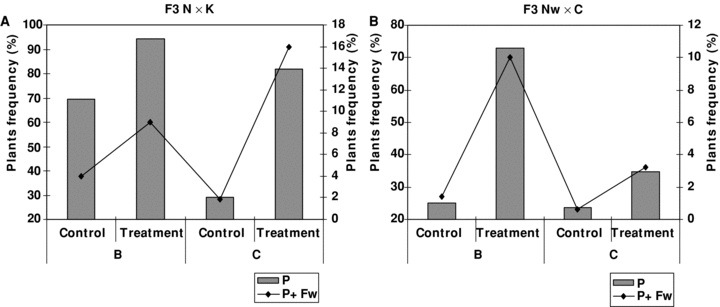
The proportion (%) of early-maturing forms with a large fruit weight (>100 g) in the F3 generation. For each bar diagram, index values for columns appear on the right, and those for lines on the left. B and C groups represent descendents from F2 plants with 20–50 and 50–100 g fruit weight, respectively. P represents the proportion of plants with early fruit ripening and P + Fw – that of plants that combine early fruit ripening and large fruit weight (>100 g).
Earliness of ripening was often associated with a smaller fruit weight in the control (and in the parental form K) in the F3 of the N × K cross, while in the treatment variant of this cross earliness of ripening was observed in plant forms with a larger fruit weight (a combination of traits inherited from both parental forms). The above traits differed dramatically compared with the control in the B group in the F3 of the Nw × C cross and less so in the C group (Figure 4A,B). As for the population that had not been subdivided into fruit weight categories, the frequency of plants combining earliness of ripening and large fruit weight increased from 2.22 up to 8.24 in the treatment variant. In the treatment variant of the F3 population of the La × V cross, along with earliness of ripening noted for this population, a proportion of plants exhibited very late maturity (>120 d) – something that was not observable in the control.
Generally, a shorter “seeds germination–fruit maturing” period results in lower fruit biochemical indices since most of these indices are negatively correlated with early maturity (Raijadhav et al. 1996). In view of the above, of interest are individuals that combine such traits as large fruit weight, early maturity and increased soluble solids (SS) content. anova revealed a significant contribution (P < 0.001) of the factors “fruit weight” and “virus” to the variation in SS in populations descending from three virus-infected F1 hybrids (Table 7).
| Genotype | Source ofvariation | df | Sum ofsquares | Meansquares | F-ratio |
|---|---|---|---|---|---|
| F2 N × K | Virus (V) | 1 | 0.016 | 0.016 3 | 84.39*** |
| Fruit weight (FW) | 2 | 0.253 | 0.126 4 | 653.84*** | |
| Interaction V-FW | 2 | 0.157 | 0.078 4 | 405.64*** | |
| F2 Nw × C | Virus (V) | 1 | 0.072 | 0.072 2 | 205.57*** |
| Fruit weight (FW) | 2 | 0.023 | 0.011 6 | 33.06*** | |
| Interaction V-FW | 2 | 0.093 | 0.046 4 | 132.18*** | |
| F2 La × V | Virus (V) | 1 | 0.087 | 0.087 | 142.71*** |
| Fruit weight (FW) | 2 | 3.334 | 3.317 | 5 436.00*** | |
| Interaction V-FW | 2 | 0.097 | 0.498 | 816.76*** |
- *** significant at P≤ 0.001. “Fruit weight” represents fruit with a weight of 20–50 g, 50–100 g and >100 g for F2 of hybrid combinations N × K and Nw × C, whereas <20 g instead of >101 g for F2 of La × V. “Virus” represents variants descending from virus-infected plants as compared with those from healthy plants.
According to some evidence (Eshed and Zamir 1996; Tanksley et al. 1996; Bernacchi et al. 1998; Causse et al. 2002), the SS content is negatively correlated with fruit weight. At the same time, in the treatment variant of F2 population the correlation between fruit weight and SS content was not always similar to that in the control. Sometimes, this correlation was positive as well, as in Grandillo and Tanksley (1996). Thus, in the progeny comprising the treatment variants, fruit with large weight often had a higher SS content as compared with the large-weighted fruit in the control or with the smaller weight fruit in the same treatment variant. Similarly, the values of coefficients of correlation between SS and pH or fruit weight and pH changed in the treatment variant populations with those of the control (data not shown).
Besides the facts mentioned, we have to add that in recombinant forms the favorable combinations of traits were frequently associated with less desired ones, such as fasciated or cracking fruits.
Discussion
The effect of biotic stress on processes occurring in plants is still not clearly understood, especially due to the fact that it is difficult to precisely assess the response of virus-exposed plants at a particular moment and its physiological consequences are diverse. One of the objectives of the present study was to evaluate some economic traits in the progeny of tomato infected plants. On the basis of the data obtained, we assume that the observed effects could be due to a peculiar behavior of the infected plant genetic material which, in our case, manifested itself as modified chiasma frequency per PMC and per bivalent. Although viral particles did not occur in the progeny of the infected plants, the observed deviation and the duration of their manifestation (three generations) suggest that the genetic material of the host plant has been affected.
The recombination effects on meiotic stage of F1 hybrids can be manifested in modification of the population structure in subsequent generations (Zhuchenco and Korol 1985). In F2–F4 populations derived from F1 hybrids infected with TMV + PVX modifications of the population structure have also been observed. Distribution curves for some traits indicate the viral infection causes either broadening or narrowing of variability spectrum. The differences could be attested for some 40.91–90.48% of analyzed traits, depending of genotypes (Table 5). In the progeny of infected hybrids, recombinant forms occurred that are lacking or rare in the control. Among these occurred forms with commercially valuable traits: larger fruits, increased content of soluble solids in fruit and early ripening.
It is not inconceivable that TMV + PVX could contribute to modification of the expression of some genes controlling the above traits, but the effects of viral infection can persist for a number of generations, similarly to the phenomenon of aberrant ratio or production of unstable genotypes (Sprague and McKinney 1966; Yan et al. 2000). According to the latest publications (Molinier et al. 2006; Boyko et al. 2007) the basis for the described transgeneration effect could be epigenetic because the whole population changes its behavior; whereas a mutation would affect only very few plants.
Corroboration of fruit weight increase or decrease and/or of earlier or later fruit maturing in the progeny of virus-infected cultivars and hybrid populations would be a point in favor of this idea. Examination of two successive generations (G1 & G2) descendant from six virus-infected parental forms (N, K, Nw, C, V, and La) revealed no significant differences in fruit ripening (in earliness) in parental forms with medium maturity (1–2 d differences observed for N), whereas no difference was noted for late (V, Nw) and early (C, K) ripening cultivars (data not shown). At the same time, no deviation was observed for fruit mean weight (Table 6) and SS content (data not shown) in the treatment and control variants.
The distribution of plant frequencies for the trait “fruit weight” was not the same in the control and the treatment variant of hybrid populations; a fact that could be negligible in cultivars populations (Table 6). Also, differences in mean values of fruit weight and ripening stem from modified within-population distribution of individuals (Figure 3). Also, the differences noted in hybrid populations were observable over the course of three successive generations – F2–F4 (with a few exceptions). Enhanced variance and modified genotype frequencies caused the fruit weight mean values to increase by 4.8 to 137.8% in F3 of three hybrid combinations and their fruit matured 1 to 12 d earlier than in the control.
Previous studies (Chiriac and Bujoreanu 1996) have shown that populations derived from a single hybrid plant infected with TMV – one produced from seeds harvested from the first cluster before viral infection, and the other from seeds collected from the second cluster in which meiosis proceeded under the influence of viral infection – differed in the mean values of some valuable traits, such as fruit number on the first cluster and leaf number on the main stem.
Our experimental data are in good agreement with evidence in the literature (Stokes et al. 1988) as far as the explanation of the observed differences is concerned, the differences being attributable to the effect of viral infection on meiotic processes occurring in the infected plants.
An important observation is an increased proportion of plants in which large fruit weight was combined with earlier maturity in the treatment population as compared with the control (Figure 4) as, according to some publications (Banerjee and Kalloo 1989; Kemble and Gardner 1992), there is a negative association between earliness and fruit weight.
A two-way anova revealed a significant contribution of the “fruit weight” and “virus” factors to the SS variation (Table 7). Besides, differences in the degree of correlation between traits in the treatment and control populations of the F2 hybrids in question cannot be ignored.
Recent studies confirm our assumptions that the frequency of mitotic crossing-over is modified in infected plants, with modification of recombination frequency being stable in subsequent generations too (Kovalchuk et al. 2003).
The effect on chromosome behavior during meiosis and the occurrence of deviations, such as chromosome bridges and lagging, were observed in both hybrids and cultivars (Marii L. unpubl. data, 2000) – a fact mentioned by other authors (Kostoff 1933; Caldwell 1952) too. Examining chromosome pairing was also of interest to us. Chiasma expression represents the cytological effect of crossing-over and may indicate on modification of the recombination's number (Sherman and Stack 1995).
In our studies, viral infection induced an increase in chiasma frequency per bivalent and PMC (Tables 1, 2). Concurrently, an increase in the number of bivalents with three chiasmata (TTI bivalents) was noted which, according to Rees and Dale (1974), enhanced genetic variability. An increase in the proportion of univalents was observed for both hybrid and cultivar genotypes. Distinctive chromosome behavior during chromosome pairing was also evidenced in tomato plants infected with tomato aspermy virus or with TMV and PVX applied separately (Chiriac et al. 2006).
In conformity with some reports in the literature, it appears that, like other factors, viral infection can induce an increase in the incidence of recombination in the progeny of treated plants, thus manifesting meiotic stability (Lebel et al. 1993; Puchta et al. 1995; Ries et al. 2000; Lucht et al. 2002; Kovalchuk et al. 2003; Filkowski et al. 2004).
The above led us to assume that the given combination of viruses or each of the viruses (Chiriac et al. 2006) can modify recombination frequency in the d-aw segment. Similar results were obtained in the case of sorghum hybrids infected with the sugarcane mosaic virus (Stokes et al. 1988). In line with the results of phenological studies, viral infection did not affect the expression of genes d, aw, c, m-2, h, ful and hl (the last four genes not being involved in the present study) in the progeny of virus-infected homozygous genotypes, as well as the ratio of dominant to recessive phenotype was within the expected range 3:1 (Table 3). Thus, it is conceivable that modified frequencies of genotypes dd/Aw- and D-/awaw are due to altered recombination frequencies, rather than being a product of gene expression. The modified numbers of recombinants were noted for genes located on chromosome 2, but not for those on chromosome 6. In line with Singh's evidence (Singh 1981) and our preliminary results (Chiriac et al. 2006), the same chromosome responds differently to recombinogenic factors, and different chromosome segments respond in a different manner to the same factor.
Association between various traits is often an attribute of linkage between two or more quantitative trait loci: in that case recombination will bring about separation of desirable traits from the undesirable ones. Manifestation of this phenomenon of association between traits in a large number of crosses will be due to reduced crossing-over rates (Paterson et al. 1990; Lippman and Tanksley 2001). It is not improbable that in virus-infected tomato plants, the genes controlling the traits under study exhibit linkage disequilibrium as a result of modification of meiotic crossing-over, and, in particular, of recombination frequency and spectrum. According to some reports (Villanueva and Kennedy 1990; Wu et al. 2002), linkage disequilibrium has a direct effect on genetic variability, heritability and correlation between traits.
The foregoing suggests that the mechanism of action of viral infection does not produce differential effects in either infected hybrid or cultivar genotypes. At the same time, it is evident that changes are observable in the progeny of virus-infected F1 hybrids, but not in the cultivars. These peculiarities are attributable to the fact that variability in self-pollinating species is reduced (Baudry et al. 2001). In our case, cultivars appear to be “homozygous”, but recombination in that case would be meaningless, whereas hybrids, being heterozygous, could manifest distribution of genetic material at meiosis through variation in traits in subsequent generations.
Stephan and Langley (1998) reported strong correlation between recombination frequency and variability in Lycopersicon species. Similarly, chiasmata frequency and quantitative traits variation were found to be correlated in cotton (Srivastava 1980) and grasshopper (Westerman 1983) species. According to Tanksley et al. (1992), intrachromosomal heterogeneity of crossing-over is of both practical and evolutionary significance.
It has been demonstrated that rearrangements in a transgene of infected plants could potentially be transmitted to the next generation (Kovalchuk et al. 2003; Boyko et al. 2007). Molinier et al. (2006) attributes high levels of homologous recombination in the progeny of the treated plants (exposed to biotic and abiotic stress) to the fact that plants “memorize” their previous exposure, but the nature of this phenomenon remains to be ascertained.
The foregoing suggests that populations descending from virus-infected hybrids exhibit genetic variation in the traits under study. Chromosome pairing modification noted in virus-infected F1 plants should be regarded as a major factor contributing to the within-population redistribution of individuals, the direct effect being deviations for a number of statistical parameters. Moreover, increased frequency of forms combining traits of both parents, as well as deviations in correlation between traits in the progeny of heterozygous hybrids and the lack of these in “homozygous” cultivars, are further proof of modified recombination. The observed variability can be used in plant improvement programs.
Materials and Methods
Basic experimental materials
The material used in the present study consisted of four tomato (Solanum lycopersicum L.) intraspecific hybrids F1 Nota × Krasnoyarskii rannii (N × K), Novichok × Colocolchik (Nw × C), La 0651 × Victorina (La × V), Mo 500 × Krasnoyarskii rannii (Mo × K), parental forms (N, Nw, C, K, V, La, and Mo) and two additional cultivars – Fakel and Prizior. The genotypes used in producing hybrids differed in the traits under study. Tomato F1 hybrids were obtained by artificial cross pollination.
Two of the F1 hybrids – Mo × K and La × V – heterozygous for genes D, Aw, C, M-2 and C, M-2 respectively, were used to determine the recombination frequency (rf) in the marked segments. The genotypes La 0651 (c-m-2/ c-m-2) and Mo 500 (d-aw/ d-aw; c-m-2/ c-m-2) served as maternal parents. The genes d-aw (d– dwarf and aw– without anthocyan) and c-m-2 (c– potato leaf and m-2– mottled-2) are located on chromosome 2 and chromosome 6, respectively. Locations and designations of these genes are according to Atherton and Rudich (1986).
Inoculation of plants
In our previous research (Chiriac et al. 2006) we have attested the effect of TMV or PVX alone on chiasmata frequency in virus-infected plants as well as inducing variability in virus-free progenies. Synergetic effect was attested in some cases of mixed infection. A double infection inoculum – TMV + PVX, was used in our case.
Tomato plants were inoculated by rubbing the TMV + PVX inoculum into two leaves at the stage of the first truss differentiation. Under similar conditions, plants of the control variants were inoculated with a tissue extract from healthy plants. Viruses were isolated from tomato and potato plants infected with TMV and PVX, respectively. The TMV + PVX combination causes the inoculated plants to develop visual symptoms manifested as diffuse mosaic. The progeny lacked the symptoms. The presence of virions in the infected plants and their absence in the progeny of these plants as well as in the control variants was ascertained through the immunosorbent electron microscopy (ISEM) test (Derrick and Brlansky 1976).
Field experiments
Experiment 1
Tomato hybrid plants, parental forms and additional cultivars were inoculated with TMV + PVX. At meiotic prophase I, flower buds were excised from both infected and non-infected plants and analyzed for chiasma frequency. The infected plants were examined concomitantly with non-infected ones but on different experimental plots to avoid infection of healthy plants. F1 hybrid plants were grown in the field, next to parental plants. Some 12–15 plants were infected for hybrid combinations and 10–20 plants for parental forms.
Experiment 2
The seeds collected from fruits of the first cluster from infected plants were used to produce the first generation of progeny from infected plants (F2 for hybrid combinations and G1 for parental forms herein referred to as the treatment plants and those collected from non-infected plants comprised the control).
Progenies of F2 and F3 plants, both the control and treatment, were used to produce the F3 and F4 generations, respectively. Under similar conditions, descendants of healthy and infected plants of parental forms were used to produce the first (G1) and second (G2) generations for the control and treatment, respectively.
With a view to inactivating viral particles, the seeds representing the control and the treatment and coming from healthy and virus-infected plants, respectively, were subjected to thermal treatment at 70 °C for 72 h (Wolf and Schmelzer 1972). Heat treatment had no significant influence on seed germination rate.
The seeds derived from selfing of four F1 hybrids (N × K, Nw × C, La × V, and Mo × K) and of parental forms of the control and treatment were sterilized with 1% KMnO4 for 10 min, rinsed in running water for 30 min, germinated in Petri dishes and then sown in the soil in a greenhouse.
Thirty five days after germination, some 250–424 F2 seedlings, depending of genotype, were transplanted in the field. Plants of both variants – the control and treatment – were planted in the field in three replications with a minimum between-variant disturbance.
The F 2 generation of plants derived from infected and healthy F1 hybrids were examined for fruit weight and classed into a few categories: <20, 20–50, 50–100, >100 g. Seeds from fruits of each plant were selected separately and then pooled into groups according to the weight of fruit from which they had been extracted. These seeds were used to produce F3–F4 plants, for separate groups, according to their derivation in F2 from plants with a particular fruit weight: the 20–50 and 50–100 groups (mentioned as B and C groups, respectively) for the N × K and Nw × C hybrids. The exception was La × V for which the <20 and 20–50 groups (mentioned as A and B groups, respectively) were included in the study for only F3 plants. Included in the study were groups that were more representative numerically in F2. Each plant group was analyzed separately.
The F2 hybrids Mo × K and La × V were evaluated for the expression of qualitative traits c, m-2, d, and aw at the stage of two true leaves.
Quantitative traits
Trait values were assessed for each plant with subsequent estimation of the mean value per group. Approximately 20 traits were examined for each plant (of hybrid populations and cultivars): internode length, number of internodes to the first truss, number of trusses per main stalk, leaf length, plant height, number of fruit per plant per growing season, number of flowers and fruit in the first, second and third cluster, mass flowering and fruit ripening; fruit weight, diameter and height, and number of locules according to Brezhnev (1982). Besides, soluble solids content and pH were measured (Ermakov et al. 1987).
At the fruit ripening stage, five fruits were harvested from each plant. These were used to determine the mean weight and other fruit parameters. In order to determine biochemical characteristics of fruit (soluble solids content and pH), five fruits were homogenized and filtered through a cotton cloth. Soluble solids (SS) content was measured with the aid of a refractometer and pH level – with a pH-meter (Ermakov et al. 1987), each parameter being estimated with three replications.
Flowering: The number of plants with at least one flower in the first truss was counted every 4–6 d for a period of 5 weeks, and mass flowering was represented by the number of day at which 60% of the plants were in flower.
Ripening: The number of d from germination to the appearance of ripe fruit in the first cluster in 60% of the plants.
Cytological analysis
Genotypes of three tomato hybrids (N × K, Nw × C, and La × V) and of two cultivars (Fakel and Prizior) were used to ascertain the correlation between the presence of infection in the plants and chiasma frequency. The hybrid genotypes were the same as those for which quantitative traits had been examined. The cultivars were chosen from a similar and parallel study based on the estimation of chiasma frequency in non-hybrid genotypes.
Chiasma frequency was measured in 43–53 PMCs from buds of five infected plants and five non-infected plants for each genotype. Flower buds corresponding to stage II (4–5 mm) of the meiotic prophase I (Sawhney and Bhadula 1988) were collected from the first truss of infected and non-infected plants. The flower buds were fixed according to Clark's procedure (Pausheva 1988).
Chiasma numbers were determined in temporary mounts produced by staining with acetocarmine and pretreated with iron alums. The mounts were visualized under a light microscope BIOLOR, at resolutions of ×100 (objective) and ×20 (ocular).
The bivalents visualized (12 pairs) were classed according to the number and position of chiasmata at diakinesis (Imai and Moriwaki 1982): 1 interstitial (I), 2 interstitial (II), 1 terminal (T), 1 interstitial + 1 terminal (IT), 1 terminal + 2 interstitial (TII), 2 terminal (TT), 2 terminal + 1 interstitial (TTI), and univalents (0).
Analysis of recombination frequency in marked segments
F2 populations of the Mo × K and La × V hybrids were produced by selfing virus-infected and healthy F1 plants.
Heterozygous F1 plants exhibited uniformity in dominant traits. Recombination frequency in marked segments was measured in segregating populations and it is expressed as a percentage. Included in the analysis were 4 795 plants for the treatment variant and 2 581 for the control of the F2 hybrid Mo × K, and 1 552 and 1 235 plants for the treatment and the control, respectively, of the F2 La × V genotype.
Mathematical data processing was carried out using the RISS software package of the Institute of Genetics, Academy of Sciences of Moldova. The segregation was tested by χ2 analysis for their goodness-of-fit statistics to the expected Mendelian segregation ratio (3:1). Two methods were used to calculate the recombination fraction: the maximum likelihood method and the product ratio method.
Statistical analysis
Mean values of traits and error or standard deviation were estimated for F1–F4 hybrid plants and parental forms. The data were processed using the software package Statgraphics Plus for Windows (version 2.1; Microsoft Corp., Redmond, WA, USA) and Microsoft Excel. Significant differences in mean values of traits were established using the t-test. anova of SS was carried out by means of a two-way anova (Clewer and Scarisbrick 2001).
Cluster analysis was carried out by the Nearest Neighbor method based on comparing distances between the parameters analyzed and placing these in clusters by the closest distance between them. The distance is expressed in Squared Euclidean units.
(Handling editor: Daoxin Xie)
Acknowledgements
The authors are grateful to Mr G.K. Lakhman for translating the manuscript into English.




