Vegetative Storage Protein with Trypsin Inhibitor Activity Occurs in Sapindus mukorassi, a Sapindaceae Deciduous Tree
Supported by the National Natural Science Foundation of China (No. 30460107)
Abstract
A vegetative storage protein (VSP) with trypsin inhibitor activity in a deciduous tree, Sapindus mukorassi, was characterized by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western-blot, immuno-histochemical localization, light- and electro-microscopy, together with analysis of proteinase inhibitor activity of the purified VSP in vitro. There were two proteins with molecular masses of about 23 and 27 kDa in a relatively high content in the bark tissues of terminal branches of S. mukorassi in leafless periods. The proteins decreased markedly during young shoot development, indicating their role in seasonal nitrogen storage. Immuno-histochemical localization with the polyclonal antibodies raised against the 23 kDa protein demonstrated that the 23 kDa protein was the major component of protein inclusions in protein-storing cells. The protein inclusions were identified by protein-specific staining and should correspond to the electron-dense materials in different forms in the vacuoles of phloem parenchyma cells and phloem ray parenchyma cells under an electron microscope. So, the 23 kDa protein was a typical VSP in S. mukorassi. The 23 and 27 kDa proteins shared no immuno-relatedness, whereas the 23 kDa protein was immuno-related with the 22 kDa VSP in lychee and possessed trypsin inhibitor activity. The 23 kDa protein may confer dual functions: nitrogen storage and defense.
On the basis of the characteristics of seed storage proteins, Staswick defined vegetative storage proteins (VSPs) as those that sequestered in vacuoles in relatively high contents and their accumulation and degradation solely accorded to the availability of excess N (Staswick 1994). In this sense, the proteins found in the vacuoles of the paraveinal mesophyll cells in soybean leaves (Franceschi et al. 1983; Wittenbach 1983a) and those in perennial organs of other herbaceous plants (Volenec et al. 1996) are of typical VSPs. These proteins serve as transient nitrogen reserves during normal pod development (Staswick 1994) based on the fact that pod removal results in a significant increase in the level of VSPs (Wittenbach 1983b; Staswick 1989) and are positively related to the vigor of spring regrowth but poorly to winter survival (Dhont et al. 2006). The fact that VSPs play a very small direct role in overall plant productivity in the transgenic soybean plants in which VSP synthesis is suppressed by a vspA antigene may be the result of partial compensation by increased level of other proteins and/or non-protein N (Staswick et al. 2001). In a broad sense, the trypsin inhibitors accumulated in the leaves of Solanaceae species under biotic or abiotic stresses (Ryan 1990; Moura and Ryan 2001) and those in the flower/flower buds of Arabidopsis (Berger et al. 1995) are also considered as VSPs because of their vacuolar localization. Furthermore, the VSPs in the leaves of soybean and many of those in the perennial organs of other herbaceous plants are characterized to be bioactive (Graham et al. 1986; Andrews et al. 1988; Ryan 1990; Tranbarger et al. 1991; De Wald et al. 1992; Volenec et al. 1996; Yeh et al. 1997; Moura and Ryan 2001; Flores et al. 2002; Meuriot et al. 2004), and the role of VSPs in Arabidopsis in defense against insects have been demonstrated (Liu et al. 2005).
Vegetative storage proteins, characterized by their vacuolar localization, relatively high contents and seasonal fluctuation, have been identified in evergreen trees and deciduous trees since the mid-1980s (Clausen and Apel 1991; Stepien et al. 1994). Although evergreen trees are generally poor in VSPs (Wetzel and Greenwood 1991; Wetzel et al. 1991; Roberts et al. 1991; Arora et al. 1992), lychee (Litchi chinensis), a subtropical evergreen fruit tree, was recently demonstrated to be rich in a 22 kDa VSP with trypsin inhibitor activity (Tian et al. 2007). VSPs are commonly found in abundance in many temperate deciduous trees (for review, see Stepien et al. 1994) and tropical deciduous trees (Wu and Hao 1986, 1991; Hao and Wu 1993; Tian et al. 1998, 2002, 2003; Tian and Hu 2004). The best-characterized VSP in deciduous trees is the 32 kDa protein in poplar (for review, see Stepien et al. 1994). In addition, WIN4 and PNI288 are also considered to be VSPs in poplar (Cooke and Weih 2005). These three proteins are thought to play a role in defense against herbivores except for nitrogen storage on the basis of the upregulation of their genes by mechanical wounding (Davis et al. 1993; Cooke and Weih 2005). However, the 32 kDa VSP in poplar has no enzymatic or other biological activities (Stepien and Martin 1992; Stepien et al. 1994) and there are not any reports on the biological activities of the characterized VSPs so far in other deciduous trees. To find VSPs with special biological activities in deciduous tree species, we characterized the VSPs in Sapindus mukorassi by means of cytological and biochemical techniques.
Results
Seasonal fluctuation of the proteins in branches within an annual growth cycle
Two proteins with molecular masses of about 23 and 27 kDa fluctuated seasonally (Figure 1A,B). They existed in abundance in the bark tissues of terminal branches during the leafless period (Figure 1A, 1) and decreased obviously during young shoot development (Figure 3A, 2), suggesting that they were used to support the growth of young shoots. The proteins increased evidently when the leaves of young shoots had just matured (Figure 1A, 3), and thereafter, kept a similar level (Figure 1A, 4–7) until an obvious increase in mid-November when the trees began shedding their leaves (Figure 1A, 8).

Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses of the seasonal fluctuation of vegetative storage proteins in terminal branches (A) and young shoots (B).For terminal branches, samples were collected by late January (1), at the beginning of March (2), April (3), May (4), July (5), September (6) and October (7), and in mid-November (8). For young shoots, samples were collected in mid-February (1), by late February (2), at the beginning of March (3), April (4), May (5), June (6), July (7), August (8), September (9), October (10) and November (11), and in mid-November (12). Arrow heads, the 23 and 27 kDa protein; S, protein standards.
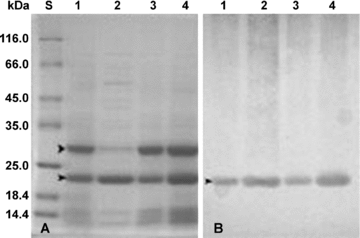
Distribution of vegetative storage proteins revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (A) and Western-blotting (B). 1 and 2, the bark (1) and xylem (2) tissues of terminal branches. 3 and 4, the bark tissues of trunk (3) and large roots (4). 12% SDS-polyacrylamide gels were used and 10 μg protein were loaded per lane. S, protein standards.
Although the 23 and 27 kDa proteins could not be certainly detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in the growing apexes soon after sprouting (Figure 1B, 1), the two proteins occurred in small amounts in the stems of young shoots 10 d after sprouting when leaves were purple (Figure 1B, 2), increased gradually as leaves turned light green (Figure 1B, 3), and reached the highest level when leaves had just matured (Figure 1B, 4). Thereafter, the level of the 23 and 27 kDa proteins decreased to some extent during flowering (Figure 1B, 5–6) and fruit development (Figure 1B, 7–10) and increased slightly in November when the trees began shedding their leaves (Figure 1B, 11–12).
Similar seasonal fluctuation patterns of the proteins occurred in the xylem tissues of young shoots and terminal branches (data not shown).
Protein-storing cells
In paraffin sections stained with mercury-bromophenol blue, dense vacuolar inclusions in clear blue were present in parenchyma cells. Thus, the inclusions were proteinaceous and the cells containing the proteinaceous inclusions were called protein-storing cells (PSCs) (Tian et al. 1998). The PSCs were ordinary parenchyma cells such as cortex parenchyma cells, phloem parenchyma cells (Figure 2A) and xylem parenchyma cells (Figure 2B).
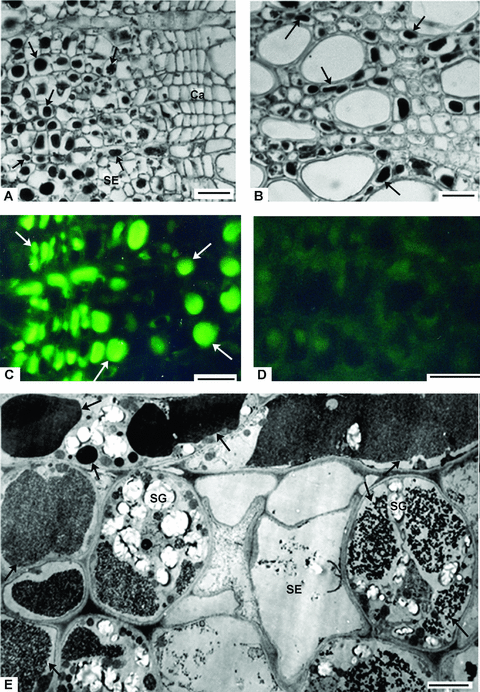
Identification of protein-storing cells (PSCs).(A–B) Light micrographs of the cross-sections of terminal branches, showing PSCs with vacuolar protein inclusions (black arrows) in bark (A) and xylem (B).(C–D) Indirect immunofluorescence localization of the 23 kDa protein. Sections were treated with the polyclonal antibodies raised against the 23 kDa protein (C) and preimmune-serum (D).(E) Electron micrograph of the cross-sections of terminal branches, showing PSCs with different forms of vacuolar electron-dense masses (black arrows) in the secondary phloem. White arrows, the vacuolar inclusions labeled by fluorescein isothiocyanate (FITC)-specific fluorescence. SE, sieve element; SG, starch grain. Bars = 20 μm in (A) and (B), 12.5 μm in (C) and (D) and 8 μm in (E).
Indirect immunofluorescence localization demonstrated that fluorescein isothiocyanate (FITC)-specific blue-green fluorescence existed in the sections that were treated with the polyclonal antibodies raised against the 23 kDa protein (Figure 2C), but was not observed in the sections treated with preimmuno-serum (Figure 1D). So, the 23 kDa protein was the component of the vacuolar protein inclusions in PSCs.
Under an electron microscope, electron-dense masses in different forms were observed in the secondary phloem parenchyma cells and secondary phloem ray parenchyma cells (Figure 2E). The electron-dense masses occurred in vacuoles of various sizes (Figure 2E) and should correspond to the vacuolar protein inclusions of PSCs observed under a light microscope (Figure 2A). There were more than three distinguished forms of the electron-dense masses, but the forms in the same cells were identical (Figure 2E). The parenchyma cells containing vacuolar electron-dense masses also accumulated a large number of starch grains in cytoplasm (Figure 2E).
Tissue-specific expression of the 23 and 27 kDa proteins
During the leafless period, the 23 and 27 kDa protein was present in large amounts in the bark tissues of terminal branches (Figure 3A, 1), trunk (Figure 3A, 3) and large roots (Figure 3A, 4) while the 27 kDa protein was relatively poor in the xylem tissues of terminal branches (Figure 3A, 1) and both the 23 kDa protein and the 27 kDa protein were absent from the xylem tissues of trunk and large roots (data not shown).
Western-blot analysis showed that the polyclonal antibodies raised against the 23 kDa protein from the bark tissues of the terminal branches reacted strongly with the 23 kDa protein in the bark and xylem tissues of terminal branches (Figure 3B, 1 and 2), the bark tissues of trunk (Figure 3B, 3) and large roots (Figure 3B, 4), but the antibodies could not recognize the 27 kDa protein in these organs (Figure 3B), indicating that the two proteins shared no immuno-relatedness.
The 23 kDa protein possessed trypsin inhibitor activity
The 23 and 27 kDa proteins were separated and purified by size-exclusion chromatography (Figure 4A), indicating that they are of different proteins other than different sub-units of the same protein. Polyclonal antibodies raised against the 23 kDa protein could not recognize the purified 27 kDa protein (Figure 4B). As lychee (Litchi chinensis Sonn.), a Sapindaceae evergreen tree species, was rich in a 22 kDa VSP (Figure 4C), which was characterized as a Kunitz trypsin inhibitor (Tian et al. 2007). Immuno-relatedness between the 23 and 27 kDa proteins in S. mukorassi and the 22 kDa VSP in L. chinensis was analyzed by Western-blot with the antiserum raised against the 22 kDa VSP in L. chinensis. The 23 kDa protein, but not the 27 kDa protein in S. mukorassi, was strongly immuno-related to the 22 kDa VSP in L. chinensis (Figure 4D). The assays for the trypsin inhibitor activity of the 23 and 27 kDa proteins were carried out in vitro. A typical purification of the 23 kDa protein is summarized in Table 1. The 23 kDa protein was effective at inhibiting trypsin with a concentration of 4.9 μg/mL at half maximum velocity of inhibition (Figure 4E). The inhibitory effect of the 27 kDa protein on trypsin could not be detected (data not shown).

Biochemical properties of vegetative storage proteins.(A–B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (A) and Western-blotting (B) treated with the polycolonal antibodies raised against the 23 kDa protein. 1–3, soluble proteins from bark tissues of terminal branches (1), the purified 27 kDa protein (2) and 23 kDa protein (3). Twenty micrograms of protein were loaded in lane 1 while 2 μg proteins were loaded in lane 2 and lane 3, respectively. 12% SDS-polyacrylamide gels were used for SDS-PAGE. S, protein standards.(C–D) SDS-PAGE (C) and Western-blotting (D) treated with the polyclonal antibodies raised against the 22 kDa vegetative storage protein (VSP) in lychee. Bark soluble proteins from the terminal branches of Chinese soapberry (1) and lychee (Litchi chinesis) (2). 12% SDS-polyacrylamide gels were used for SDS-PAGE and 10 μg protein were loaded per lane. S, protein standards.(E) Inhibitory effect of the purified 23 kDa protein on trypsin.
| Fraction | Total activity (UI) | Protein (mg) | Specific activity (UI/mg) | Yield (%) | Purification (-fold) |
|---|---|---|---|---|---|
| Crude extract | 2 568.1 | 12.99 | 198 | 100.0 | 1.00 |
| SephacrylS-100HR | 1 161.0 | 3.87 | 300 | 45.2 | 1.52 |
| DEAE-sepharose | 437.0 | 1.15 | 380 | 17.0 | 1.92 |
Discussion
Vegetative storage proteins in trees are defined by their localization in vacuoles, their relatively high abundance, and their seasonal fluctuation (O'Kennedy and Titus 1979; Clausen and Apel 1991). According to these three criteria the 23 kDa protein belongs to this class of proteins. (i) As shown by cytological and immuno-histochemical localization the protein is the component of the protein inclusions in PSCs associated with the electron-dense masses in the vacuoles of phloem parenchyma cells; and (ii) the protein accumulates in large amounts in leafless periods and decreases obviously during new shoot development as revealed by SDS-PAGE. Moreover, the 27 kDa protein is possibly a VSP considering that its relatively high abundance and seasonal fluctuation although its localization in vacuoles remains to be elucidated.
A traditional concept of the seasonal fluctuation pattern of N reserves (VSPs) in temperate trees is that seasonal nitrogen storage takes place during autumn senescence when nitrogen transports from senescing leaves to perennial tissues while the N reserves in woody perennial roots and stems are mobilized to support the early season development of expanding buds in spring (Cooke and Weih 2005). That is to say, VSPs in different perennial parts are mobilized simultaneously in spring during young shoot development and VSPs are also accumulated simultaneously in the different perennial parts in autumn when leaves become senescent. We have reported a seasonal fluctuation pattern of VSPs in Swietenia macrophylla, a tropical deciduous tree species, which is quite different from this traditional concept (Tian et al. 2003). The VSPs stored in the stems of branches are mobilized dramatically, while those stored in the bark tissues of trunk and large roots remain unchangeable in abundance during young shoot development (Tian et al. 2003). The mobilization of VSPs in trunk and large roots is associated with the secondary growth of these organs (Tian et al. 2003). On the other hand, the young shoots as well as the terminal branches initiate VSP accumulation soon after new leaves have just matured in spring (Tian et al. 2003). It should not be a special case in S. macrophylla because a similar seasonal fluctuation pattern of VSPs occurs in poplar, a temperate deciduous tree species (Tian et al. 2005). In the present study, we reveal again a similar seasonal fluctuation pattern of the VSPs in the young shoot and terminal branches of S. mukorassi in an annual growth cycle. Thus, we conclude here that a fundamental seasonal fluctuation pattern of VSPs, which is characterized by the differentially spatial-temporal mobilization and accumulation, occurs in different taxa of tree species.
Lots of VSPs have been identified in temperate trees (for review, see Stepien et al. 1994). Their diversity in biochemistry promotes a hypothesis that VSPs should be present with special bioactivity in trees (Stepien et al. 1994). However, all of the identified VSPs so far in temperate trees are not bioactive, suggesting that there may be few VSPs with special bioactivities in temperate trees. VSPs are also found in many tropical trees (Wu and Hao 1986, 1991; Hao and Wu 1993; Tian et al. 1998, 2003; Tian and Hu 2004). In contrast to the VSPs in temperate trees, we recently characterize the 22 kDa VSP in L. chinesis, a subtropical evergreen tree species, to be a Kunitz trypsin inhibitor (Tian et al. 2007). In the present study, the 23 kDa protein in S. mukorassi is demonstrated to be immuno-related to the 22 kDa VSP in L. chinesis and trypsin inhibitor active. Moreover, the 67 kDa VSP in Hevea brasiliensis, a tropical deciduous tree species (Tian et al. 1998; Tian and Hao 1999) is an active cyanogenic beta-glucosidase (Tian et al., unpubl. data, 2008). Taken together, VSPs with special bioactivities exist in tropical trees and may play dual roles in nitrogen storage and defense.
Materials and Methods
Plant materials
Fifteen-year-old Chinese soapberry (Sapindus mukorassi Gaertn.), cultivated in the Hainan Tropical Botanical Garden on Hainan Island was used for this study. Samples were collected from five trees that were nearly the same in size, growth vigor, and phenological phase (phenophase). The trees began shedding their leaves in mid-November, remained leafless for about 3 months and then sprouted in mid-February the next year. To trace the seasonal fluctuation of VSPs in branches, the stems of terminal branches were collected at the periods when the trees were leafless (late January); the new leaves were light green (by early March) and had just matured (by early April). Thereafter, samples were collected every one or two months until the trees began shedding their leaves (mid-November). To trace the seasonal fluctuation of VSPs in young shoots, the stems of young shoots were collected at the periods when the trees began sprouting (mid-February), the new leaves were purple (by late February), turned light green (by early March) and had just matured (by early April). Thereafter, samples were collected every month until the trees began shedding their leaves (mid-November). Trunk and large root samples, together with the branch samples, were harvested in mid-November to demonstrate the distribution of VSPs in the whole trees. Trunk samples were taken at 1 m up from the ground level and the large root samples were taken at a distance of 50 cm from the trunk base from exposed parts of the roots (above ground level). Samples from different trees at each time point were analyzed respectively, and the representative data were given in the results.
Light microscopy
The samples were fixed in 4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) at 4 °C for 24 h. Paraffin blocks were prepared after dehydration through graded series of ethanol. Microtome sections (thickness 10 μm) were stained with mercury-bromophenol blue solution (1% HgCl2, 0.05% bromophenol blue, 2% acetic acid) which is specific for protein staining (Wu and Hao 1986). The antiserum production referred to Tian et al. (2003). The 23 kDa protein was extracted from the bark tissues of branches, purified by preparative SDS-PAGE, and emulsified with Freund's adjuvant (Sigma, St. Louis, MO, USA). This emulsion was used to immunize two New Zealand male rabbits by hypodermic injection. The antiserum was purified by ammonium sulfate precipitation. The indirect immunohistochemical localization referred to Tian and Hao (1999). The secondary antibodies labeled by FITC were purchased from Sino-America Biotechnology Co. (SABC, Shanghai, China). Sections were examined in a Leica DMLN microscope (Leica, Wetzlar, Germany).
Electron microscopy
The samples were fixed in 4% glutaraldehyde in 0.1 M phosphate buffer at pH 7.2 at 4 °C for 24 h and postfixed in 2% OsO4 in the same buffer for 6 h. The samples were then dehydrated in ethanol and embedded in Epon 812 resin (Serva Feinbiochemica Co., Heidelberg, Germany). Ultrathin sections were cut with an LKB-V ultratome (LKB, Bromma, Sweden), stained with 1% uranyl acetate and lead citrate, and examined using a JEM100CX-II electron microscope (Electron Microscope Ltd., Tokyo, Japan).
Soluble protein extraction
Samples (2 g fresh weight) were mixed with 0.5 g polyvinylpolypyrrolidone (PVPP) (Sigma) and ground to a homogenate in 5 mL of extraction buffer (0.1 M Tris base, 0.05 M sodium borate, 0.05 M ascorbic acid, 1%β-mercaptoethanol, 0.1% Triton X-100, 1 mM phenylmethyl sulfonyl fluoride, pH 8.4). Homogenates were centrifuged with a Himac CR 22G high-speed refrigerated centrifuge (Hitachi Ltd., Tokyo, Japan) at 17 500 g for 30 min at 4 °C and the supernatants were collected. Proteins in the supernatants were determined according to the method of Bradford (1976) using bovine serum albumin (BSA) as the standard.
SDS-PAGE and Western-blot
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out by using 14% SDS-polyacrylamide gels (or else, demonstrated in figures), and a running buffer (25 mM Tris base, 192 mM glycine, 0.1% SDS, pH 8.3) at 25 mA/gel. Thirty micrograms of protein were loaded per lane (or else, demonstrated in figures). Gels were stained with 0.125% Coomassie brilliant blue R-250.
Electrophoretic transfer of proteins from SDS-polyacrylamide gels to unmodified nitrocellulose (BBI, Ontario, Canada) was based on Towbin et al. (1979). The other steps referred to Tian et al. (2003). The secondary antibodies labeled by AP (alkaline phosphatase) were purchased from Sino-America Biotechnology Co. (SABC, Shanghai, China).
Assays for proteinase inhibitor activity
The vegetative storage proteins in the protein supernatants were purified by Sephacryl S-100HR (Amersham Pharmacia Biotech, Uppsala, Sweden) and DEAE-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden). Assays for the inhibitory activity of the purified proteins on trypsin were referred to Erlanger et al. (1961). Inhibitory activity was measured against 16.2 μg of trypsin (Sigma, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer (pH 8.0) containing 0.01 M CaCl2. Synthetic substrate (N-α-benzoyl-DL-arginine-p-nitroanilide hydrochloride) for trypsin was purchased from Sigma. Inhibitory activity was expressed as a percentage of the remaining proteinase activity after being incubated with the purified proteins for 30 min to the proteinase activity of control (BSA instead of the purified proteins). One trypsin inhibitor unit (UI) is defined as a decrease of 0.01 absorbance units at 410 nm per 1 min in 1 mL of reaction mixture under the assay conditions (Erlanger et al. 1961).
(Handling editor: Jinxing Lin)




