Tufted Hairgrass (Deschampsia caespitosa) Exhibits a Lower Photosynthetic Plasticity than Antarctic Hairgrass (D. antarctica)
Supported by the Foundation for Polish Science (FNP) for the Domestic Stipend for Young Scholars (“START”, awarded in 2008).
Abstract
The aim of our work was to assess photosynthetic plasticity of two hairgrass species with different ecological origins (a temperate zone species, Deschampsia caespitosa (L.) Beauv. and an Antarctic species, D. antarctica) and to consider how the anticipated climate change may affect vitality of these plants. Measurements of chlorophyll fluorescence showed that the photosystem II (PSII) quantum efficiency of D. caespitosa decreased during 4 d of incubation at 4 °C but it remained stable in D. antarctica. The fluorescence half-rise times were almost always lower in D. caespitosa than in D. antarctica, irrespective of the incubation temperature. These results indicate that the photosynthetic apparatus of D. caespitosa has poorer performance in these conditions. D. caespitosa reached the maximum photosynthesis rate at a higher temperature than D. antarctica although the values obtained at 8 °C were similar in both species. The photosynthetic water-use efficiency (photosynthesis-to-transpiration ratio, P/E) emerges as an important factor demonstrating presence of mechanisms which facilitate functioning of a plant in non-optimal conditions. Comparison of the P/E values, which were higher in D. antarctica than in D. caespitosa at low and medium temperatures, confirms a high degree of adjustability of the photosynthetic apparatus in D. antarctica and unveils the lack of such a feature in D. caespitosa.
Climate change is an important issue that the global society has to face at the beginning of the twenty-first century. Warming of the climate is evident from observations of increases in global average air and ocean temperatures, widespread melting of snow and ice and rising global ocean levels – which might potentially endanger lowland coastal territories (“Climate Change” 2007; Domingues et al. 2008). A 100-year linear warming trend (1906–2005) of 0.74 °C has recently been reported (“Climate Change” 2007). However, despite such a strong evidence of the warming trend, recently publicized in scientific literature (Forest and Reynolds 2008; Rosenzweig et al. 2008; Thompson et al. 2008; Zwiers and Hegerl 2008), it is clear that the global temperature is fluctuating and short periods of temperature decrease normally occur (Przybylak 2002). The polar zone and in particular Antarctica are considered to be the most vulnerable places that could be affected by the forecasted climate change (Wasley et al. 2006).
Recent studies also suggest that the increasing temperature is responsible for significant changes in physical and biological systems around the world (Walther et al. 2002; Massot et al. 2008; Rosenzweig et al. 2008). Antarctic flora and fauna are often regarded as indicators of what happens with the global climate and researchers have associated climate change with biological and ecological changes that are observed in this continent (Convey and Smith 2007). Constant low temperatures and episodes of high light are typical conditions during the growing season in the polar regions (Pérez-Torres et al. 2004). Terrestrial photosynthetic organisms in Antarctica are exposed during the summer to high light operating simultaneously with low temperatures. It is evident that plants living in Antarctica had to evolve comprehensive adaptation mechanisms to cope with the harsh environmental conditions. Among these adaptations are high freezing resistance, high resistance to light stress and high photosynthetic capacity at low temperatures (Alberdi et al. 2002). These adaptations distinguish species growing in Antarctica from the related species from other climatic zones.
Only two vascular plants, Deschampsia antarctica and Colobanthus quitensis, have been able to colonize some of the coastal areas. Exposure to the harsh polar environment makes these plants particularly interesting for the study of adaptation mechanisms. To date, few studies aimed to identify specific physiological adaptations by matching data obtained for species originating from contrasting climatic zones. The present study fills this gap and compares the photosynthetic performance of two hairgrass species: Tufted hairgrass (D. caespitosa) and Antarctic hairgrass (D. antarctica). D. caespitosa is a species related to D. antarctica (Souto et al. 2006) characterized by a wide distribution around the world.
D. antarctica seems to be well adapted to the rapidly changing growth conditions and various abiotic factors, such as high and low radiation, deficient precipitation, drought, flooding, salinity, variable temperatures, frost, snow, ice, etc. (Giełwanowska et al. 2005). It is clear that the species also had to evolve its photosynthetic apparatus, so as to take advantage of the illumination available in the Antarctic environment. D. antarctica is characterized by relatively high photosynthesis rates at low temperatures (Xiong et al. 1999; Xiong et al. 2000; Bystrzejewska 2001).
Unlike Antarctic hairgrass, Tufted hairgrass (D. caespitosa) is characteristic of the temperate zone. It is a common perennial bunchgrass occupying a wide range of habitats: from the coldest to moderately warm. It has a holarctic distribution, extending around the globe, although very rarely to the north of the Arctic Circle (Lawrence 1945). There is a great variability of D. caespitosa and it is believed that its genetic diversity may correspond to differences in ecological conditions throughout its wide geographic distribution (Lawrence 1945).
A basic requirement of all photosynthesizing organisms is a balance between overall energy supply through temperature-independent reactions – and on the other side – energy consumption through the temperature-dependent biochemical reactions of photosynthetic electron transport and contiguous metabolic pathway (Hunner et al. 1996). Due to the inherent sensitivity to variations of redox potentials, chloroplasts, in addition to its role in energy transduction, may also be considered as primary sensors of environmental change. In photosynthesis, reaction centers of two photosystems convert photon energy into redox energy (Heber et al. 2001). Photosystem (PS) II plays a central role – not only in energy transduction – but also in monitoring of the molecular redox mechanisms involved in signal transduction for acclimation to environmental stresses (Anderson et al. 1997). PSII is considered to be one of the most stress-sensitive sites in plants (Öquist and Wass 1988), and as such, damage to PSII will often be the first manifestation of stress in a leaf (Maxwell and Johnson 2000). Therefore chlorophyll fluorescence and photosynthesis are commonly used indicators of plant stress.
By measuring the yield of chlorophyll fluorescence, information about changes in the efficiency of photochemistry and heat dissipation can be gained (Maxwell and Johnson 2000). Fluorescence yield can be quantified by exposing a leaf to light of defined wavelength and measuring the amount of light re-emitted at longer wavelengths (Maxwell and Johnson 2000). The fast fluorescence rise starts from F0 and reaches a maximum value Fm. The variable chlorophyll fluorescence (Fv) is correlated with the availability of quinone molecules (Öquist and Wass 1988). Oxidized quinone will provide a minimal fluorescence, while reduction of quinone will increase fluorescence until all quinone molecules are reduced and the fluorescence reaches its maximum (Fm) (Öquist and Wass 1988).
The Fv/Fm ratio is proportional to the quantum yield of photochemical reactions. Fv/Fm represents the maximum efficiency of PSII photochemistry and is frequently used to test performance of the photosynthetic apparatus (Fu et al. 2007). A change in Fv/Fm is due to a change in the efficiency of non-photochemical quenching (Maxwell and Johnson 2000). A well functioning photosynthetic apparatus has Fv/Fm equaling ∼0.8 (Öquist and Wass 1988; Maxwell and Johnson 2000). Lower values indicate a damage of PSII. The t1/2 parameter is the half-rise time from the minimal fluorescence (F0) to the maximal fluorescence (Fm). It represents the rate of photochemical reaction and the pool size of electron acceptors on the reducing side of PSII, including the plastoquinone pool (Öquist and Wass 1988).
As pointed out, it is especially interesting to compare the Antarctic plant (D. antarctica), with a related temperate zone species (D. caespitosa). A similar approach has successfully been used to study adaptation of photosynthetic CO2 assimilation and carbon recovery in the two distinct environments (Bystrzejewska 2001). D. caespitosa has evolved to grow in a temperate climate; hence, it is not expected to have acquired cold-tolerance mechanisms like those identified in D. antarctica (Giełwanowska et al. 2005; Giełwanowska and Szczuka 2005). Based on this, we hypothesize that D. antarctica has a greater photosynthetic plasticity than D. caespitosa. The second hypothesis of this study is that the photosynthetic water-use efficiency is an important parameter to assess adjustment of the investigated species to a temperature change. Both hypotheses are verified in the present work by carrying out a comparative study of photosynthesis of the Antarctic and the temperate climate hairgrass, D. antarctica and D. caespitosa, respectively. Gas exchange parameters and chlorophyll fluorescence are determined at continuous light, characteristic of Antarctic summer, at temperatures ranging from 4 to 25 °C. We also make an attempt to discuss the potential adjustment and adaptation abilities of both hairgrass species with regard to the forecasted climate change.
Results
Chlorophyll fluorescence measurements
The parameters of chlorophyll a fluorescence, Fv/Fm, were significantly different in D. antarctica than in D. caespitosa at the temperature of 4 °C (Figure 1). During the consecutive 4 d of continuous illumination, Fv/Fm did not change in D. Antarctica, whereas it did decrease in D. caespitosa. The Fv/Fm ratio increased in both plants following incubation in higher temperatures (8 and 13 °C). It was the highest and invariable at 13 °C during all 4 d of continuous illumination without any significant differences between both species. At 21 °C, the Fv/Fm in D. antarctica was initially lower than in D. caespitose, but it increased following 1 d acclimation.
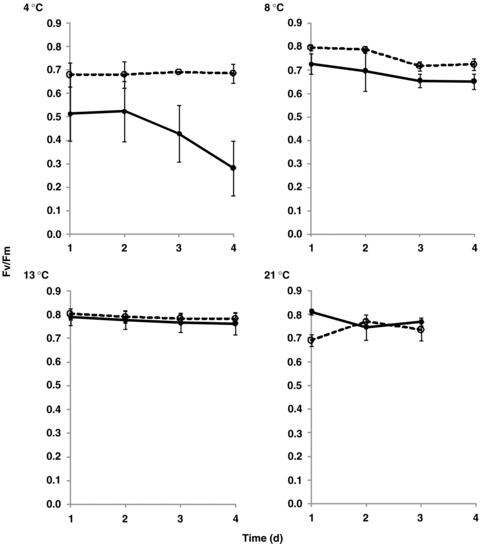
Ratio of variable-to-maximum chlorophyll a fluorescence (Fv/Fm) in relation to the temperature during 4 d of continuous light.Deschampsia antarctica ( ); D. caespitosa (
); D. caespitosa ( ). Photosynthetically active radiation (PAR) ∼200 μmol/m2 per s. The potential efficiency of photosystem II (PSII) was determined in dark-adapted intact plants by means of the ratio of variable-to-maximum chlorophyll a fluorescence emission. The measurements were repeated at least 10 times for each treatment.
). Photosynthetically active radiation (PAR) ∼200 μmol/m2 per s. The potential efficiency of photosystem II (PSII) was determined in dark-adapted intact plants by means of the ratio of variable-to-maximum chlorophyll a fluorescence emission. The measurements were repeated at least 10 times for each treatment.
The fluorescence half-rise times, t1/2, were almost always higher in D. antarctica than in D. caespitosa, irrespective of the incubation temperature (Figure 2). The highest values were observed at the temperature of 13 °C in both plants.
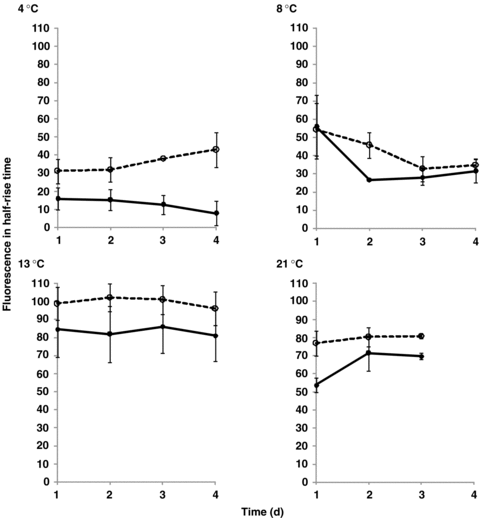
Changes in the fluorescence half-rise time (t1/2) in relation to the temperature during 4 d of continuous light.Deschampsia antarctica ( ); D. caespitosa (
); D. caespitosa ( ). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The half-rise time, t1/2– measured as an interval between the minimal, Fo, and maximal, Fm value. The measurements were repeated at least 10 times for each treatment.
). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The half-rise time, t1/2– measured as an interval between the minimal, Fo, and maximal, Fm value. The measurements were repeated at least 10 times for each treatment.
Gas exchange measurements
The photosynthetic CO2 assimilation rate at 4 °C was much higher in D. antarctica than in D. caespitosa (Figure 3). The photosynthesis rates in both species were similar at 8 °C and reached maximum at 13 °C. A reduction of photosynthesis rate was observed at the highest temperature. The stomatal conductance was significantly higher in D. antarctica than in D. caespitosa in a wide range of temperatures from 4 to 13 °C (Figure 4). At higher temperatures, stomatal conductance went down in D. antarctica. The transpiration rate reached a local maximum of 8 °C in D. antarctica, while in D. caespitosa, it went up with the temperature increasing from 4 to 25 °C (Figure 5). The photosynthetic water-use efficiency (P/E) in D. antarctica was much higher than in D. caespitosa at temperatures of 4 and 13 °C (Figure 6) and it decreased at higher temperatures. The values obtained for the temperature of 8 °C are associated with a high standard deviation, and therefore are not further discussed. At 25 °C, the P/E parameter was equal for both studied hairgrass species.
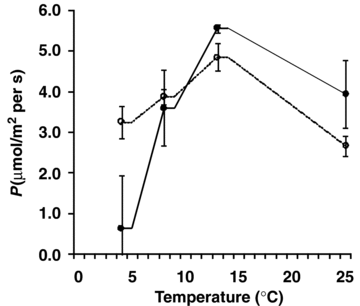
Photosynthetic assimilation of CO2 (P) in relation to temperature.Deschampsia antarctica ( ); D. caespitosa (
); D. caespitosa ( ). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The measurements were carried out four times for each set of conditions.
). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The measurements were carried out four times for each set of conditions.
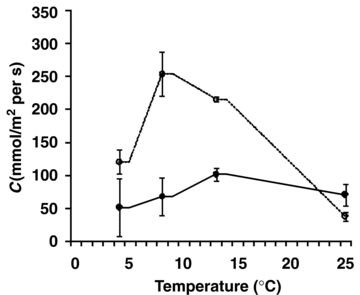
Stomatal conductance (C) in relation to temperature.Deschampsia antarctica ( ); D. caespitosa (
); D. caespitosa ( ). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The measurements were carried out four times for each set of conditions.
). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The measurements were carried out four times for each set of conditions.
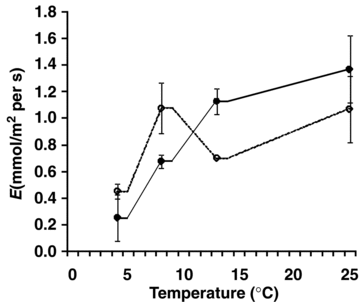
Transpiration (E) in relation to temperature.Deschampsia antarctica ( ); D. caespitosa (
); D. caespitosa ( ). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The measurements were carried out four times for each set of conditions.
). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s. The measurements were carried out four times for each set of conditions.
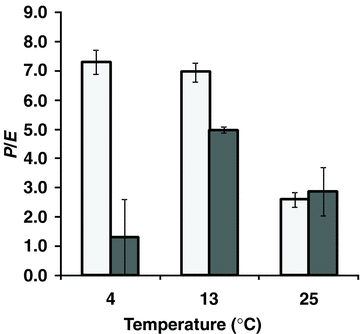
Photosynthetic water-use efficiency (P/E) in relation to temperature.Deschampsia antarctica (□); D. caespitosa (▪). Photosynthetically active radiation (PAR) ∼ 200 μmol/m2 per s.
Discussion
Chlorophyll fluorescence
The chloroplastic redox sensing mechanism is an important component for sensing abiotic stress (Hunner et al. 1996). An early study of chlorophyll a fluorescence of D. antarctica was carried out by Xiong et al. (1999). While Xiong et al. (1999) compared D. antarctica with another Antarctic vascular plant, Colobanthus quitensis; the present study focused on a comparison of photosynthetic performance of D. antarctica and that of another species belonging to the Poaceae family: tufted hairgrass (D. caespitosa). To our knowledge, no comprehensive data on chlorophyll fluorescence and photosynthesis of D. caespitosa have been published to date.
Differential susceptibility of PSII to light stress in light-acclimated D. antarctica and shade-acclimated D. caespitosa plants is evident (Figures 1 and 2). The potential efficiency of PSII was determined in dark-adapted intact plants by means of the ratio of variable-to-maximum chlorophyll a fluorescence emission (Fv/Fm). The results also indicate synergistic interaction of low temperature and continuous light on the level of PSII, which is especially well represented within the Fv/Fm result obtained for D. caespitosa at 4 °C (Figure 1). In leaves of D. antarctica, the Fv/Fm was ∼0.7 at 4 °C and ∼0.8 at 8, 13 and 21 °C, and it did not decrease during the following 4 d of growth under continuous illumination (Figure 1). But the exposure of this chilling-sensitive species, D. caespitosa, to the low temperatures (8, and in particular, 4 °C) resulted in a decline of Fv/Fm, in comparison with the chilling-tolerant plant, D. antarctica: the Fv/Fm ratio went down during the 4 d of growth under continuous illumination at 4 °C (Figure 1). Thus, our data demonstrate that in D. caespitosa, PSII may become either inhibited or damaged at low temperature and continuous illumination. Overall, the continuous illumination at the photosynthetically active radiation (PAR) intensity of ∼200 μmol/m2 per s does not exert a damaging effect on functionality of PSII in D. antarctica at low and temperate temperatures, as opposed to the effect observed in D. caespitosa.
During the first day of the experiment with the temperature of 21 °C, the Fv/Fm ratio measured in D. antarctica was initially lower than in D. caespitosa (∼0.7) but later it recovered the normal value (∼0.8). This shows the plant's ability to adjust to the temperatures far from native and illustrates inherent functional flexibility of the PSII. Although high temperatures are known to induce structural changes in thylakoid membrane, which would also affect PSII and the Fv/Fm ratio (Lazár and Ilik 1997), in other work, temperatures higher than 21 °C were applied and such treatment did not prove to be damaging for the photosynthesis in D. antarctica (Bystrzejewska 2001).
Xiong et al. (1999) observed that the optimal temperature for photochemical quenching and the quantum yield of PSII electron transfer in D. antarctica was 7 °C. Supraoptimal temperatures had little effect on the Fv/Fm ratio, which is also reflected in our results (Figure 1). However, in contrast to the present study, Pérez-Torres et al. (2004) observed a decrease of Fv/Fm ratio in D. antarctica under high light and at low temperature. It was proposed that photochemical quenching and the high level of antioxidants help D. antarctica to resist photoinhibitory conditions (Pérez-Torres et al. 2004). In other work, it has also been reported that the maximum yield of PSII (Fv/Fm) is more sensitive to temperature in D. antarctica than in other species from warmer regions (Salvucci and Crafts-Brandner 2004). Even though, in our study the Fv/Fm remained stable during 3 d of incubation of Antarctic hairgrass at supraoptimal temperature (21°C) (Figure 1). Comparison of these studies, conducted according to diverse experimental designs, shows that the results obtained and the conclusions drawn are different. This observation points to the importance of applying uniform conditions, especially when comparing performance of photosynthetic apparatus in different species, as in the present work.
The effect of increased temperature and continuous illumination in both Antarctic and temperate zone plants was also observed in the chlorophyll fluorescence half-rise time characteristics (Figure 2). The half-rise time, t1/2, confirms ability of the investigated plants to tolerate low temperatures. It is striking that D. antarctica maintains a higher value of t1/2 than D. caespitosa at all temperatures tested. It is also apparent that all of the profiles seem to be flat for the time range tested without any evident extrema (Figure 2). The observation that the t1/2 values were greater for D. antarctica than for D. caespitosa in the tested temperature range (Figure 2) reveals differences in the length of energetic antennae and plastoquinone pools in PSII. According to the t1/2 results (Figure 2), D. antarctica is characterized by the presence of relatively small PSII antennae and a large pool of plastoquinone. On the other hand, the shade-plant, D. caespitosa, is believed to possess a smaller pool of plastoquinone.
Following a prolonged exposition of D. caespitosa to continuous irradiation, the leaves of this grass revealed symptoms of chlorosis (data not shown), which is yet further proof of synergistic action of continuous light and non-optimal temperature. In other work it was observed that the incidence of leaf chlorosis under continuous illumination was strongly dependent on the light quality and quantity, and the temperature regime which interact to exert their effects through changes in the leaf photosynthetic activity and the overall carbon metabolism (Murage et al. 1997).
Chilling can substantially reduce photosynthesis in the plant species adapted to a warm climate (Allen and Ort 2001). Excess photosynthetically active radiation or an elevated absorption of light energy can induce radiative stress. When plants absorb more light than can be used for photosynthesis, the excessive energy can cause photoinhibition and even photooxidation of photosynthetic apparatus. Hence, the symptoms of chlorosis observed in D. caespitosa can be attributed to photoinactivation of PSII followed by photooxidation of chlorophyll molecules. Chlorosis was not observed in D. antarctica and the Fv/Fm remained at a high level during continuous irradiation (Figure 1). This is believed to be due to photoprotection, presence of protective pigments and photorespiration (Bystrzejewska 2001). The relatively high antioxidant capacity of D. antarctica may be crucial for avoiding photooxidation (Pérez-Torres et al. 2004; Pérez-Torres et al. 2007) which would also lead to chlorosis. On the contrary, D. caespitosa is believed to have less efficient mechanisms protecting its photosynthetic apparatus. Extremely intensive or prolonged irradiation may contribute to destruction of thylakoid structure and decomposition of pigments which will also lead to a decrease of the intensity of photosynthesis. It must be noted that the protective pigments play the biggest role in photoprotection at lower temperatures, whereas photooxidation becomes more relevant at relatively high temperatures (Bystrzejewska 2001).
Photosynthesis
The results obtained in the present study indicate that the responses of CO2 assimilation rate and photochemical efficiency (1, 2 and 3) to temperature significantly vary among ecologically different species of Deschampsia. When leaves were exposed to low temperatures, the photosynthesis was found to be lower in D. caespitosa than in D. antarctica. Photosynthetic CO2 assimilation rate (Figure 3) in D. caespitosa, several-fold lower than in D. antarctica at 4 °C, points to the strong reduction of the energy-consuming carbon reduction processes. The profile in Figure 3 is in agreement with previous observations that the optimal leaf temperature for photosynthesis rate in D. antarctica is ∼13 °C (Xiong et al. 1999): it has been noted that lowering of the photosynthesis rate at supraoptimal temperatures appeared to be mainly due to high rates of temperature-enhanced respiration.
High photochemical efficiency of the PSII (Figure 1) requires the existence of sinks for the trapped energy (Bravo et al. 2001). The high rate of photosynthetic CO2 assimilation (Figure 3) assures reception of energy which has been produced during the light phase of photosynthesis. D. antarctica has a very high content of sucrose in leaves (Zúñiga-Feest et al. 2005) which may play a protective role in cold tolerance but also constitute an important energy sink (Morgan-Kiss et al. 2006). The rate of photosynthesis in D. antarctica is regulated by temperature in the step of RuBP carboxylation/oxygenation (Bystrzejewska 2001): increased photorespiration (at supraoptimal temperatures) can protect the photosynthetic apparatus from photoinhibition. Considering the recent report on ultrastructural studies of chloroplasts (Giełwanowska et al. 2005), we believe that this protective mechanism is enhanced by the proximity of peroxisomes (and mitochondria) to the chloroplasts. It is known that the mitochondrial oxidative electron transport and phosphorylation protect chloroplasts against photoinhibition – the beneficial interaction between chloroplasts and mitochondria (Padmasree et al. 2002).
The measurements of stomatal conductance at lower temperatures demonstrate a decrease of stomatal opening in D. caespitosa plants subjected to continuous irradiation (Figure 4). The response of D. antarctica is faster than that of D. caespitosa. After an increase of temperature, it quickly regulates stomatal conductance. At higher temperatures, the stomata become closed, which contributes to a decrease of transpiration (Figure 5). This further affects the photosynthetic water-use efficiency. While the stomata in leaves become closed due to the temperature-related stress, the chloroplasts continue absorbing energy and both ATP and NADPH are synthesized. Excess of the redox power in chloroplasts is the reason for modification of the photosynthetic carbon metabolism, including stimulation of photooxidation (Bystrzejewska 2001): this process can lead to a discharge of chloroplast and transfer of the excess energy to other cell compartments.
Photosynthetic and respiratory responses of plants to experimental warming were also investigated by Zhou et al. (2007): photosynthesis increased significantly in spring, decreased in early autumn, and did not change in summer and late autumn in the four species under warming while respiration of the four species increased significantly until mid-summer and then did not change under warming. Plants from contrasting temperature environments normally show considerable differences in their photosynthetic response to temperature (Kaurin et al. 1983): as observed in the present study (Figure 3), species from the polar zones tend to have lower temperature optima and lower light requirements for photosynthesis saturation than alpine species, a feature attributed to genetic constitution.
Photosynthetic water-use efficiency
Photosynthetic water-use efficiency (P/E) is expressed as a ratio of carbon gain in photosynthesis (P) and water loss in transpiration (E) (Figures 3 and 5) and it is normally well correlated with the water-use efficiency of productivity (Lambers et al. 1998). This parameter, as described by Lambers et al. (1998), is an effect of optimization of photosynthesis and transpiration, and as such is frequently used in the evaluation of osmotic stress in plants (Bystrzejewska-Piotrowska and Urban 2003; Urban and Bystrzejewska-Piotrowska 2003; Bystrzejewska-Piotrowska et al. 2004). A plant's photosynthetic water-use efficiency depends on stomatal conductance and on the difference in vapor pressure in the leaf's intracellular air spaces and that in the air. Because temperature affects the vapor pressure in the leaf, temperature also has an effect on plant water-use efficiency (Lambers et al. 1998). On the other hand, reduced air and leaf temperature reduces the evaporative demand of the plant (Allen and Ort 2001).
P/E ratio in D. antarctica was highest at 4 and 13 °C and reached a local maximum of ∼7 (Figure 6). In D. caespitosa, it reached a maximum at 13 °C although the absolute value was still significantly lower than in D. antarctica. At 25 °C, the P/E did not significantly differ in both species. Although the transpiration pattern was comparable in both plants (Figure 5), the photosynthesis rate in D. antarctica was significantly lower (Figure 3), which affected the photosynthetic water-use efficiency (Figure 6). Despite the closure of stomata in D. antarctica, resulting in a comparable stomatal conductance (Figure 4), the transpiration rate was not limited in both species (Figure 5). The comparable transpiration and photosynthesis rates in D. antarctica and D. caespitosa led to the convergence of the P/E ratio at 25 °C. Building on our results, we suggest that the P/E ratio is a useful parameter for evaluation of the adjustment of plants to grow at different (non-optimal) temperatures.
A high value of photosynthetic water-use efficiency at low and moderate temperatures is indicative of good hydration of the photosynthesizing cells. It is necessary to point out that the leaf anatomy in D. antarctica exhibits features typical of xerophytes (Giełwanowska and Szczuka 2005), hence it is adapted to the environment with poor availability of water. D. caespitosa may lack appropriate mechanisms necessary for efficient transport of water in leaves, which may also lead to desiccation when the availability of water in the environment is lower than normal. The significantly decreased, at lower temperatures, photosynthetic water-use efficiency in D. caespitosa (Figure 6) may be succeeded by further (secondary) disturbance of the carbon reduction and other processes.
Climate change and its possible impact on hairgrass populations
Climate change is capable of modifying the geographic distributions of species (Fitzpatrick et al. 2008). For example, Niu et al. (2008), investigating vegetation of a temperate steppe of northern China, concluded that the climate warming may be responsible for changes in plant photosynthesis and its temperature dependence. In fact, photosynthesis has already been proposed as a temperature indicator in Quercus ilex (Gratani et al. 2000). Chilling sensitivity of photosynthesis plays a critical role in limiting the geographical range of plant species (Zúñiga-Feest et al. 2005).
Although numerous studies report the warming trend (“Climate Change” 2007; Forest and Reynolds 2008; Rosenzweig et al. 2008; Thompson et al. 2008; Zwiers and Hegerl 2008), it is not certain whether the climate will warm up or cool down in the short-term (i.e. during the forthcoming years). The mean annual values of ground temperature near the Arctowski Station in Antarctica ranged in 1996 from 0.4 to 0.7 °C (Kejna and Láska 1999) and the mean annual air temperature on King George Island, over the period 1944–1996, was −2.8 °C (Kejna 1999). During this period, the air temperature increased by 1.5 °C (i.e. 0.28 °C/10 years) (Kejna 1999). However, some stations in Antarctica report a cooling trend (Daly 2000).
A possible temperature decrease, for example due to a transient fluctuation in the overall temperature dynamics, could affect photosynthesis of the plants with high photosynthetic temperature optima. According to the above-presented results, D. caespitosa, could be one of them (Figure 3). On the other hand, D. antarctica, can reach a high photosynthesis efficiency in a wide temperature range. Therefore, the photosynthesis rate in this species is not supposed to be influenced by a minor decrease of temperature. Nonetheless, the vegetation period could be altered and the overall biomass production could be decreased.
A possible increase of temperature could potentially exert much greater effect on the growth of both studied Deschampsia plants. According to the previous (Bystrzejewska 2001) and the present (1, 3 and 6) results, both species can cope with the increasing temperature. However, an increase of temperature could also lead to structural changes in the native habitats. For example, Edwards (1972) observed that D. antarctica is increasing in abundance and suggested that it is colonizing ground as it becomes deglaciated: the seedlings establish slowly and the growth rates are relatively low. In the case of D. caespitosa, changes in its biotic interactions could become more relevant. Hence, it is more likely that the impact of climate change on hairgrass populations will be secondary rather than primary.
Phenotypic plasticity can greatly enhance capacities of plants, including those growing in subpolar zones, to colonize new territories (Frenot et al. 1999). However, due to relatively high uniformity of the habitat conditions in the Maritime Antarctica, and the distribution limited to the polar region, the D. antarctica species is not supposed to be rich in ecotypes. In fact, long-distance gene flow is very limited in this species, which causes a very low diversity in the sub-Antarctic islands (van de Wouw et al. 2008). It has been noted that the populations of D. antarctica have experienced a recent evolutionary bottleneck (van de Wouw et al. 2008). A possible divergence of species into ecotypes could facilitate colonization of new areas in the changing environmental conditions. Further ecophysiological studies on hairgrasses are crucial to learn more about their capacities to adjust to the changing environment.
The survival of D. caespitosa in any one of the environments it occupies depends on its physiological fitness to that environment, and this fitness is part of its inheritance (Lawrence 1945): the individual and racial reactions in uniform environments as well as the plasticity of individuals and races are thought to be hereditary. Steiner et al. (2001) investigated interspecific interference between D. caespitosa and Festuca arundinaceae and concluded that the latter species had greater PSII efficiency and glutathione reductase stability under elevated temperatures. This explains why F. arundinaceae has been able to dominate wetland landscapes of the temperate Pacific Northwest (Steiner et al. 2001).
Based on the present results, one may conclude that D. antarctica has a greater photosynthetic plasticity than D. caespitosa. Since D. antarctica has also the potential to colonize areas with different pH, salinity and nitrogen levels, and is tolerant to different nitrogen forms, it also has a greater potential than D. caespitosa to colonize unfavorable habitats. On the other hand, its interaction with other organisms, which do not occur in Antarctica, are not known. One possible limitation for colonization of new areas by D. antarctica can be the ecological barrier. D. antarctica grows in the zone with literally no competition. It is able to cope with abiotic stresses, the feature characteristic of extremophiles, but may not necessarily be able to handle biotic interactions.
Although the previous work (Bystrzejewska 2001) discussed the role of photorespiration as a protection against photoinhibition of the photosynthetic apparatus in D. antarctica and D. caespitosa, herein we compared the effects of continuous irradiation and increased temperature on PSII as well as photosynthesis and photosynthetic water-use efficiency in both species. D. caespitosa exhibits a lower photosynthetic plasticity than D. antarctica in the broad temperature range of 4–25 °C. This conclusion is supported by a higher PSII efficiency, fluorescence half-rise time and photosynthesis rate observed in D. antarctica at lower temperatures. D. antarctica reaches higher photosynthesis rates, equaling those of D. caespitosa recorded at higher temperatures. Due to a greater photosynthetic plasticity and robustness of its photosynthetic apparatus, D. antarctica easily adjusts to temperature fluctuations. It is also able to adjust to relatively high growth temperatures. Such adjustments are attributed to the process of photorespiration (Bystrzejewska 2001). One of the reasons of this observation may be the presence of mechanisms protecting the photosynthetic apparatus from photoinhibition, especially when low temperature and continuous irradiation act synergistically to induce a response of the plant. Such interpretation points to the inherent robustness of the photosynthetic apparatus in D. antarctica.
Comparison of the photosynthetic water-use efficiency reveals a distinct life strategy of the investigated species. The P/E ratio in D. antarctica was the highest at lower temperatures, while in D. caespitosa, it reached a maximum at 13 °C, although its absolute value was still significantly lower than in D. antarctica. At a higher temperature (25 °C), P/E ratios were similar in both species. Therefore, it is apparent that the P/E ratio is an important parameter demonstrating the existence of appropriate mechanisms, which enable functioning of the investigated plant species in a wide temperature range.
Materials and Methods
Plant collection and culture
The Deschampsia antarctica Desv. plants were collected in the Admiralty Bay region (King George Island, Antarctica; λ= 58° 28.10' W, ϕ= 62° 9.41′S) near Arctowski Station (Polish Academy of Sciences). The station is situated on the West shore of Admiralty Bay (Rakusa-Suszczewski 2003). The climatic conditions in this site have been described elsewhere (Zwolska 2001). Subsequently, the plants were transplanted into 10 pots (0.4 m2) with native soil, transported to Poland and grown in plant growth chambers with controlled temperature and photoperiod. The Deschampsia caespitosa (L.) Beauv. plants were collected in the Las Bielański forest (Bielany, Warsaw; λ= 21° 0′E, ϕ= 52° 15′N). Before the experiments the plants were cultured in a 12:12 h light: dark (LD) cycle. During the experiments, the plants were illuminated continuously. The PAR intensity was set to ∼200 μmol/m2 per s. The temperatures ranged from 4 to 24 °C (see figure labels). The relative humidity was ∼40%.
Gas exchange measurements
The net photosynthesis rate (P), transpiration (E) and stomatal conductance (C) were measured using leaves of intact plants as described by Bystrzejewska (2001). Laboratory measurements of gas exchange rates in the light were made using a closed gas-exchange system (CO2 analyzer with two channels, CI-301 PS; CID, Camas, WA, USA). Such parameters as: temperature inside the leaf probe (6.25 cm2), outside the leaf chamber and PAR were controlled by sensors. The measurements were carried out inside plant growth chambers. The temperature measured inside the chamber differed by less than 1 °C from the ambient temperature. The ambient humidity was kept between 30 and 50%. Illumination was provided by a halogen lamp with neutral density filters and water filter. The measurements were normally carried out four times for each set of conditions and the mean values were calculated.
Chlorophyll fluorescence measurements
A detailed description of the method can be found in Öquist and Wass (1988), Lichtenthaler and Miehé (1997) as well as Maxwell and Johnson (2000). The plants were divided into four groups and grown in different temperature regimes: 4, 8, 13 and 21 °C. 10 d after acclimation to the temperature conditions, plants were further grown for 4 d at continuous light. Leaves adapted to laboratory conditions were placed in darkness for 30 min to ensure that all energy-dependent quenchings had relaxed (Krause and Weis 1984; Poskuta et al. 1998). Measurements of chlorophyll a fluorescence ratios, Fv/Fm, and fluorescence half-rise time (for transition from F0 to Fm), t1/2, were carried out using a time-resolved fluorescence apparatus (Plant Stress Meter; Biomonitor S.C.I. AB, Umea, Sweden). The measurements were repeated at least 10 times for each treatment.
(Handling editor: Chengcai Zhang)
Acknowledgements
We thank Professor Stanisław Rakusa-Suszczewski (Polish Academy of Sciences) for the generous gift of the Antarctic hairgrass and for his assistance with plant cultivation.




