Development of a Low-cost Polymerase Chain Reaction-based Method for Studying Differentially Expressed Genes in Developing Rice Leaves
Supported by the Area of Excellence Grant on Plant and Fungal Biotechnology from the University Grants Committee of the Hong Kong SAR Government.
Abstract
Gene expression studies are important for revealing gene functions putatively involved in biological processes. We were interested in identifying differentially expressed genes during leaf development in rice. We combined the RNA arbitrarily primed-polymerase chain reaction (RAP-PCR) and dot blot hybridization methods to screen a rice leaf primordium cDNA library. Three developmental stages during vegetative growth were examined. The cDNA clones showing different hybridization patterns were further analyzed and verified. Here we demonstrate that the combination of RAP-PCR and dot blot hybridization could provide an efficient and relatively low-cost cDNA library screening approach to discover genes not previously known to be associated with leaf development in rice. We believe that the findings described here will help to elucidate the molecular mechanism(s) underlying the developmental processes of rice leaf.
Gene expression studies play an important role in revealing gene functions that are putatively involved in various biological processes, such as cell differentiation and development. Of particular interest is the identification of differentially expressed genes (DEGs), which are responsible for programming molecular events that are necessary for many cellular activities to occur. Methods of searching for DEGs include differential screening of cDNA libraries (St John and Davis 1979) and subtractive hybridization (Sargent and Dawid 1983). Although both techniques have been applied successfully, these screening methods have certain drawbacks, being labor intensive, time consuming, and requiring a large amount of mRNA to produce representative cDNA libraries. Polymerase chain reaction (PCR)-based methods, such as RNA arbitrarily primed PCR (RAP-PCR, Welsh et al. 1992) and differential display (Liang and Pardee 1992), have gained popularity for studying differential gene expression recently because they require smaller amounts of starting materials.
RNA arbitrarily primed-PCR and differential display (DD) share similarities in generating semi-quantitative RNA fingerprints to allow simultaneous analysis of multiple samples. These two methods have been applied in the identification of genes differentially expressed in normal and tumor cells in mammary epithelium (Liang et al. 1992) and ovarian epithelium (Mok et al. 1994), differentially regulated genes restricted to rheumatoid arthritis synovial fibroblasts (Neumann et al. 2002), and sex-specific genes from the parasite Oesophagostomum dentatum (Boag et al. 2000). These methods have also been used to search for DEGs in two common human parasites, Trypanosoma (Murphy and Pellé 1994) and Plasmodium falciparum (Thélu et al. 1994).
RNA fingerprinting by RAP-PCR depends on the use of an arbitrary primer (or a primer pair) to generate cDNA sequences that are subsequently amplified by low-stringency PCR using the same chosen primer (or primer pair). Unlike the requirement of oligo-dT primer by DD during first-strand cDNA synthesis, RAP-PCR can be used on RNAs without polyadenylated tail, such as the RNAs found in bacteria. RAP-PCR provides a simple and fast method to analyze differential gene expression. However, subsequent verification of the screening result is necessary, as RAP-PCR tends to generate a high incidence of false positive results (McClelland et al. 1995).
We were interested in identifying DEGs during various stages of leaf development and growth in rice. Without prior knowledge of any candidate gene in leaf development, either microarray or cDNA library screening approach seemed to be obvious for our objective. Despite its ability to screen genes on a larger scale, the microarray approach suffers from high costs associated with commercially available microarrays and specialized equipment. Furthermore, the genes of interest found by microarray still need to be cloned for further study, a process that can be both tedious and labor-intensive. As opposed to the microarray approach, the cDNA library screening makes cloned genes of interest immediately available, but suitable genes for making labeled probes will be required. We therefore resorted to generating probes by RAP-PCR to screen a cDNA library with a “dot blot hybridization” method (Kafatos et al. 1979). Clones from a cDNA library were randomly picked and cDNA inserts amplified by PCR were dot blotted onto membranes in replicates. Probes generated from RAP-PCR amplicons representing various developmental stages were then hybridized on these dot blots to search for DEGs. Here we demonstrate that the combination of RAP-PCR and dot blot hybridization can provide an efficient and low-cost cDNA library screening approach to discover genes not previously known to be associated with leaf development in rice.
Results
Detection of differentially expressed genes
We made use of a combinatorial approach of RAP-PCR and dot blot hybridization to screen a rice leaf primordium cDNA library (Figure 1). RNA samples from three developmental stages of the third leaf (primordium, half expanded, and fully expanded) during vegetative growth were examined by means of RAP-PCR. Amplicons displaying variations of band intensity or pattern were used as screening probes on the cDNA library to subsequently search for differentially expressed genes during leaf development. The third leaf was chosen for its vegetative status before tiller formation on the fifth leaf. Also, primordium, half and fully expanded leaves could be prominently distinguished for sample collection purposes. In general, amplicons from RAP-PCR ranged from 200 to 1 200 bp. Band patterns as resolved on 3% agarose gel, although at first sight similar, revealed some fragments representing differentially expressed genes. Figure 2A illustrates a representative gel image produced from RAP-PCR with the CK52U primer.
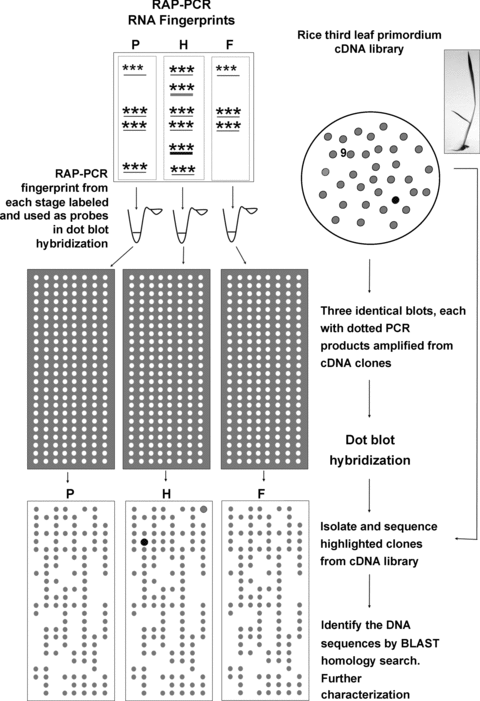
A schematic showing the workflow of RNA arbitrarily primed-polymerase chain reaction (RAP-PCR)/dot blot hybridization.RAP-PCR fingerprints for three developmental stages (F, fully expanded leaf; H, half expanded leaf; P, primordium) were labeled to use as probes to screen a third leaf primordium cDNA library. Amplicons from cDNA clones were dot blotted onto three identical sets of membranes. Subsequent dot blot hybridization revealed two genes (dot-shaded highlights in the figure) differentially expressed in H. Retrieval of the highlighted clones from the cDNA library allowed further characterization of these two genes. Inset shows the appearance of a rice plant with third leaf primordium still embedded within the second leaf.
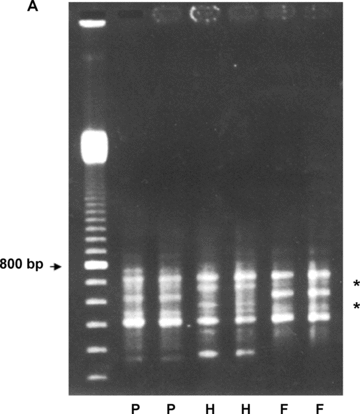
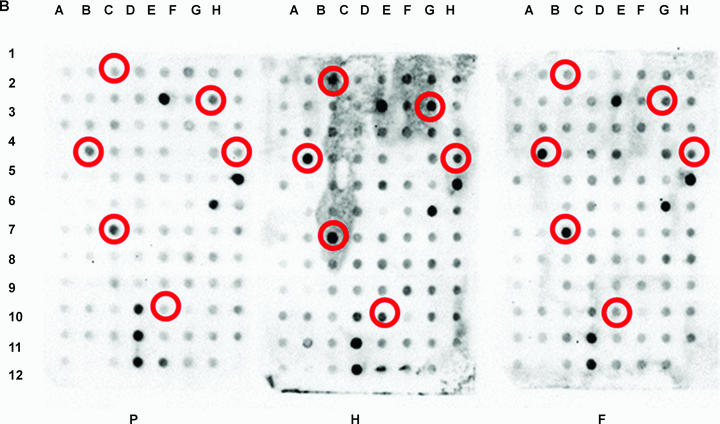
RNA arbitrarily primed-polymerase chain reaction (RAP-PCR) dot-blot hybridization result.(A) A representative RNA fingerprint as resolved by 3% agarose gel electrophoresis. RNAs from primordium (P), half (H) and fully (F) expanded third leaf of Pei'ai 64S were analyzed by RAP-PCR using CK52U primer. Areas showing different banding pattern are denoted by *. Size markers are a 100-bp DNA ladder.(B) A typical dot-blot hybridization result. Dot-blotted PCR amplicons from randomly picked cDNA clones were screened with RAP-PCR probes representing the three developmental stages. Detection was accomplished by chemiluminescence. Circles indicate the clones exhibiting different hybridization patterns. Position 12H on each membrane refers to negative control, where water was dotted.
In total, 2 500 randomly picked cDNA clones were screened, 205 of which showed different hybridization patterns among the studied developmental stages (Figure 2B). The 205 cDNA clones were readily retrieved and subjected to DNA sequencing for gene identification. After conducting BLAST searches, the number of usable clones was substantially reduced by the occurrence of a highly redundant gene (RUBISCO small subunit). A list of unique genes putatively showing differential expression in the developing third leaf is provided in Table 1. Approximately 18% of the sequenced clones (36 clones) represented genes that did not match significantly any known gene. Although other methods, such as serial analysis of gene expression (SAGE) (Virlon et al. 1999), are effective in providing sequence information to expedite gene identification, downstream procedures to clone the full-length cDNAs can be costly and labor intensive. The immediate availability of cDNA clones for further characterization is therefore a key advantage offered by this combinatorial approach.
| Gene ID | Species | P value | Stage expressed |
|---|---|---|---|
| 14-3-3 like protein (7271252) | Oryza sativa | 0.0 | P |
| Histone H4 (70774) | Triticum aestivum | 5 × 10−20 | P |
| Chaperonin 10-like protein (7443827) | O. sativa | 3 × 10−39 | P |
| Hemoglobin 2 (14701799) | O. sativa | 1 × 10−110 | H, F |
| Actin depolymerizing factor (12957717) | O. sativa | 3 × 10−11 | H [H, F] |
| Chlorophyll a/b binding protein (3126854) | O. sativa | 8 × 10−46 | H [P, H] |
| Ferredoxin-NADP reductase (7433381) | O. sativa | 1 × 10−48 | H |
| Geranylgeranyl reductase (3821254) | Nicotiana tabacum | 0.0 | H |
| RUBISCO small subunit (132105) | O. sativa | 4 × 10−13 | H [H, F] |
| Heme oxygenase (14485571) | O. sativa | 3 × 10−16 | F |
| Polyubiquitin 1 (1574943) | O. sativa | 3 × 10−9 | P, H |
| NADH dehydrogenase ND3 | O. sativa | 3 × 10−52 | P, H [P, H, F] |
- F, fully expanded leaf; H, half expanded leaf; P, primoridum. Dot-blot and northern blotting suggested the same stage, except as noted by square brackets for northern blotting results. Numbers in round parentheses after gene IDs are Gi numbers.
Verification of putative development-related genes
Northern blot analysis was carried out to verify the differential expression patterns revealed by dot blot hybridization. Although a relatively old technique (first reported in Alwine et al. 1977), northern blotting is probably the only technique that allows size determination of an RNA of interest simultaneously with the detection of this RNA on the blot. Northern blotting results for some of the genes listed in Table 1 are shown in Figure 3. Northern blotting results for some genes were not satisfactory, either because there were insufficient amounts of transcripts present on the RNA blots or because the extraneous bands produced by some labeled probes made interpretation difficult.
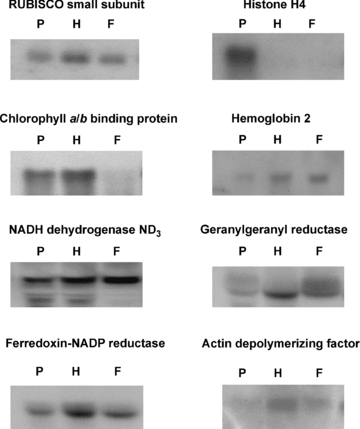
Northern blot analysis of eight genes showing differential pattern in dot-blot hybridization.Ten to fifteen micrograms of RNA were analyzed for each gene. 17S and 25S rRNA bands were used as loading control and did not differ significantly among the samples representing the three developmental stages (data not shown). F, fully expanded leaf; H, half expanded leaf; P, primordium.
Figure 3 indicates the differential expression patterns of eight genes verified by northern blot analysis. Four genes conformed to the pattern as revealed by dot blot hybridization; histone H4 was expressed more highly in primordium than the other two stages, while ferredoxin-nicotinamide adenine dinucleotide phosphate (NADP) reductase and geranylgeranyl reductase both were upregulated in half expanded leaves. Hemoglobin could be found more highly expressed in half and fully expanded leaves. Northern blotting in fact provided more information on the expression level than the dot blot approach. Actin depolymerizing factor, chlorophyll a/b binding protein and RUBISCO small subunit were shown to be differentially expressed in half expanded leaves by dot blot hybridization, but northern blotting results indicated that these three genes could also be found in fully expanded leaf. NADH dehydrogenase ND3 was expressed throughout the three developmental stages, but dot blot hybridization indicated expression only in primordium and half expanded leaves. The incomplete differential patterns revealed by dot blot hybridization as opposed to northern blot analysis demonstrates the importance of verifying the differential expression pattern provided by a crude screening technique.
Discussion
Inference for rice leaf development
A combinatorial approach using RAP-PCR and dot blot hybridization has allowed screening of genes that are differentially expressed during development of the third leaf in rice. Of the 2 500 cDNA library clones screened, gene expression pattern of eight genes has been further investigated using northern blot analysis. Most of these eight genes obtained by screening a leaf primordium cDNA library are differentially expressed in half and fully expanded leaves. Studies of genes involved in the development of rice leaves have been scarce, with a few papers reporting leaf initiation (Sato et al. 1996) and leaf senescence (Lee et al. 2001). Leaf elongation is crucial to physiological processes (e.g. photosynthesis), but genes related to leaf elongation have rarely been described. As the grain yield of rice plants mainly depends on photosynthetic contribution by the leaves, the findings reported here will provide new insights into the genetic mechanism(s) underlying the developmental processes of rice leaves.
During initiation of primordium, it is plausible to see increased activities in cell division and enlargement. Higher expression of genes for signal transduction and chromosome biosynthesis will therefore be required to meet the demand for this growth spurt. Histone H4 and 14-3-3 like protein are differentially expressed in primordium, with histone H4 interacting with DNA to regulate replication and transcription (Martinson et al. 1979). Signaling molecules such as 14-3-3 like protein have been shown to inhibit protein kinase C activity in vitro, suggesting a role for this type of protein in plant signal transduction (Marra et al. 1994).
As the leaf starts to expand (or elongate) after primordium formation, oxygen requirement increases and efficient transport of oxygen to the site of development is vital to cells undergoing active processes to build up cellular components. Hemoglobin plays a pivotal role in binding oxygen and other gaseous ligands during development. Hemoglobin, as a heme-containing protein, can be found in both dicot and monocot plants (Lira-Ruan et al. 2001), and rice (Arredondo-Peter et al. 1997). Hemoglobin 2 is differentially expressed in expanding leaves, suggesting increased metabolic activity and hence oxygen demand during elongation of the leaf. As photosynthesis commences in a new green leaf, genes related to photosynthesis (eg. RUBISCO small subunit) are also differentially expressed. Chlorophyll a/b binding protein can act as an antenna pigment in the photosystem to capture light effectively, whereas ferredoxin-NADP reductase catalyzes the final step in the photosynthetic electron transport chain (Knaff and Hirasawa 1991). Expression of genes associated with biosynthesis of photoreceptors is higher in fully expanded leaves. Heme oxygenase I, for example, is involved in the biosynthesis of a group of phytochromes, plant red/far-red light photoreceptors. This phytochrome group is important in mediating chloroplast differentiation (Quail et al. 1995) and photoperiodic regulation of flowering in rice (Takeshi et al. 2000). Establishment of a proper cytoskeletal structure is also important to cells undergoing division and growth, therefore actin related genes (such as actin depolymerizing factor) are sensibly found to show higher expression in elongating leaves.
From the description of genes associated with leaf development, it can be generally concluded that genes related to chromosome biosynthesis and signal transduction are more prominent during early stages of leaf development, whereas genes for photosynthesis and photo-pigments likely predominate during leaf expansions. This demonstrates concerted expression of genes to govern an orderly change of a new leaf from primordium to a fully expanded form. Development is a complicated process involving the expression of many genes that await discovery. This study, aimed at elucidating the differential expression of development-related genes, makes an important initial contribution to a better understanding of the fundamental events involved in the development of leaves. Further work can include the investigation of proteins, especially the ones encoded by genes found to exhibit differential expression in this report.
In summary, we report an efficient approach combining the strength of RAP-PCR and dot blot hybridization to search for genes putatively involved in the development of rice leaves. This approach has two main advantages. It is relatively low-cost, and it makes immediately available cDNA clones for verification and further characterization work. We believe that the findings described here will help to elucidate the molecular mechanism(s) underlying the developmental processes of rice leaves.
Materials and Methods
Strain and growth conditions
Rice strain, Pei'ai 64S (PS), was used in this study and all seeds were supplied by Hunan Hybrid Rice Research Center (Changsha, China). Seeds were allowed to germinate on pieces of moist tissue at 37 °C inside a totally dark chamber for 48 h. Seedlings were then planted in homogenized soil and grown outdoors under ambient conditions in Hong Kong in springtime (temperature 26–33 °C, relative humidity ∼80%). Samples of the third leaf at various stages (primordium, half expanded, and fully expanded) were excised from the plants and immediately frozen in liquid nitrogen. These samples were then kept at −80 °C until required for further experimentation.
Preparation of RNA samples
Samples of total RNA from the excised leaf were extracted using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) according to the manufacturer's instructions. Pulverized leaf samples (0.1–0.5 g) were placed into the Tri-Regent. A high salt solution (0.8 M sodium citrate/1.2 M sodium chloride) was used to facilitate the removal of polysaccharide present in the samples. All RNA samples were eventually dissolved in diethylpyrocarbonate (DEPC)-treated water. Quantification of RNA samples was achieved spectrophotometrically and RNA integrity was assessed with formaldehyde-agarose gel electrophoresis.
RAP-PCR
RNA arbitrarily primed-PCR was carried out in accordance with the protocols described by Welsh et al. (1992) and Szeto et al. (2007) using the RNA samples as described above. Briefly, the RNA samples were treated with DNase I (Invitrogen, Carlsbad, CA, USA) to remove traces of genomic DNA, in accordance with the manufacturer's instructions. First-strand cDNA was generated using a SuperScript cDNA Synthesis System (Invitrogen) with an arbitrary primer for each cDNA reaction (primers used: CK52U, 5′-CGGGTTGTGAGCAGAAGT-3′; CK64U, 5′-GAGCCGCATCAATCACAT-3′; CK73U, 5′-TCGTCCTTCTTCATAGCA-3′; CK74L, 5′-AGAAAGGCAATGATGAGG-3′). The resultant cDNA was subsequently placed in a reaction mixture containing 1× PCR buffer (Promega, Madison, WI, USA), 2.5 mM MgCl2, 0.1 mM dNTP, 0.5 μM primer (the same as for cDNA synthesis), and 2.5 U Taq DNA polymerase (Promega). PCR was carried out using thermal cycling parameters as follows: one low stringency cycle (94 °C for 5 min, 32 °C for 5 min, 72 °C for 5 min), then 39 high stringency cycles (94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min). RAP-PCR products were resolved on standard ethidium bromide 3% agarose gel. RAP-PCR products from the three developmental stages were subsequently subjected to a PCR purification kit (Qiagen, Hilden, Germany) for probe labeling with the digoxigenin (DIG)-High Prime kit (Roche Applied Science, Basel, Switzerland).
Construction and manipulation of cDNA Library
A cDNA library representing the third leaf primordium was constructed using the Smart cDNA Library Construction Kit (Clontech, Mountain View, CA, USA), in accordance with the manufacturer's instructions. Briefly, 2–3 μg total RNA was used in the production of first-strand cDNA, which was further amplified by 23 cycles of long-distance (LD) PCR. The resultant double-stranded DNAs were cloned into the kit-provided λTripEx2 vector, which was subsequently packaged using Gigapack III Gold Packaging Extract (Stratagene, La Jolla, CA, USA). The primary cDNA library was amplified once and the titer was determined in accordance with the manufacturer's instructions. The λTripEx2 recombinant clones were converted to pTripEx2 recombinant plasmid clones using the protocol recommended by the library kit manufacturer. Single colonies were randomly picked to prepare bacterial lysate. Each centrifuge-cleared lysate (1 μL) was added to a PCR reaction containing 1× PCR buffer (Promega), 2 mM MgCl2, 0.1 mM dNTP mix, 0.2 μM T3 (5′-AATTAACCCTCACTAAAGGG-3′) and T7 (5′-TAATACGACTCACTATAGGG-3′) primers, and 2.5 U Taq DNA polymerase (Promega). PCR was carried out to obtain the cDNA inserts with conditions as follows: 94 °C, 6 min; 29 cycles of 94 °C, 50 s; 60 °C, 50 s; 72 °C, 50 s; and final extension at 72 °C for 10 min.
Dot blot hybridization
Polymerase chain reaction-amplified inserts (10 μL) were denatured in 0.2 M NaOH for 30 min at room temperature. The denatured products were applied using a dot blot apparatus (Bio-Dot Microfiltration Apparatus, Bio-Rad, Hercules, CA, USA) onto a nylon membrane (Hybond N, GE Healthcare, Piscataway, NJ, USA). A total of 2 500 clones were dot blotted to three sets of identical blots. Membranes were prehybridized in a solution composed of 5× standard saline citrate (SSC), 1× blocking solution (Roche Applied Science), and 0.1%N-lauroylsarcosine at 68 °C for 2 h. Afterwards each blot set was hybridized with the same solution containing a labeled RAP-PCR probe representing each developmental stage at 68 °C for 16–20 h. Membranes were washed sequentially in SSC/sodium dodecyl sulfate (SDS) solutions and a Tween 20 wash buffer before signal detection with the DIG Luminescent Detection Kit (Roche Applied Science), in accordance with the manufacturer's protocol.
DNA sequence determination and sequence analysis
Plasmid DNA samples representing clones showing differential expression as revealed by dot blot hybridization were prepared using a Wizard Plus SV Minipreps DNA Purification System (Promega). DNA sequences were determined using Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) on the Prism 3100 Genetic Analyzer (Applied Biosystems) following the manufacturer's procedures. Comparison of obtained DNA sequences with a public database was accomplished by the BLAST algorithm available at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi).
Northern blot analysis
Confirmation of putative genes expressed differentially during leaf development was done using Northern blot analysis. Briefly, total RNA samples (10–15 μg) prepared from primordium, half expanded, and fully expanded leaves were resolved on 1% formaldehyde agarose gel and transferred to a nylon membrane (Roche Applied Science). Each membrane was subsequently hybridized with a probe (labeled by the PCR DIG Probe Synthesis Kit) representing each differentially expressed gene in a solution consisting of 50% formamide, 5× SSC, 2% blocking solution, 50 mM sodium phosphate, 0.1% N-lauroysarcosine and 7% SDS at 42 °C for 16 h. Membranes were washed sequentially in SSC/SDS solutions and a Tween 20 wash buffer before signal detection with the DIG luminescent detection kit (Roche Applied Science) in accordance with the manufacturer's protocol.
(Handling editor: Yongbiao Xue)
Acknowledgements
We thank Victor Natareno and David Wilmshurst for editing the manuscript.




