Ultrastructure of the Mature Egg and Fertilization in the Fern Ceratopteris thalictroides
Supported by the National Natural Science Foundation of China (30670128).
Abstract
The ultrastructure of the mature egg and fertilization in the fern Ceratopteris thalictroides (L.) Brongn. were observed by transmission electron microscopy. The results revealed that the mature egg possesses an obvious egg membrane at the periphery of the egg. Furthermore, a fertilization pore was identified in the upper egg membrane of the mature egg. The structure of the pore is described for the first time. The fertilization experiment indicated that spermatozoids crowd into the cavity above the egg through the neck canal of the archegonium; however, only one of these can penetrate into the egg through the fertilization pore. Immediately on penetration of the spermatozoid, the egg begins to shrink. The volume of the fertilized egg decreases to almost one-half that of the unfertilized egg. As a result, the protoplasm of the fertilized egg becomes dense and opaque, which may lead to a situation where the organelles of both the egg and the fertilizing spermatozoid become indistinguishable. Simultaneously, abundant vesicles containing concentric membranes or opaque materials appear near the fertilization pore in the cytoplasm of the fertilized egg. These vesicles are considered to act as a barrier that prevents polyspermy. The present study provides a new insight into the ultrastructure of the mature egg and the cytological mechanism of fertilization in ferns.
The processes of fertilization, which are usually considered to include the approach of sperm to the egg, the fusion of gametes, and the prevention of polyspermy, are well described in animals, seed plants, and some algae (Friedmann et al. 1968; Braten 1971; Callow et al. 1978; Lord and Russell 2002; Primakoff and Myles 2002). However, the details of these processes are little known in ferns, which are an important group in the plant kingdom. Myles (1978) has described the fine structure of fertilization in the heterosporous fern Marsilea vestita; however, the reproductive biology of this species differs greatly from that of most other ferns (homosporous ferns). In the homosporous fern Pteridium aquilinum, certain fertilization phenomena, including the formation of a fertilization cavity, nuclear fusion, and the degeneration of spermatozoid organelles, have been described (Duckett and Bell 1972; Bell 1975). However, in a later review paper, Bell and Duckett (1976) indicated that the fertilization cavity they described may in fact have been an artifact. The fertilization of another homosporous fern, Athyrium filix-femina, was described from both living material and ultrastructural observations (Fasciati et al. 1994a,b). It is considered that the egg of this species possesses a receptive spot at which the spermatozoids can dock (Fasciati et al. 1994a). One or several spermatozoids can penetrate through a pore in the venter coat that covers the top of the egg and enter the fertilization vesicle that sequesters the spermatozoids from the egg (Fasciati et al. 1994b). However, the structure of the receptive area is still unclear. Although some fertilization events have been described in the fern C. richardii (Lopez-Smith and Renzaglia, 2008), no mating structure is discovered in the mature egg. In general, certain critical events in the process of fertilization, including egg penetration and the prevention of polyspermy, are still unclear. In the present investigation, the detailed ultrastructures of the mature egg, the egg penetration event, and certain cytological changes in the gametes occurring during fertilization in the fern C. thalictroides (L.) Brongn. were investigated by transmission electron microscopy (TEM). A mating structure, which we describe as the fertilization pore, was discovered in the mature egg. The role of this pore in the fertilization process and the mechanism of fertilization in C. thalictroides are described.
Results
The results reported in this study are based on the examination of over 10 archegonia used to investigate the ultrastructure of the mature egg and 30 fertilized archegonia used to study fertilization. The figures selected for presentation are representative of the results as a whole.
The archegonium and the mature egg
The archegonium is the female reproductive organ of the fern gametophyte. In C. thalictroides, the archegonium is usually produced on the upper surface just behind the apical notch of the asymmetrical gametophyte. When the archegonium matures, it contains three cells; an egg cell, a ventral canal cell, and an elongated binucleate neck canal cell (Figure 1). The mature egg is usually hemispheroidal and depressed slightly at the top, with a height of approximately 15 μm and a cross diameter of approximately 28 μm. A conspicuous feature of the mature egg is that it is surrounded by a distinctive layer of osmiophilic membrane, referred to as the extra egg membrane (Duckett and Bell 1972), which is not present around the ventral and neck canal cells (Figures 2,4). The membrane in the upper part of the egg is particularly prominent since it is clearly thicker than that occurring in the side and lower parts. Moreover, the thickness of the upper egg membrane is not uniform; it is thickest in the centric region, where it can exceed a width of 0.5 μm, and becomes thinner gradually toward the periphery, where the thickness is only approximately 70 nm (Figures 2,3). The structure of the upper egg membrane can be distinguished clearly under TEM. It usually consists of many layers of sheets, which are similar to the endoplasmic reticula immediately below it, and the plasma membrane, which is the outermost layer of the egg membrane. The side and lower parts of the egg membrane is a relatively thin layer of osmiophilic membrane, with a thickness of approximately 50–60 nm (Figures 2,4).
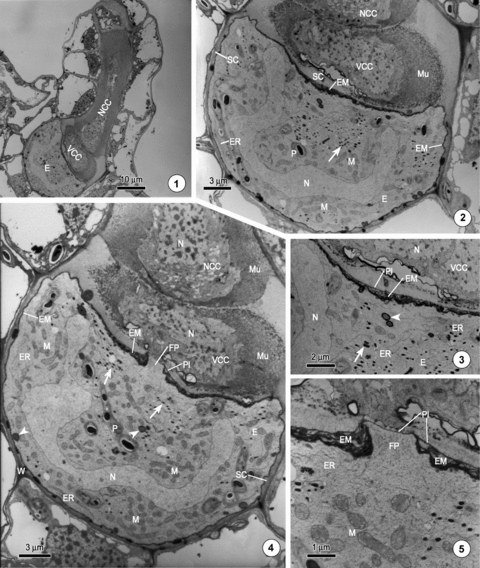
The mature archegonium of Ceratopteris thalictroides.1. A mature archegonium, which contains a mature egg (E), a ventral canal cell (VCC), and an elongated neck canal cell (NCC).2. The mature egg (E) is surrounded by a layer of egg membrane (EM), which is considerably thicker in the upper part than in the side and lower parts. The cytoplasm contains abundant organelles, including mitochondria (M), endoplasmic reticula (ER), plastids (P), and vesicles containing osmiophilic material (arrow). A separation cavity (SC) forms around the egg. The nucleus (N) exhibits a cup shape. Mucilaginous granular material (Mu) is secreted from the canal cells.3. A magnified image of Figure 2 showing the upper egg membrane (EM), which consists of many layers of sheets similar in appearance to the endoplasmic reticula (ER). The outmost layer of the egg membrane is the plasmalemma (Pl). The vesicles containing osmiophilic material (arrow) and lipid droplets (arrowhead) are clearly visible.4. The same archegonium as shown in Figure 2; a fertilization pore (FP) is apparent in the center of the upper egg membrane (EM) and is covered by only a layer of plasmalemma (Pl). The organelles, including well-developed mitochondria (M), degenerate plastids (P), numerous vesicles containing osmiophilic material (arrow), lipid droplets (arrowhead), and endoplasmic reticula (ER), which are usually parallel to the egg membrane, are abundant. Considerable amounts of mucilaginous granular materials (Mu) are secreted from the canal cells.5. A magnified image of Figure 4 showing the structure of the fertilization pore. The plasmalemma (Pl), which is the outmost layer of the egg membrane (EM), covers the fertilization pore (FP). The sheets around the fertilization pore appear to be interwoven into a knot.
When the archegonium is cut vertically and continuously, a pore is revealed in the center region of the upper egg membrane (Figures 4,5). The presence of a pore in each of the specimens examined suggests that this structure is an inherent feature of the mature egg (Figures 6–8). The pore, with a maximum diameter of about 2.5 μm, is only slightly larger than the cross diameter of a spermatozoid, which is approximately 2.0 μm at its widest part (Figure 12). The subsequent fertilization experiment indicated that this pore is an entrance by which spermatozoids enter the egg. We accordingly named this pore the fertilization pore. The fertilization pore protrudes outwards and lacks the sheets of egg membrane. The only membrane covering the fertilization pore is the plasmalemma, which is the outmost layer of the egg membrane (1–5, 6–11). The sheets around the fertilization pore appear to be interwoven into a knot, forming the border of the pore (1–5, 6–11).

The mature archegonium of Ceratopteris thalictroides.6. A mature egg showing the fertilization pore (FP).7. A magnified image of Figure 6 showing the structure of the fertilization pore (FP) and the egg membrane (EM).8. A further section of the specimen depicted in Figure 4, showing the structure of the fertilization pore (FP).9. Part of the mature egg of the same cell depicted in Figure 4, showing the stacks of endoplasmic reticulum (ER).10. Part of the mature egg and the ventral canal cell of the mature archegonium showing the shriveled nucleus of the ventral canal cell (VCC) and a thick layer of egg membrane (EM) covering the egg.11. The neck canal cell of the mature archegonium showing the organelles and the mucilage in the canal (Mu).
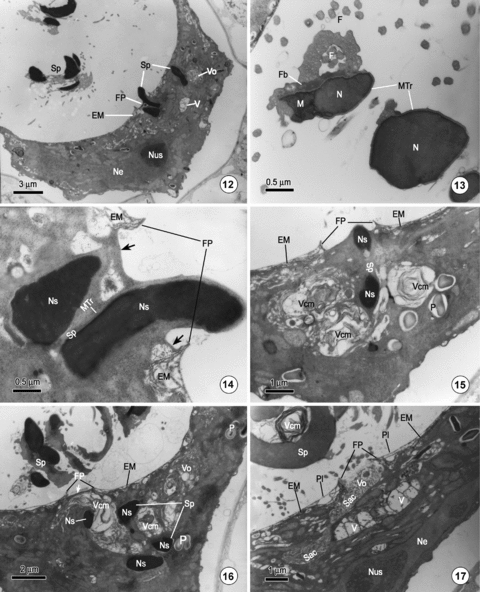
Observations on fertilization in Ceratopteris thalictroides.12. The egg shrinks markedly as a spermatozoid (Sp) penetrates through the fertilization pore (FP). Other spermatozoids (Sp) remain in the cavity above the egg. Vesicles containing transparent (V) or opaque material (Vo) appear around the fertilizing spermatozoid.13. Part of a longitudinal section of a helical spermatozoid above the fertilized egg, showing the spermatozoid with only a rod-like nucleus (N), and some motile apparatus, including flagellar band (Fb), microtubular ribbon (MTr), numerous flagella (F), and mitochondria (M).14. A magnified image of Figure 12 shows a spermatozoid penetrating the egg through the fertilization pore (FP). A layer of protoplasm (arrow) connects the surface of spermatozoid with the border of the fertilization pore of the egg. Ns, nucleus of spermatozoid.15. A specimen showing a spermatozoid (Sp) that has just penetrated an egg. Vesicles comprising many layers of concentric membranes (Vcm) occur near the fertilization pore (FP).16. A fertilized egg showing a spermatozoid that has completely entered the egg. A vesicle containing concentric membranes (Vcm) has migrated to a position just beneath the fertilization pore (FP). A layer of protoplasm still covers the fertilization pore (arrow).17. After the spermatozoid has entered the egg, a sac (Sac) containing opaque material (Vo) is seen connected to the fertilization pore (FP). A transparent vesicle (V) lies just below the fertilization pore. Ne, nucleus of egg.
The cytoplasm of the mature egg contains numerous organelles, which are densely packed and non-randomly distributed. Mitochondria, the most abundant and well-developed organelle, are distributed virtually throughout the egg (Figures 2,4). The endoplasmic reticula, often forming stacks, are mainly distributed at the periphery of the egg (1–5, 6–11). Numerous small vesicles, which contain heavy osmiophilic materials, with a diameter approximately 0.3 μm, are mainly distributed in the upper central part of the egg (1–5, 6–11, arrow). Plastids, which have lost their internal structures, contain only a few starch grains (1–5, 6–11). Occasionally, several lipid droplets can be seen at the periphery of the cytoplasm (Figures 3,4, arrowhead). In the mature egg, the nucleus usually becomes cup-shaped and the chromatin is dispersed homogeneously (1–5, 6–11).
The ventral canal cell and the neck canal cell conspicuously degenerate as the egg matures. Their nuclei become irregular and pycnotic. The chromatin in the nuclei of the canal cells becomes noticeably heterogeneous (Figures 10,11). Abundant organelles, mostly the endoplasmic reticula and numerous vesicles, are observed in the cytoplasm. At the same time, considerable mucilaginous granular material is secreted from the canal cells (1–5, 6–11).
Fertilization
As the archegonium matures and meets with water containing the spermatozoids, it discharges its internal contents, which includes the mucilage and the residue of the degenerating canal cells. At the moment of neck opening, a large number of spermatozoids, undoubtedly attracted by the substance secreted by the archegonium, swim toward the archegonium. Of those spermatozoids in the vicinity of the archegonium, only a few can squeeze into the cavity above the egg through the canal of the archegonium. Before a spermatozoid enters the archegonium, it will exclude most of its cytoplasm including all of the plastids, small isolated mitochondria, and some vesicles. Thus, the spermatozoids in the cavity above the egg possess only a conspicuous rod-like nucleus and some necessary motile apparatuses, including a multilayered structure, a single elongate coiled mitochondrion, a microtubular ribbon, a flagellar band, flagella, and some membranous vesicles (Figures 12,13,17).
Although there are several spermatozoids in the cavity above the egg, only one of these can enter the egg (most likely the first spermatozoid to enter the archegonium). Penetration of the egg by more than one spermatozoid was not encountered in any of the material examined. The process by which the spermatozoid enters the egg was previously unknown. In the present investigation, 30 fertilized archegonia were examined and our observations revealed that the spermatozoid enters the egg through the fertilization pore (Figures 12,14,15). Figure 12 shows a spermatozoid penetrating the egg and a majority of the spermatozoid has entered the egg through the fertilization pore; only the posterior of the nucleus remains in the fertilization pore. The fertilizing spermatozoid, which nucleus is cut three times, still retains its helical form within the cytoplasm of the egg (Figure 12). As the spermatozoid penetrates, a layer of protoplasm connects the surface of spermatozoid with the border of the fertilization pore of the egg (Figure 14, arrow). Figure 15 shows a fertilizing spermatozoid that has just entered the egg through the fertilization pore, and Figure 16 shows a fertilizing spermatozoid that has entered the egg completely. The latter spermatozoid, which nucleus is cut four times, still retains a helical form within the cytoplasm of the egg, and a layer of protoplasm still covers the fertilization pore (Figure 16, arrow).
The ultrastructures of the motile apparatus of the fertilizing spermatozoid are unclear compared with those of the spermatozoids above the egg. The microtubular ribbon, flagellar band, and mitochondria of the non-fertilizing spermatozoid can all clearly be identified (Figure 13). The nucleus of the fertilizing spermatozoid, when just entering the egg, is still rod-like and its chromatin remains condensed (Figures 14–16).
As soon as the egg is fertilized, it shrinks markedly. The fertilized egg, which has a height of approximately 7 μm and a cross diameter of approximately 24 μm, becomes deeply depressed at the top surface. Thus, the volume of the fertilized egg decreases to almost one-half that of the unfertilized egg. The protoplasm of the fertilized egg becomes dense and opaque. The organelles, particularly the mitochondria and endoplasmic reticula of the egg and most of the motile apparatus of the fertilizing spermatozoid, including the flagella, microtubular ribbon, multilayered structure, and mitochondrion, are barely identifiable. Only the starch grain-containing plastids remain prominent in the egg cytoplasm (Figures 15,16). The egg nucleus, containing an obvious nucleolus, lies close to the bottom of the egg. The chromatin of the egg nucleus is almost homogenous with the cytoplasm of the egg (Figure 17). The egg membrane is still intact except in the region of the fertilization pore where the plasmalemma has been broken up after the spermatozoid enters the egg (Figures 14,15,17). The structure of the sheets that constitute the egg membrane is still clearly identifiable (Figures 14–17).
Another conspicuous feature of the fertilized egg observed during the process of egg penetration is the occurrence of numerous vesicles in the cytoplasm. There are principally three types of vesicle (or sac) that occur successively in the cytoplasm of the fertilized egg as the spermatozoid penetrates the egg. First, vesicles containing concentric membranes, which are similar to the vesicles in the spermatozoid (Figure 17, Vcm), always occur around the fertilizing spermatozoid or near the fertilization pore (Figure 15, Vcm). As the fertilizing spermatozoid enters the egg completely, this type of vesicle migrates rapidly to a location just beneath the fertilization pore (Figure 16, Vcm). Second, sacs containing opaque material are also frequently observed near the fertilization pore. Occasionally, this type of sac can be observed fusing with the fertilization pore (Figure 17, Sac). Third, certain large transparent vesicles, which may be newly formed by the merging of a number of smaller vesicles, are also often found below the fertilization pore (Figure 17, V). In the fertilized egg, these large vesicles migrate toward the fertilization pore as the spermatozoid penetrates the egg. It is conceivable that these vesicles function as a barrier that prevents other spermatozoids penetrating the egg.
Discussion
The features of the mature egg
The mature egg of C. thalictroides– possessing a conspicuous egg membrane, well-developed mitochondria, stacks of endoplasmic reticulum in the cytoplasm, and a cup-shaped nucleus – is generally similar to that of the other ferns so far reported, including P. aquilinum (Bell and Duckett 1976), Histiopteris incise (Bell 1980), Dryopteris crassirhizoma (Bao et al. 2005), and C. richadii (Lopez-Smith and Renzaglia 2008). However, the egg membrane is usually considered to be formed outside the plasmolemma of the egg (Bell and Mühlethaler 1962a,b; Bell 1980; Bao et al. 2005). Nevertheless, the present investigation has revealed that the egg membrane is formed inside the plasmolemma of the egg, and it appears most likely the endoplasmic reticula are involved in the formation of the egg membrane. As the egg matures, both the ventral canal cell and the neck canal cell conspicuously degenerate. The nucleus becomes shriveled and pycnotic. The organelles in the degenerated canal cells are mostly the Golgi bodies, endoplasmic reticula and numerous vesicles, which may be involved in the formation of mucilage. All of these features indicate that the egg has fully matured.
The fertilization pore and its function
The present investigation has also demonstrated that a pore exists in the upper egg membrane of the mature eggs of C. thalictroides. Although a similar pore has been observed in the venter coat (equivalent to the egg membrane described in the present paper) of a fertilized egg of A. filix-femina, this type of pore is considered to be formed by the drilling of a spermatozoid at a receptive spot (Fasciati et al. 1994b). Moreover, a similar structure has also appeared in a fertilized egg of the fern C. richardii, but it was not described as a mating structure (Lopez-Smith and Renzaglia 2008). Hitherto, such a pore has not been reported in the mature egg of ferns so far investigated (Bell and Mühlethaler 1962a,b; Cave and Bell 1974; Myles 1978; Bell 1980, 1986; Fasciati et al. 1994b; Bao et al. 2003, 2005; Lopez-Smith and Renzaglia 2008). The present investigation describes the pore for the first time. What role the pore played is of special interest. The diameter of the pore is only slightly larger than the cross diameter of a spermatozoid. The experiments on fertilization have proved that the pore is an entrance, through which the spermatozoid can penetrate the egg (Figure 12). Consequently, the pore is named as a fertilization pore. After the spermatozoid enters the egg, the egg membrane is still intact, which indicated that the spermatozoid entry is restricted exclusively to the fertilization pore. Moreover, the phenomena that the spermatozoids are first taken up in a fertilization cavity, and then one of them enters the cytoplasm of the egg, as observed in P. aquilinum (Duckett and Bell 1972) and in A. filix-femina (Fasciati et al. 1994b), was never observed in the present investigation. Indeed, in a later review paper, Bell and Duckett (1976) also questioned whether the fertilization cavity is an authentic feature of viable eggs.
The observations of live specimens revealed that fertilization in C. thalictroides is a rapid process. The prolonged (more than 15 min) gyration of spermatozoids observed at the surface of the eggs of A. filix-femina without subsequent penetration (Fasciati et al. 1994a) was never observed in the present study. In P. aquilinum, spermatozoid penetration is also considered to be a convulsive process (Bell and Duckett 1976). Therefore, it is difficult to capture the very point at which a spermatozoid penetrates an egg. In order to capture the penetrating process, almost all gametophytes that are used in fertilization experiments are fixed immediately on observing spermatozoids crowding into the archegonium in the suspension of spermatozoids (under a stereo microscope). In all of the specimens observed, only a few spermatozoids were captured in the process of penetrating. This mechanism of penetration differs markedly from that of other organisms, including some algae and animals, which usually exhibit a stage of protracted membrane fusion during syngamy (Friedmann et al. 1968; Braten 1971; Callow et al. 1978; Mcculloh and Chambers 1992; Primakoff and Myles 2002). The discovery of the fertilization pore is undoubtedly of considerable significance in elucidating the cytological mechanism of fertilization in ferns. Moreover, this mode of fertilization may also be typical of archegoneate plants as a whole. The evolutionary significance of this characteristic fertilization is also noteworthy.
Viability and cytological changes in the fertilized egg
A conspicuous feature of the fertilized egg of C. thalictroides is the marked shrinkage during fertilization. The volume of the fertilized egg decreases to almost one-half that of the unfertilized egg, and the egg structure becomes dense and opaque. Does this mean that the fertilized eggs shown in Figures 12–17 are degenerate or abnormal? In the present investigation, more than 30 fertilized archegonia were fixed and examined. All of the fertilized eggs exhibited the same reaction. In contrast, the fertilized eggs that were not fixed for observation eventually developed into the normal embryos and sporophytes. This indicates that the shrinkage of the fertilized egg may be a normal physiological reaction. Therefore, the fertilized eggs shown in Figures 12–17 should be viable cells. Bell (1975) also indicated that the fertilized eggs of P. aquilinum in the earliest fixings were shrunken and that the structures were dense and opaque. Then the fertilized eggs became fully expanded and recovered their clear structure within 25 min after insemination. The present study revealed that the shrunken fertilized eggs of C. thalictroides also become expanded and recover their clear structure within 30 min after insemination (JG Cao, unpubl. data, 2008). The shrinkage of the fertilized eggs may be the irritability in C. thalictroides as suggested in the fern P. aquilinum (Bell 1975). It is possible that the shrinkage of the protoplasm of the fertilized egg renders certain organelles, particularly the mitochondria and endoplasmic reticula of the egg, and almost all of the motile apparatus of the fertilizing spermatozoid, indistinguishable. Only the starch grain-containing plastids are recognizable. Clearly, the cytological changes that take place during the fertilization of C. thalictroides eggs are very complicated. The detailed process of fertilization during the course of development following insemination and the cytological features of the zygote will be reported in a further paper.
The prevention of polyspermy
In the heterosporous fern M. vestita, it is believed that two structures (i.e. a thickened polysaccharide wall and a newly formed extracellular layer), may be responsible for the prevention of polyspermy (Myles 1978). In the homosporous fern P. aquilinum, although the phenomenon of polyspermy prevention has been discussed, the mechanism underlying this phenomenon is still unknown (Duckett and Bell 1972; Bell 1975; Bell and Duckett 1976). In the present investigation, several spermatozoids were observed in the cavity above the egg; however, it appears that only one of these is able to enter the egg. Penetration of the egg by more than one spermatozoid was not encountered in any of the specimens examined. The mechanism by which polyspermy is prevented may be attributable to the numerous vesicles observed in the vicinity of the fertilizing spermatozoid or near the fertilization pore when the spermatozoid penetrates the egg (Figures 15–17). In the present investigation, three principle types of vesicle (or sac) appeared successively in the cytoplasm of fertilized eggs during the process of spermatozoid penetration. First, vesicles containing concentric membranes always occurred near the fertilization pore (Figure 15), and then migrated to the fertilization pore (Figure 16). Second, sacs containing opaque material were also frequently observed in the vicinity of the fertilization pore. Occasionally, a sac was observed fusing with the fertilization pore (Figure 17). Third, large transparent vesicles, which may be newly formed by the merging of smaller vesicles, were also observed below the fertilization pore. These three types of vesicle undoubtedly function as a barrier that blocks the fertilization pore, and thus prevents other spermatozoids entering the egg through the pore. Moreover, the shrinkage of the fertilized egg may also result in the pyknosis of the protoplasm, which may also contribute to the prevention of polyspermy.
Materials and Methods
The spores of Ceratopteris thalictroides (L.) Brongn. were collected from plants in the botanic garden of Shanghai Normal University. The spores were sterilized with 5% sodium hypochlorite solution for 3 min. After rinsing three times with distilled water, the spores were sown on a modified Knop's solution, solidified with 1.5% agar in culture dishes. These dishes were placed in an artificial climate chamber under conditions of 25 °C in the light (18 h) and 20 °C in the dark (6 h). After 3 to 4 weeks, archegonia had developed over the surface of the gametophytes, mostly on the upper surface, but with a few formed on the lower surface.
For studying the ultrastructure of the mature egg, the gametophytes containing mature archegonia were picked up and fixed with 3% glutaraldehyde in 0.1 mol/L phosphate buffer at room temperature for 6–8 h. For studying fertilization, the gametophytes containing near-mature archegonia were plunged into a suspension of spermatozoids (water containing numerous mature male gametophytes, designed to ensure a continuous release of spermatozoids). The maturation process may take up to 3 h or more prior to neck opening. As soon as the spermatozoids were observed crowding into the archegonium through the open neck (under a stereomicroscope), the gametophytes were immediately fixed as described above. More than 10 mature eggs (mature archegonia) and 30 fertilized eggs (fertilized archegonia) were fixed for observation. The specimens were subsequently washed three times with the phosphate buffer, post-fixed in osmic acid (1% solution in phosphate buffer) for 2 h, and then washed again three times with the phosphate buffer. After dehydration in a graded acetone series, the materials were infiltrated with a mixture of acetone and Spurr's resin, and then embedded in pure Spurr's resin. Specimens were initially thick-sectioned for the presence of archegonia and then thin-sectioned with a diamond knife on an Ultracut-E ultramicrotome (Reichert-Jung, Germany). The thin sections were double-stained with uranyl acetate and lead citrate. All specimens were observed with an H-600 electron microscope (Hitachi, Japan).
(Handling editor: Shuang-Quan Huang)
Acknowledgements
The authors are grateful to Professor Wen-Mei Bao for the encouragement and for suggesting this paper and Ms. Hui-Qi Zhang for her assistance in ultrastructural section.




