Variation in Floral Sex Allocation and Reproductive Success in Sequentially Flowering Inflorescence of Corydalis remota var. lineariloba (Fumariaceae)
Supported by the National Natural Science Foundation of China (30430160).
Abstract
In hermaphroditic plants, female reproductive success often varies among different positions within an inflorescence. However, few studies have evaluated the relative importance of underlying causes such as pollen limitation, resource limitation or architectural effect, and few have compared male allocation. During a 2-year investigation, we found that female reproductive success of an acropetally flowering species, Corydalis remota Fisch. ex Maxim. var. lineariloba Maxim. was significantly lower in the upper late developing flowers when compared with the lower early flowers. Supplementation with outcross pollen did not improve female reproductive success of the upper flowers, while removal of the lower developing fruits significantly increased female reproductive success of the upper flowers in both years, evidencing resource limitation of the upper flowers. Female production in upper flowers was greatly improved by simultaneous pollen supplementation of the upper flowers and removal of the lower fruits, suggesting that, when resources are abundant, pollen may limit the female reproductive success of the upper flowers. The less seed mass in the upper flowers didn't increase in all treatments due to architecture. In the upper flowers, ovule production was significantly lower and the pollen : ovule ratio was significantly higher. These results suggest that male-biased sex allocation in the upper flowers may lead to increased male reproductive success, whereas the lower flowers have higher female reproductive success.
It is well known that flowers of an inflorescence are not created equal. Even for hermaphrodite plants considerable variation exists within an inflorescence in investment in attractive traits (e.g. petals, nectar) and reproductive potential (reviewed in Stephenson 1981; Diggle 1995). Most studies have found that, in species with acropetal inflorescence, the probability of fruit set and/or the number of seeds per flower is often lower for the distal/late-opening flowers than for the proximal/early-opening flowers (Solomon 1988; Herrera 1991; Ehrlen 1992, 1993; Guitian 1994; Guitian and Navarro 1996; Navarro 1996; Medrano et al. 2000; Vallius 2000; Kliber and Eckert 2004; Tremblay 2006).
Three hypotheses have been proposed to account for within-inflorescence variation in female reproductive success: pollen limitation, resource limitation, and architectural effect. First, both low pollen quality and quantity may influence the probability of fruit and seed maturation in the upper flowers (Lee 1988; Thomson 1989). The stereotypic upward movements of pollinators result in decreased transport of outcross pollen to the upper flowers, which may result in lower quality offspring in self-compatible species (Harder et al. 2000) or lower ovule fertilization and seed maturation in self-incompatible species (Kudo et al. 2001). Additionally, fruit set in the higher flowers may also decrease due to ineffective pollen deposition as flowers decrease in size (Tremblay 2006). Second, a decrease in fruit and seed set for distal/late flowers may be associated with limited resources. Resource competition between early and late flowers and fruits is a common phenomenon (Stephenson 1981; Nakamura 1986; Lee 1988; Solomon 1988; Thomson 1989; Guitian 1994; Medrano et al. 2000; Vallius 2000; Kliber and Eckert 2004). As early initiated fruits captured most of the resources, insufficient resources may be available for fruit maturation of late opening flowers. Third, the potential for fruit development may vary with flower position due to the inherent inflorescence architecture (Wyatt 1982; Lee 1988; Wolfe 1992; Diggle 1995, 1997). In contrast to the flowers or fruits in basal positions, those produced distally are borne on stems of smaller diameter that contain less vascular tissue. Thus, the supply of resources to these distal flowers or fruits may be lower (Wolfe 1992; Diggle 1995).
The above hypotheses emphasize lower female reproductive success for distal/late-opening flowers, but largely ignore male reproductive success. In hermaphroditic plants, the male and female contributions to fitness are each due to the sum of contributions from each of the plant's flowers (Brunet and Charlesworth 1995). Recently, studies began to pay attention to the male function of distal or later flowers (Brunet and Charlesworth 1995; Brunet 1996; Ashman and Hitchens 2000; Tremblay 2006; Vallius and Salonen 2006). Brunet (1996) suggested the low fruit and seed set of late flowers reflected the fact that late flowers specialized as males, while the early flowers got higher female success. Factors such as protandry can modify the probability of pollen transfer among flowers and select for male specialization of late flowers (Brunet and Charlesworth 1995). Pollinators’ directional movement on an inflorescence may also influence the probability that pollen from given positions reaches stigmas at different positions (Brunet and Charlesworth 1995). Sex-differentiation of intra-inflorescence patterns may maximize plant fitness through females at positions where resources for seed production are likely to be most abundant (Ashman and Hitchens 2000), and males at positions where pollen is most likely to be dispersed.
We aimed to answer the following questions: (i) is female reproductive success significantly lower in the upper, late developing flowers within an inflorescence of the sequentially flowering species Corydalis remota var. lineariloba? (ii) which of the three hypotheses (pollen limitation, resource availability, and architectural effect) best explains the lower female reproductive success of upper late developing flowers? (iii) do the late upper flowers allocate relatively more resources to male function at the expense of female function?
Results
Female reproductive success variation
In 2004, we successfully collected 50 naturally pollinated infructescences. The fruit set and seed set of the upper flowers were significantly lower than those of middle and lower flowers (fruit set: χ2 = 14.74, d.f. = 2, P < 0.001; Figure 1A; seed set: F2, 148 = 19.43, P < 0.001; Figure 1B). In 2005, fruit set and seed set from 61 infructescences decreased significantly from lower to upper (fruit set: χ2 = 16.17, df = 2, P < 0.001; Figure 1A; seed set: F2, 182 = 58.05, P < 0.001; Figure 1B). Seed production per fruit decreased significantly from the lower to upper flower in both years (F2, 148 = 40.33, P < 0.001 in 2004; F2, 182 = 91.82, P < 0.001 in 2005; Figure 1C). In 2004, the mean seed mass of the lower fruits was significantly higher than that of the middle and upper fruits (F2, 135 = 12.56, P < 0.001; Figure 1D).
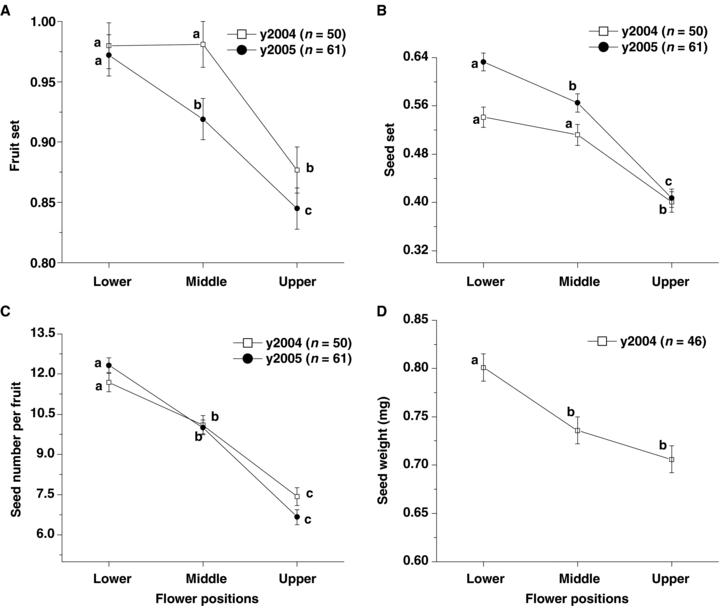
Female reproductive success variation among different positions within inflorescences.(A) Fruit set.(B) Seed set.(C) Seed number per fruit.(D) Seed mass.
Sex allocation and pollen viability within inflorescences
Ovule production decreased significantly from lower to upper flowers (F2, 83 = 67.44, P < 0.001 in 2004; F2, 134 = 78.74, P < 0.001 in 2005; Figure 2A). However, pollen production did not vary significantly between the flower positions in both years (F2, 32 = 1.00, P = 0.390 in 2004; F2, 134 = 1.71, P = 0.187 in 2005). The pollen : ovule ratio (P/O ratio) increased significantly from lower to upper in 2005 (F2, 134 = 78.74, P < 0.001; Figure 2B), and it was significantly higher in the upper flowers than in the lower flowers in 2004 (F2, 32 = 5.56, P = 0.012; Figure 2B). The corolla is typically considered to be part of the male reproductive system and was also significantly decreased in mass from lower to upper flowers (F2, 146 = 55.49, P < 0.001; Figure 2C).
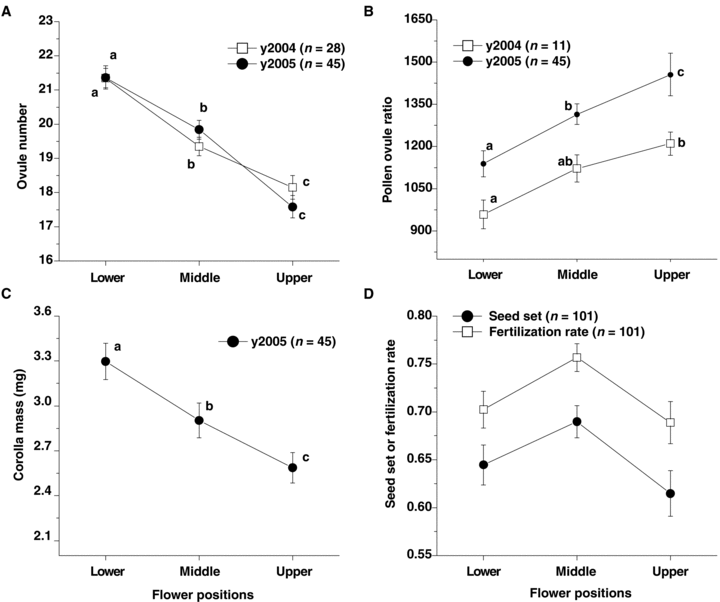
Variation in floral sex allocation among different positions within an inflorescence.(A) Ovule number.(B) Pollen : ovule ratio.(C) Corolla mass per flower.(D) Seed set and fertilization rate of lower flowers pollinated by outcross pollen from different flower positions.
We collected data from 101 inflorescences to determine pollen viability. The majority of the pollinated lower flowers set fruits. Seed set (F2, 100 = 1.67, P = 0.193; Figure 2D) and fertilization rate (F2, 100 = 1.53, P = 0.222; Figure 2D) of the three groups of lower flowers that were pollinated by outcross pollen from different positions did not vary significantly. These results suggest that pollen viability was similar at all of the positions.
Resource and pollen limitation
When naturally pollinated (control), the upper flowers had a significantly lower fruit set, seed set and seed number per fruit than the middle and lower flowers in both years (Figure 1A–C). Supplementing outcross pollen (S treatment) neither improved fruit set nor seed set for upper flowers in both years. However, removing the lower developing fruits (R treatment) resulted in significantly higher seed set compared with the controls and S treated flowers (see Table 1 for marginal means comparison and number of successfully collected infructescences for analysis; Figure 3). There was a significant interaction between pollen supplementation and removal of the lower fruit for seed set in the upper flowers (F1, 153 = 6.59, P = 0.011; Figure 3C) in 2004, which was not reflected by the fruit set (Figure 3A). A marginal means comparison suggests that the seed sets of the upper flowers with outcross pollen supplementation and lower developing fruit removal simultaneously (S + R treatment) were significantly higher than other treatments (Table 1; Figure 3C). Such an interaction was not observed in 2005 (Table 1; Figure 3D); however, the Mann-Whitney Test indicated that the upper flowers in this treatment had the highest fruit set (Figure 3B). These results suggest that C. remota var. lineariloba is generally resource limited, but may also be limited by pollen availability when resources are abundant. All three treatments (S, R and S + R) had an equivalent seed mass (S = 0.69 ± 0.02 mg, n = 45; R = 0.74 ± 0.04 mg, n = 38; S + R = 0.70 ± 0.04 mg, n = 15) in the upper flowers and did not differ from the controls (0.71 ± 0.03 mg, n = 46).
| Year | Treatment | Sup | Del | n | Seed set mean ±SE | 95% confidence interval of seed set | |
|---|---|---|---|---|---|---|---|
| 2004 | Control | No | No | 50 | 0.401 ± 0.030 | 0.341 | 0.461 |
| S | Yes | No | 46 | 0.419 ± 0.032 | 0.356 | 0.481 | |
| R | No | Yes | 42 | 0.526 ± 0.033 | 0.461 | 0.591 | |
| S + R | Yes | Yes | 16 | 0.740 ± 0.054 | 0.634 | 0.846 | |
| 2005 | Control | No | No | 60 | 0.407 ± 0.025 | 0.359 | 0.456 |
| S | Yes | No | 45 | 0.420 ± 0.029 | 0.364 | 0.477 | |
| R | No | Yes | 54 | 0.567 ± 0.026 | 0.516 | 0.619 | |
| S + R | Yes | Yes | 52 | 0.567 ± 0.027 | 0.514 | 0.619 | |
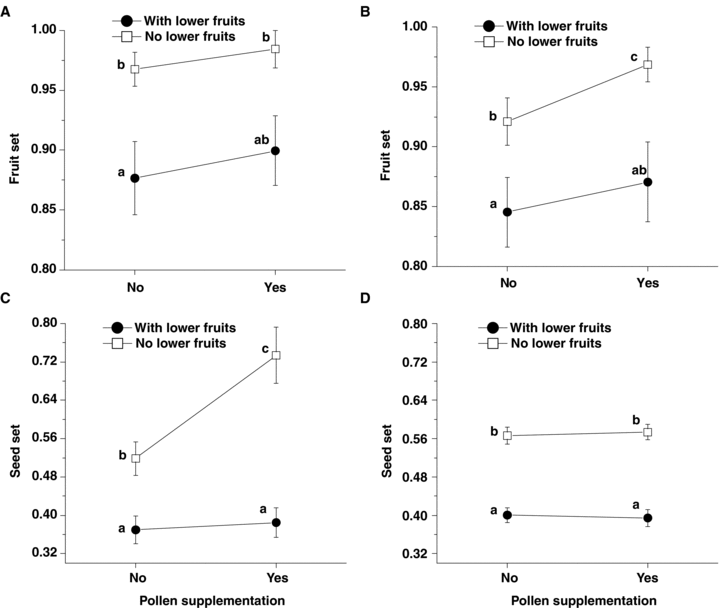
Female success comparison of upper flowers among control (no outcross pollen supplementation and with lower fruits present), pollen supplementation (with outcross pollen supplementation for upper flowers and with lower fruits present), fruit removal (no outcross pollen supplementation and with lower fruits absent) and simultaneous pollen supplementation and fruit removal (with outcross pollen supplementation and with lower fruits absent).(A) Fruit set in 2004, n = 50, 46, 42 and 16 for above treatments, respectively.(B) Fruit set in 2005, n = 61, 45, 54 and 52 for above treatment respectively.(C) Seed set in 2004, n = 50, 46, 42 and 16.(D) Seed set in 2005, n = 61, 45, 54 and 52. The cross of two lines suggest the interaction effects.
Discussion
Female reproductive success was significantly lower for the late distal (upper) flowers in the sequentially flowering plant C. remota var. lineariloba. Our results suggest that resource competition is largely responsible for this phenomenon as evidenced by the improved fruit set and seed set of the upper flowers after removal of the lower fruits (R treatment). However, female reproductive success of the upper flowers was greatest in the S + R treatment, suggesting that lower deposition of outcross pollen on the stigma may also influence the female reproductive success of the upper flowers of C. remota var. lineariloba when resources are abundant. Ovule investment declined significantly from lower to upper flowers; and the P/O ratio was significantly increased.
Pollen limitation versus resource competition
Removal of the lower developing fruits significantly increased the fruit set and seed set of the upper flowers in both years. Our results indicate that the production of seeds in the late developing upper flowers is limited by resource competition with the earlier developing flowers. Resource competition has also been reported previously in plants with similar flowering patterns (Stephenson 1981; Ehrlen 1992; Guitian 1994; Navarro 1996; Medrano et al. 2000; Vallius 2000; Kliber and Eckert 2004). The lower fruits appear to have both spatial and temporal advantage in competition for resources in this kind of flowering plant species. The resources getting from leaf and root must pass the lower fruits en route to the younger upper fruits along the inflorescence. When resources are limited, the reproductive structures located furthest from the source of resources are shed first (Stephenson 1981).
Supplementing with outcross pollen on the upper flowers did not improve seed set in either study year, suggesting that the lower seed set of upper flowers was not a function of pollen limitation. Similar results were also found in other species studies (Sutherland 1987; Ehrlen 1992; Medrano et al. 2000). However, supplementing pollen onto the upper flowers and removing the lower fruits simultaneously resulted in greatly improved seed set among the upper flowers in 2004 and fruit set in 2005. The interaction effect between pollen supplementation and removal of the lower fruits was significant, suggesting that when resources are not limited, the supply of outcross pollen may limit female reproductive success of the upper flowers of C. remota var. lineariloba. The pollinators’ upward movement during feeding is similar to the developmental progression of flowers within an inflorescence and may not allow for transport of sufficient outcross pollen to the upper flowers. This may result in pollen-limited fertilization of the upper flowers.
Pollen limitation for the whole inflorescence, especially for upper flowers in C. ambigua, another species of the same genus, was confirmed in Japan (Kudo et al. 2001). In contrast, we did not observe pollen limitation for the lower flowers of C. remota var. lineariloba (outcross pollination during the pollen viability trial did not improve female reproductive success of the lower flowers compared with natural pollination). Besides, pollen limitation was minimal among the upper flowers of our species. These two species share many common traits: they have similar flower morphology and inflorescence patterns, and they have the same life cycle, flowering time and identical pollinator behavior, although the species of pollinators do differ. However, the total flowering period of C. ambigua is approximately twice that of C. remota var. lineariloba. Furthermore, the development period of flowers within an inflorescence of C. ambigua is much more compressed. Given this, the competitive advantage enjoyed by earlier developing flowers may not be as obvious in C. ambigua.Haig and Westoby (1988) presented a graphical model proposing that, at an evolutionary equilibrium female fecundity would be limited by both pollen and resources. When pollen limits seed set, selection would favor increased allocation to pollinator attraction at the expense of ovule investment, but in species receiving excess pollen, a shift from attractive investment to ovules would be favored. The differences in flower allocation between C. ambigua and C. remota var. lineariloba support this hypothesis. The C. ambigua has relatively larger flowers, fewer ovules per flower and a longer flowering period for a single inflorescence. In addition, as sequentially flowering species, the overlap in flowering time between early and late flowers can be adjusted according to environmental conditions. For example, when pollinators are scarce, resulting in pollen limitation, selection favors a larger flower display by increasing the overlap within an inflorescence (e.g. C. ambigua). When resources limit seed production, the ability to separate flowering time within an inflorescence allows the plant to maximize fitness by sex spatial separation (e.g. C. remota var. lineariloba).
Architectural effect
The flowers at different positions within an inflorescence appeared equally capable of setting seed in the absence of other fruits in C. remota var. lineariloba. However, this was not the case for seed maturation. Neither the removal of the lower fruits and/or pollen supplementation (S, R or S + R) had an effect on the weight per seed of upper flowers. Such an effect cannot be explained by resource limitation raising the possibility that fruit and seed maturation of the upper flowers may be simultaneously limited by both resource competition and inflorescence architecture (Kang and Primack 1991; Wolfe 1992; Diggle 1995).
It has been suggested that the vasculature of inflorescence might regulate fruit and/or seed maturation (reviewed in Wolfe 1992; Diggle 1995). In contrast to the reproductive structures in basal positions, those produced distally are borne on stems of smaller diameter that contain less vascular tissue. Thus, the supply of resources to these distal structures may be lower (Diggle 1995). It was reported that the growth of distal pods was slower than that of proximal ones in Lupinus luteus, even when basal pods were removed (Van Stevenick 1957). The C. remota var. lineariloba is an ephemeral species whose leaves begin to turn yellow at the end of the flowering season. It is possible that seeds from the upper late developing flowers do not have sufficient time to mature given that photosynthesis is critical for seed maturation (Smith et al. 1986).
Mating environment and sex allocation
Sex allocation theory assumes hermaphroditic plants should allocate resources to male and female in a pattern that maximizes individual fitness. Variation in the mating environments of flowers can select for differences in sex allocation between flowers (Brunet and Charlesworth 1995). Both resource competition and architectural effect decreased the female reproductive success of the upper late flowers within C. remota var. lineariloba inflorescences. Reduced ovule production for the upper late flowers may be an adaptive strategy rather than just a result of architectural effects. Architectural effects should reduce both the production of ovule and pollen; however, pollen production does not differ among three positions. The relatively increased male allocation (P/O ratio) for the upper late flowers in this species further supports the mating environments theory of Brunet and Charlesworth (1995). The movement of pollinators from the lower flowers towards the upper flowers increased the possibility of pollen transfer of upper flowers to other individuals. Protandry can also increase the male reproductive success of the late flowers by modifying the probability of pollen transfer, and male biased allocation may be favored for the late flowers. The opposite pattern of floral sex allocation has been reported in protogynous species such as Sagittaria trifolia (Huang et al. 2002) and Aquilegia yabeana (Huang et al. 2004). Although female reproductive success (fruit set, seed set and seed number per fruit) was lower in the upper late flowers of C. remota var. lineariloba, they may maximize fitness by fertilizing more ovules in other plants. Thus, sexual separation within an inflorescence of the species like C. remota var. lineariloba would favor more female investment for lower early flowers and more male investment for upper late flowers.
Materials and Methods
Study species and study site
All experiments were carried out in the understory of the deciduous forest on Dongling Mountain, 100 km west of Beijing, China (39°58′N, 115°26′E, 1070 m). During early spring before the leaves of the canopy expand, many Corydalis remota var. lineariloba emerge and bloom in the valleys and hillsides of Dongling Mountain.
The spring ephemeral, Corydalis remota var. lineariloba, is a self-incompatible clone species with one to three vertical inflorescences in each ramet. An inflorescence contains 4–20 flowers, each of which has a spur with nectar. The anthers and stigma are posited within an inner petal and are protected by it. Anthers completely dehisce before the flower opens, and pollen presents as a layer on the stigma surface. The flowers of an inflorescence are produced in tiers that develop acropetally. A flower lasts 3–5 d. The mean flowering period of an inflorescence is 9 d (range 6–12 d). Tetralonica chinensis and Habropoda tananicola macilla lieftinck are the major pollinators of C. remota var. lineariloba. Both pollinators tend to move upward, following the developmental sequence of the flower, while they absorb nectar and collect pollen. In the study population, the flowering of C. remota var. lineariloba starts in late April and lasts until mid May. Seeds mature during late May, and are dispersed secondarily by ants when they fall from the chapped pods (Zeng pers. obs. 2004).
We classified flower positions into lower, middle or upper according to the total flower number in an inflorescence (Table 2) (Kudo et al. 2001).
| Number of flowers per plant | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position class | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | … |
| Lower | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 4 | 5 | 5 | … |
| Middle | 2 | 3 | 2 | 3 | 4 | 3 | 4 | 5 | 4 | 5 | 6 | 5 | 6 | … |
| Upper | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 4 | 5 | 5 | … |
Female reproductive success variation
The variation in female reproductive success within an inflorescence under field conditions was evaluated by comparing mean fruit set, seed numbers per fruit and seed set among the three positions in 2004 and 2005, and by comparing mean seed mass (weight per seed) among the three positions in 2004.
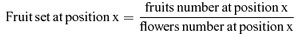

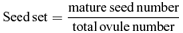
Floral sex allocation and pollen viability
We quantified the sequential variation in ovules, pollen and the pollen : ovule ratio (P/O ratio) under field conditions. During late April 2004, we randomly marked and bagged 28 inflorescences with various total numbers of flower buds. Each flower was classified with respect to its position within the inflorescence (Table 2). We collected every flower produced just after opening, counted ovules for each flower of the 28 inflorescences, and estimated pollen production for each flower of 11 inflorescences by complete suspension of the anther in 1 mL water and counting pollen in five replicate 0.005 mL subsamples under a microscope. During the blossom period of 2005, we collected data on the number of ovules, pollen production and the P/O ratio from 45 inflorescences. Corolla of each flower in these 45 inflorescences was also collected and dried with silica gel for 3 d and then weighed. The mean weight of all flowers at a given position in each inflorescence was recorded for analysis.
To compare the pollen viability among different positions, we tested the fertilization ability of pollen from the three positions. In 2005, we randomly bagged 120 inflorescences with various total numbers of flower buds. These were then divided into three groups, and the lower emasculated flowers of each group were pollinated with the outcross pollen from a different position (lower, middle and upper) when they were acceptable. We continued to bag them until all of the hand-pollinated flowers were wilted. To make sure of the pollen quality, pollen of each position was obtained by collecting recently opened stamens from more than 15 donor plants located at least 5 m away from the recipient plants. All of the pods were harvested and their seeds were counted at maturity. Fruit set, seed set and fertilization rate were measured and compared among the three groups.
Resource limitation, pollen limitation and architectural effect
The upper flowers within an inflorescence may have lower female reproductive success when compared with the lower and middle flowers due to pollen limitation, resource limitation or architectural effect. We tested the hypothesis of pollen limitation by comparing the fruit set and seed set of the upper flowers supplemented with outcross pollen or by natural pollination. We also tested whether resources were limited by measuring the fruit set and seed set improvement of the upper flowers in the presence and absence of lower developing fruit. We reasoned that if neither treatment had an effect the most likely candidate would be an architectural effect.
During the blossom period of 2004, we randomly chose 90 inflorescences. Each inflorescence was randomly assigned to one of the following two treatments with 45 replicates in each: (i) Outcross pollen was supplemented onto the upper flowers (S treatment); (ii) The lower and partial middle developing fruits were removed, while the upper flowers were still flowering (R treatment). In addition, a further 20 inflorescences had supplemental outcross pollen applied to the upper flowers and had the lower and partial middle developing fruits removed simultaneously (S + R treatment). To study yearly variation in pollen limitation and resource limitation we repeated the experiment in 2005. We increased the number of inflorescences to 55 for all of the three treatments. Fruit set and seed set for the upper flowers were calculated as above in each respective year and compared with the upper flowers that were pollinated naturally (control). In 2004, the mean seed mass (weight per seed) produced by the upper flowers was compared between treatment groups.
Statistical methods
For each position and each inflorescence of each treatment, we calculated the mean number of seeds per flower and the mean seed set. Flowers that did not set seed were excluded. Variations in mean gamete, mean seed production and mean corolla mass across flowering positions were evaluated using a univariate anova with the plant as the subject and flower position as a within-subject fixed effect, and post hoc Scheffe pairwise contrasts. Two-factor anova was used to test for interactions between pollen supplementation and removal of the lower fruit. We used a post hoc Scheffe pairwise contrast to analyze the differences in mean fruit set, mean seed set, mean seed number and mean seed mass between the different treatments. To compare the pollen viability at the different positions, we used a one-way anova comparing the mean seed set and mean fertilization rate. A non-parametric test (Mann–Whitney Test) was used to compare all of the fruit set data that could not be transformed into a normal distribution. All percentages were arcsine-transformations prior to analysis if the distribution was not normal. All analyses were carried out in SPSS 13.0 for Windows.
(Handling editor: Shuang-Quan Huang)
Acknowledgements
We thank Drs Quan-Guo Zhang for help with some field work and Wan-Jin Liao for comments on the manuscript. Professor Lawrence D. Harder provided much appreciated guidance in data analysis.




