High-throughput Procedure for Single Pollen Grain Collection and Polymerase Chain Reaction in Plants
Supported in part by Grower/processor Check-off Funds administrated by the American Sugar Cane League of the USA., Inc., Thibodaux, Louisiana, USA (to Y.-B. Pan) and the Chinese 948 Project (2003-Q06) (to P.-H. Chen).
Abstract
Single pollen grain polymerase chain reaction (PCR) has succeeded in several species, however only limited numbers of pollen grains were involved due to difficulties in pollen isolation and lysis. This has limited its application in genetic analysis and mapping studies in plants. A high-throughput (HT) procedure for collecting and detecting genetic variation in a large number of individual pollen grains by PCR is reported. The HT procedure involved the collection of individual pollen grains by a pair of special forceps and the lysis of pollen grains in a heated alkali/detergent solution followed by neutralization with a tris-ethylenediamine tetraacetic acid (TE) buffer. These resulting template solutions yielded PCR reactions involving the 5S ribosomal RNA intergenic spacers, randomly amplified polymorphic DNA, and simple sequence repeats markers. Using this procedure, one person with experience could collect and process up to 288 single pollen grain PCR reactions per day. The method worked well on sugarcane, corn, Miscanthus spp., snap bean, sorghum, and tomato. The ability to collect and conduct PCR on individual pollen grains on a large scale offers a new approach to genetic analyses and mapping studies in an easily controllable environment with a considerable cost reduction. The method will also significantly benefit studies in species that are difficult subjects for classical genetic research.
Polymerase chain reaction (PCR) has succeeded on single cells and single pollen grains for studying genetic variation (Li et al. 1990; Zhang et al. 1992; Petersen et al. 1996; Matsunaga et al. 1999; Aziz and Sauve 2003; Parducci et al. 2005), gene expression (Shoemaker et al. 2005), and genotyping (Li and Yeung 2002; Aziz et al. 2005). However, only limited numbers of pollen grains were involved in these studies (Matsunaga et al. 1999; Aziz and Sauve 2003; Parducci et al. 2005) except for one report involving 60 pollen grains (Aziz et al. 2005). Single cells, such as human sperm, could be isolated with the aid of flow cytometry (Li et al. 1990; Zhang et al. 1992), a micromanipulator (Shoemaker et al. 2005), or a capillary tube (Li and Yeung 2002), while single pollen grains were collected with laser-mediated manipulation (Matsunaga et al. 1999), micromanipulator (Aziz and Sauve 2003; Haliyo and Regnier 2003; Aziz et al. 2005), or specifically modified glass micropipettes (Parducci et al. 2005). A micromanipulator was primarily used for picking up single pollen grains; however, the efficiency was low and the number of pollen grains being picked up never exceeded 60 in a single study (Aziz et al. 2005). Laser-mediated manipulation was highly effective in isolating single pollen grains, but the instrument was expensive.
The thick and rigid walls (Santos and Mariath 1999; Parre and Geitmann 2005) and the sizes (0.005 to 0.25 mm in diameter) of mature pollen grains (Pinar and Inceoglu 1999; Perveen and Qaiser 2001; Sarkissian and Harder 2001) facilitate the picking up of individual pollen grains under a dissecting microscope by a pair of fine-tipped forceps. On the other hand, it is much more difficult to lyse pollen grains to release the nucleic acid contents when compared with animal cells. Several methods have been tested to lyse pollen grains, including a UV-laser microbeam (Matsunaga et al. 1999), lysis buffer (Matsunaga et al. 1999), pre-germination in media (Aziz and Sauve 2003; Aziz et al. 2005), or crushing with pipette tips (Parducci et al. 2005). While most laboratories have no access to a UV-laser microbeam, use of germination media may introduce more chemicals that may inhibit PCR (Aziz and Sauve 2003; Aziz et al. 2005). Pipette tips are used to crush special pollen grains from fossils (Parducci et al. 2005). Some investigations indicated that walls of pollen grains could be solubilized (Southworth 1974; Loewus et al. 1985). Recently, Xin et al. (2003) reported a high-throughput (HT) DNA extraction method that did not inhibit PCR on a number of plant species.
The objective of the present study was to develop a practical HT procedure for single pollen grain isolation and PCR.
Results
Glass slide preparation and single pollen grain collection
A pair of special forceps (DUMONT No. 11254-20) (Fine Science Tools USA, Inc., Foster City, CA, USA), a stainless steel needle, and pre-cleaned Gold Seal® microscopic glass slides (75 × 25 mm, Becton, Dickson and Company, Lincoln Park, NJ, USA) were wiped with 75% ethanol. A sterile 50-mL beaker holding the lysis solution and sterile tissue papers were used for washing and cleaning the tips of forceps before each transfer. A droplet of the dye solution was placed on a glass slide, an intact fresh anther was placed in the dye solution with a pair of forceps, and the wall of the anther was broken open to release the pollen grains into the dye solution using the dissecting microscope at a 63-fold magnification. The slide was then incubated at 28 °C in a petri dish with lid on for 20–40 min; thereafter, the slide was taken out of the dish and the dye solution was drawn to another area on the slide with the pair of forceps. Pollen grains were repeatedly washed in 15 different drops of sterile sucrose solution (Parducci et al. 2005). The pollen population size in the viewing field could be adjusted easily by drawing the solution away. Pollen grains were left to dry completely at the end.
The micromanipulator used was a three dimensional adjustable device. The glass micropipettes made by the Micropipette Puller tended to be fragile during the course of separating a single pollen grain and transferring the pollen into a PCR tube. Using this device, the overall speed was very slow. Contrarily, the DUMONT forceps had a pair of fine tips of 0.01 mm size and could be used directly to separate single pollen grains with a diameter greater than 0.01 mm from many species (Figure 1). Under a 63-fold magnified viewing field, the pollen collector could clearly see the pollen grains on the slide's surface. The pick-up of stained single pollen grains was quick and reliable and the single pollen grains could be dislodged easily from the fine tips of the forceps into the lysis solutions located at the bottoms of PCR tubes or wells of a 96-well PCR reaction plate by opening and closing the forceps tips two or three times. The tips were re-checked under the dissecting microscope prior to the next transfer to ensure pollen grains had been deposited into the lysis solutions. On average, a collector could pick up 60 pollen grains in an hour.
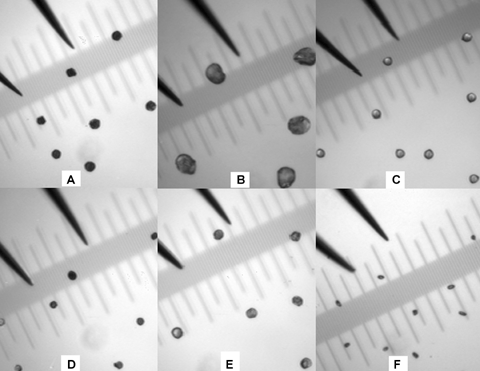
Size comparisons between pollen grains and tips of a pair of DUMONT forceps.(A) Sugarcane; (B) corn; (C) snap bean; (D) Mischanthus spp.; (E) sorghum; and (F) tomato. Ruler: OLYMPUS Objective Micrometer OB M 1/100 (a grid represents 0.01 mm).
Optimal concentration of lysis solution
All of the concentrations (0.025 M, 0.05 M, 0.1 M, 0.15 M, or 0.20 M) of sodium hydroxide (NaOH) or potassium hydroxide (KOH) in the lysis solutions tested produced PCR products. However, clearer bands without background were observed at both 0.1 M and 0.15 M, which was consistent with the findings of Xin et al. (2003). To verify this, lysis solutions with NaOH at 0.1 M were tested on single pollen grains collected from all four elite sugarcane clones. After a 17 min 30 s preheating treatment, PI/PII-primed PCR produced desirable products from the pollen samples of all four clones (Figure 2, lanes 1 to 36). Contrarily, all pollen sample tubes that were pre-heated with 1 μL water did not produce any PCR product (data not shown). Neither were there any DNA products from the negative control tubes that had 1 μL lysis solutions without a pollen grain (Figure 2, lanes 1, 10, 19, 28, and 37; Figure 4, lanes 1, 3, 7, 11, 15, and 19). Evidently, the lysis/tris-ethylenediamine tetraacetic acid (TE) solutions played an important role on the lysis of pollen grains, and the lysis function was not variety-specific. The lysis of pollen grains with KOH offers a new option for preparation for PCR.
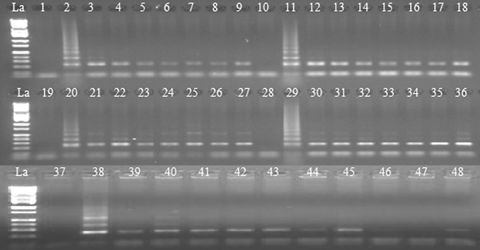
Ethidium bromide-stained polymerase chain reaction (PCR) products following amplification with primers PI and PII from individual sugarcane pollen grains.Lane designations: La (DNA ladder, Catalog #BN2050). Lanes 3–9 (clone L 03-378), 12–18 (clone HoCP 03-718), 21–27 (clone HoCP 02-618), and 30–36 (clone TCP 99-4474), PCR performance of single pollen grains under optimized conditions. Lanes 39–43, PCR performance of stained pollen grains (L 03-378). Lanes 44–48, PCR performance of unstained pollen grains (L 03-378). Lanes 1, 10, 19, 28, and 37, negative (H2O) controls. Lanes 2, 11, 20, 29, and 38, positive controls (total DNA of L03-378, HoCP03-718, HoCP02-618, TCP99-4474, and L03-378, respectively).
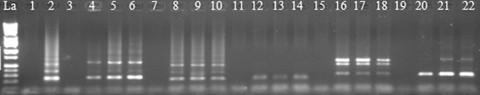
Ethidium bromide-stained polymerase chain reaction (PCR) products following amplification with primers PI and PII from single pollen grains of five plant species.Lane designations: La (DNA ladder, Catalog # BN2050). Lanes 1, 3, 7, 11, 15, and 19, negative (H2O) controls. Lane 2, positive control (HT-DNA of L03-378). Lanes 4–6, corn. Lanes 8–10, sorghum. Lanes 12–14, Miscanthus spp. Lanes 16–18, snap bean. Lanes 20–22, tomato.
Additives and performance of single pollen grain PCR
To prevent volume loss of the lysis solution during lysis at a high temperature, a 5-μL droplet of mineral oil was dispensed into each well that contained 1.0 μL lysis solution and a single pollen grain. The controls were the same except for the absence of mineral oil. PI/PII-primed PCR reactions were carried out and products were visualized by agarose gel electrophoresis. Samples with mineral oil presented clear bands (Figure 2, lanes 1 to 36); samples without mineral oil gave either faintly stained or no products at all (data not shown). Some substances like NaOH, Tween-20, and those from the pollen wall might have had adverse effects on PCR. As a stabilizing agent of Taq DNA polymerase, bovine serum albumin (BSA) is often a choice in amplifying DNA samples of lower purity (Al-Soud and Rädström 2000; Xin et al. 2003). Another polymer, polyvinyl pyrrolidone (PVP), has been reported to prevent Taq DNA polymerase from inhibition by compounds present in crude DNA preparations (Xin et al. 2003). We added 0.1% BSA and 1% PVP in the PCR mixture for PI/PII-primed amplification. There were no apparent differences from controls with absence of both BSA and PVP. However, considering the chemical substances presented in lysed pollen mixtures, it is advised that pollen PCR reactions be conducted in the presence of BSA.
Holding time in the initial step of PCR and pre-heating during lysis and number of cycles had an effect on PCR performance
Several holding times for the initial step of PCR (pre-denaturation of DNA template) were tested based on PI/PII-primed PCR. The results indicated that a 5 min pre-denaturation was the best for single pollen grain PCR because it contributed to more abundant PCR products, which is important when there are several DNA products from one PCR reaction such as those from randomly amplified polymorphic DNA (RAPD). The 5-min pre-denaturation is different from the PCR program by Xin et al. (2003), which required a 15-min pre-denaturation. Generally, Taq enzyme activity decreases when it is kept at 95 °C and the half-life for the Taq DNA polymerase is 40 min at 95 °C (Roche Applied Science 2005/2006). Obviously, single pollen grain PCR is sensitive to the holding time at 95 °C since more cycles and longer times were involved in the amplification process.
Single pollen grains are haploids that have thick walls; hence the holding time of the pre-heating during lysis and number of PCR cycles may be crucial to PCR performance. Based on the results from PI/PII-primed PCR reactions, the best pollen PCR performance was observed with the 17 min 30 s preheating during lysis and 40 cycles of PCR (Figure 2, lanes 1 to 36). These lanes showed clearer PCR products from the single pollen grains of different sugarcane varieties, indicating that the pollen grains had been lysed in 17 min 30 s and that the pollen DNA had been properly amplified after 40 cycles. None of the other combinations of holding times during lysis and number of PCR cycles produced better results.
Effect of viability of pollen grains on PCR
Some pollen grains appeared blue after staining; others were not stainable and appeared yellow. Since viable or potentially viable pollen grains absorb the dye while non-viable pollen grains do not (Pline et al. 2002), we purposely picked up the blue pollen grains, which usually were round-shaped. The yellow pollen grains, on the other hand, looked triangle-shaped and turned into flakes when picked up with a pair of forceps. Upon PI/PII-primed PCR amplification, blue stained pollen grains produced DNA products (Figure 2, lanes 1 to 43) in contrast to those yellow unstained pollen grains (Figure 2, lanes 44 to 48). The results clearly show that viable pollen grains contain DNA and that the viability of pollen grains is a major factor contributing to PCR success. To avoid pollen germination, a high concentration of aniline blue (0.7 mM) in 30 mM sucrose solution was selected to achieve a quick staining.
Reproducibility and applications of the method on other species and PCR programs
Consistent results were produced from the reproducibility experiments conducted by a different experimenter on three different dates (Figure 3). PI/PII-primed PCR products were amplified from leaf tissue (positive controls) (Lane 12), eight of 10 (top panel), eight of 10 (middle panel), and 10 of 10 (bottom panel) single pollen grains (Lanes 1 through 10). There were products from the negative controls (Lane 11). The failure to show PCR products by two of 10 single pollen grains in the top and middle panels was likely due to unsuccessful transfer of the single pollen grains during transfer from the forceps to the PCR tubes. To test the general applicability of this HT pollen PCR procedure, we also carried out PI/PII-primed PCR on single pollen grains collected from five other species, namely, corn, sorghum, Miscanthus, snap bean, and tomato, which happened to be available during the course of the study. All of the single pollen grains from these species produced PI/PII-primed PCR bands indicating successful pollen lyses (Figure 4). The method also produced PCR-amplified DNA fragments from single pollen grains of sugarcane clone HoCP 02-618 with RAPD- and simple sequence repeats (SSR) primers (Figure 5). A larger extent of genetic variations was also observed among the RAPD DNA products from the pollen grains tested. To our knowledge, this is the first molecular demonstration that individual pollen grains inherit different RAPD markers from their donor parent. We believe that pollen grains are good alternative material for genetic diversity and phylogeny studies among different plant species. Furthermore, some pollen grains produced the same RAPD markers (Figure 5, lanes 7 vs. 21; lanes 13 vs. 14), which implied the potentiality of this method in determining the frequency of each pollen genotype from the same donor. When we tested the effectiveness of NaOH- or KOH-based lysis solution on pollen RAPD-PCR, no difference was found (Figure 5, lanes 17 to 32).
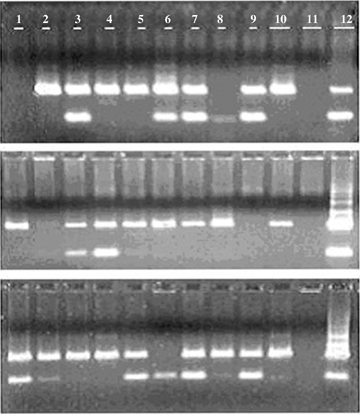
Ethidium bromide-stained polymerase chain reaction (PCR) products following amplification with primers PI and PII from 10 single pollen grains of sugarcane clone LCP 85-384 (Lanes 1 through 10), negative control (reagents only, Lane 11), and positive control (HT-DNA from sugarcane leaf tissue, lane 12).The top, middle, and bottom gel photos were taken from three reproducibility experiments.
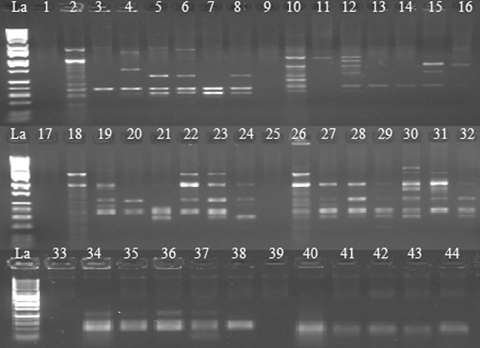
Ethidium bromide-stained polymerase chain reaction (PCR) products following amplification with randomly amplified polymorphic DNA (RAPD) or simple sequence repeat (SSR) primers from single pollen grains of HoCP02-618.Lane designations: La (DNA ladder, Catalog # BN2050). Lanes 1–8, RAPD-PCR performance with primer OPBE-04. Lanes 9–16, RAPD-PCR with primer OPA-17. Lanes 17-32, comparisons of RAPD-PCR performance using different lysis solutions (17 to 24, KOH-based lysis solution; 25 to 32, NaOH-based lysis solution). Lanes 33–38, SSR-PCR performance with marker SMC336BS. Lanes 39–44, SSR-PCR performance with marker SMC1604SA. Lanes 1, 9, 17, 25, 33, and 39 are negative (H2O) controls and Lanes 2, 10, 18, 26, 34, and 40, positive controls (HT-DNA of HoCP02-618).
The high throughput pollen-PCR procedure is summarized as follows:
- 1
Pipette 1 600 μL distilled water, 200 μL 1M NaOH (or 1M KOH), and 200 μL Tween-20 into a 2.0 mL centrifuge tube and vortex rigorously to make lysis solution.
- 2
Dispense 1 μL lysis solution into the wells of a 96-well pollen collection plate, spin briefly to settle down the lysis solution to the bottom of the wells.
- 3
Pick up under a dissecting microscope (63×) aniline blue-stained single pollen grains from the pollen spread on a glass slide with a pair of special forceps (DUMONT No. 11254-20) (Fine Science Tools USA, Inc., Foster City, CA, USA) and dislodge the pollen into the lysis solution in the well by opening and closing the forceps two to three times.
- 4
Place the forceps back under the microscope to check if the tips are clean. Dip and agitate the tips of forceps in a beaker of lysis solution prior to next pickup.
- 5
Dispense 5 μL mineral oil in each well and spin for 1 min at 1 479g.
- 6
Incubate the pollen samples at 95 °C for 17 min 30 s, cool down to 4 °C, and place on ice.
- 7
Add 1 μL TE buffer to each well, spin for 5 min at 1 479g.
- 8
Add 18 μL PCR master reaction mix to each well, and spin for 5 min at 1 479g.
- 9
Proceed with PCR with an initial step at 95 °C for 5 min, followed by 40 cycles of three temperature changes, final extension at 72 °C for 10 min, and hold at 4 °C.
- 10
Separate amplified PCR products by 1.5% agarose gel electrophoresis and visualize under UV lights by ethidium bromide staining.
Using this high throughput pollen-PCR procedure, one can collect single pollen grains into the wells of three 96-well plates and complete PCR amplifications on a regular 8-h workday.
Discussion
Picking up pollen grains with a pair of DUMONT forceps (#11254-20) has an incomparable merit in that the collection process is much quicker compared with those involving laser-mediated manipulation (Matsunaga et al. 1999), micromanipulator (Aziz and Sauve 2003; Haliyo and Regnier 2003; Aziz et al. 2005), or specifically modified glass micropipettes (Parducci et al. 2005). In addition, the collector can feel the physical characters of pollen grains like hardness and fullness, which is important since these characters contribute to the success of PCR. However, the efficiency of pickup decreased when the diameters of pollen grains were close to or less than that of the tips of the forceps, for example, the pollen grains from tomato plants (Figure 1F). In addition, pollen grains should be dried completely, otherwise they tend to be reshaped by the forceps even if the fine tips of the forceps are flexible. Pollen grains can be used on the same day of collection or can be kept frozen for later use.
The HT pollen PCR procedure worked well with a range of concentrations of alkali/detergent lysis solutions, in conjunction with a 17 min 30 s preheating and an equi-molar neutralization step. Pollen samples that were pre-heated with 1 μL water instead of the alkali/detergent solution did not produce any PCR products. Evidently, the lysis/TE solutions played an important role on the lysis of pollen grains. The lysis function was not variety- or plant species-specific. The pollen grains were either freshly collected or had been frozen. On the contrary, PCRs tended to be more sensitive to the volumes of the lysis solutions with single small pieces of leaf tissue. This might be attributed to the small sizes and cell wall composition of pollen grains. Unlike the leaf tissues that often contain poly-phenolic compounds that are inhibitory to PCR, the wall of the pollen grain in most species is constructed of the intine, the exine, and the surface coating of different chemical compositions. The intine is composed of cellulose and pectin and the exine is composed mainly of sporopollenin, a biopolymer of extremely high chemical, physical, and biological resistance. Although the chemical structure of sporopollenin is not known in detail, it is postulated that sporopollenin is a mixed polymer with a large amount of long-chain aliphatics containing additional compounds such as phenols to varying degrees (Jungfermann et al. 1997; Thom et al. 1998). However, all reported pollen PCR studies indicated that none of the chemical compounds from the pollen wall inhibit PCR.
Our HT single pollen grain PCR procedure could be repeated and worked equally well on all the plant species tested. Using either fresh or frozen pollen samples, two different experimenters reproduced the results in two different years (Figure 3 vs. 1-4 and 5). Although in both cases, some of the single pollen PCR reactions failed to produce any PCR products. This was due to failure of pollen grain transfer from the forceps to the PCR reaction tubes. However, this should not be a problem as long as the pollen samples that produced PCR products had only single pollen. Pollen grains are haploid male gametophytes derived from microspores. Due to chromosome re-assortment and crossover during meiosis, pollen grains are genetically different (except if the plant is a fixed line), each sharing a portion of the total nuclear genome from the same donor plant (Copenhaver et al. 2000). From a statistical point of view, analyzing a large number of pollen grains is crucial for any inheritance or mapping study. In general, Pollen grains are small (Pinar and Inceoglu 1999; Wen and Nowicke 1999; Su and Saunders 2003; Tel-Zur et al. 2003) and only viable from a few minutes to hours after dehiscence in natural conditions (Fritz and Lukaszewski 1989; Khatun and Flowers 1995; Luna et al. 2001). So efficiency in sampling of a large number of single pollen grains relevant to pollen viability is important for genetic analysis. Getting enough pollen grains and releasing DNA from these pollen grains without inhibiting PCR represent the two major challenges in pollen PCR studies.
Contemporary genetic or quantitative trait loci (QTL)/ association mapping studies usually involve population sizes of 50 to 250 individuals (Mohan et al. 1997); however, even larger populations are required for high-resolution mapping (Collard et al. 2005). According to Fang et al. (2001), the mapping population size is about 100 to 150 for crop plants with relatively few chromosomes. In general, the accuracy of the mapping increases with increasing size of the mapping population. Unfortunately, the workload and cost would also increase proportionally. By precisely genotyping a large number of single pollen grains, plant geneticists and breeders would be able to classify different pollen classes, predict the genotypes of female gametophytes (eggs), and simulate the population genetic structure of the future generation. This is particularly important for some aneu-polyploidy species such as sugarcane where classical genetic approaches have been difficult to follow. The HT-pollen collection and PCR method can be applied for these species.
Prior to PCR-based single pollen grain genotyping experiments, one can apply the whole genome amplification technique to obtain a 400–500-fold increase of the initial starting single pollen genomic DNA template (Blanco et al. 1989; Zhang et al. 1992; Esteban et al. 1993). As a result, one may be able to genotype with many DNA markers on any single pollen grain. Therefore, genetic variations by any DNA marker could be analyzed on a large population of single pollen grains for statistical analysis with a considerable cost reduction.
Materials and Methods
Equipment and reagents
Equipment used for this study included a micromanipulator (Model MN-153) and Joystick Manipulator (Model MN-151) from Narishige Co. Ltd. (East Meadow, NY, USA), micropipette glass tubes made on a Flaming/Brown Micropipette Puller, MODEL P-87 (Sutter Instrument Company, Novato, CA, USA), a Carl Zeiss dissecting microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA), a pair of forceps (DUMONT No. 11254-20) (Fine Science Tools USA, Inc., Foster City, CA, USA) and a Finnpipette Digital – MULTICHANNEL PIPETTE 4510 (0.5–10.0 μL) (Thermo Electron Corporation, Milford, MA, USA).
The following chemical solutions were prepared locally using chemical reagents purchased from Sigma-Aldrich (Kansas, MO, USA): 1 M NaOH; 1 M KOH; 20% Tween-20; a TE buffer (0.1 M Tris-HCl, 2 mM ethylenediamine tetraacetic acid (EDTA)); 1% (w/v) BSA; 10% (w/v) PVP-40; dye solution (0.7 mM aniline blue diammonium salt in 30 mM sucrose); sucrose solution (30 mM). Lysis solutions (either NaOH or KOH with a concentration of 0.025 M, 0.05 M, 0.1 M, 0.15 M, or 0.20 M, respectively, plus 2% Tween-20) were freshly made just before use. In addition, mineral oil, 10 mM dNTP mixture, and agarose from Sigma-Aldrich were used directly. ALL PURPOSE Hi-Lo DNA MARKERS were purchased from Bionexus Inc. (Oakland, CA, USA). Taq DNA polymerase, 10× PCR buffer, and 25 mM MgCl2 solution were purchased from Roche Diagnostics Corporation (Indianapolis, IN, USA).
Plant and pollen materials
Partial inflorescences with non-dehisced mature anthers were collected in regular brown paper bags from plants of four elite sugarcane clones (L03-378, HoCP03-718, HoCP02-618, and TCP99-4474), corn (Zea mays L.) cv. Hawaii Supersweet10, sorghum (Sorghum bicolor L.) cv. M 81-E, green bean (Phaseolus vulgaris L.) cv. Derby, and tomato (Lycopersicon esculentum L.) cv. Florida 91. The bagged flower samples were either used directly or stored in a freezer (either –20 °C or –80 °C). A description of collecting individual pollen grains is given in the first paragraph of Results. In the original series of experiments, total nucleic acids were extracted from the leaf tissues of the sugarcane clones according to Pan et al. (2000) and were used as positive controls in PCR reactions. In the reproducibility experiments, the same batch of lysis and neutralization solutions was used to extract total nucleic acids from sugarcane leaf tissues for the positive controls, while negative controls had only the reagents.
Pollen lysis and single pollen PCR
Single pollen grains were placed in individual PCR tubes or wells of a 96-well PCR reaction microplate that contained 1 μL of lysis solution. After the pollen grains were deposited, 5 μL of mineral oil was added. Two sets of tubes were used as negative controls, one set had 1 μL of lysis solution without pollen and the other set of tubes had 1 μL of water with pollen plus the mineral oil. The samples were spun briefly (1 min at 1 479g) before incubation at 95 °C for 15 min, 17 min 30 s, or 20 min to lyse the pollen grains. After lysis, equi-molars of 1 μL TE buffer were added to neutralize the samples, which were then spun briefly.
Polymerase chain reactions were conducted using five DNA markers. The nucleotide sequences (5′ to 3′) of the primers were as follows: the 5S ribosomal RNA intergenic spacers (rRNA-ITS) primers PI (TGGGAAGTCCT(C/T)GTGTTGCA) and PII ((T/G)T(A/C)G(T/C)GCTGGTATGATCGCA) (Cox et al. 1992); two RAPD markers OPBE-04 (CCCAAGCGAA) and OPA-17 (GACCGCTTGT); and two sugarcane SSR markers, SMC336BS (ATTCTAGTGCCAATCCATCTCA as forward and CATGCCAACTTCCAAACAGAC as reverse) and SMC1604SA (AGGGAAAAGGTAGCCTTGG as forward and TTCCAACAGACTTGGGTGG as reverse). The reaction volume of the PI/PII-primed amplification was 20 μL containing 1 μL lysis solution with a single pollen grain, equi-molars of 1 μL TE buffer, 50 mM KCl, 10 mM Tris.HCl (pH 8.3), 3.0 mM MgCl2, 0.2 mM each of dATP, dTTP, dGTP, and dCTP, 0.25 μM of each primer, 0.1% (w/v) BSA and 1 unit of Taq DNA polymerase.
The PI/PII-PCR program was 95 °C for 5 min (or 10 or 15 min during optimization tests), followed by 40 (or 30 or 50 during optimization tests) cycles of 93 °C for 55 s, 60 °C for 10 s, and 72 °C for 40 s, and a final extension for 10 min at 72 °C. The reaction volume of RAPD-PCR was 20 μL containing the same as that of PI/PII-PCR except for 0.25 mM each of dATP, dTTP, dGTP, and dCTP and 0.5 μM primer. The RAPD-PCR program was 95 °C for 5 min, followed by 40 cycles of 93 °C for 40 s, 40 °C for 30 s, and 72 °C for 60 s, and a final extension for 5 min at 72 °C. The reaction volume of SSR-PCR was 20 μL containing the same as that of PI/PII-PCR except for 2.75 mM MgCl2, 0.25 mM each of dATP, dTTP, dGTP, and dCTP, and 0.21 μM of each primer. The SSR-PCR program was 95 °C for 5 min, followed by 40 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s, and a final extension for 10 min at 72 °C.
Polymerase chain reaction amplifications were conducted on GENEAMP® PCR SYSTEM 9700 (Applied Biosystems, Inc., Foster City, CA, USA). PCR products were separated by electrophoreses on 1.5% agarose gels containing ethidium bromide (0.5 μg/mL) in 0.5× TBE buffer and visualized on a UV transilluminator. Gel images were captured on the GEL LOGIC 200 IMAGING SYSTEM (New Haven, CT, USA).
(Handling editor: Chun-Ming Liu)
Acknowledgements
We thank Thomas L. Tew (corn and Miscanthus spp.), Robert Cobill (sorghum), and Eric C. Petrie (snap bean and tomato) for providing access to their garden plants.




