Growing Season Ecosystem Respirations and Associated Component Fluxes in Two Alpine Meadows on the Tibetan Plateau
Supported by Field Station Foundation In the Domain of Resource and Environment, CAS and GEF program (052456 and CHA-GS-Y-4)
Abstract
From 30 June to 24 September in 2003 ecosystem respiration (Re) in two alpine meadows on the Tibetan Plateau were measured using static chamber- and gas chromatography- (GC) based techniques. Simultaneously, plant removal treatments were set to partition Re into plant autotrophic respiration (Ra) and microbial heterotrophic respiration (Rh). Results indicated that Re had clear diurnal and seasonal variation patterns in both of the meadows. The seasonal variability of Re at both meadow sites was caused mainly by changes in Ra, rather than Rh. Moreover, at the Kobresia humilis meadow site (K_site), Ra and Rh accounted for 54% and 46% of Re, respectively. While at the Potentilla fruticosa scrub meadow (P_site), the counterparts accounted for 61% and 39%, respectively. T test showed that there was significant difference in Re rates between the two meadows (t = 2.387, P = 0.022). However, no significant difference was found in Rh rates, whereas a significant difference was observed in Ra rates between the two meadows. Thus, the difference in Re rate between the two meadows was mainly attributed to plant autotrophic respirations. During the growing season, the two meadows showed relatively low Q10 values, suggesting that Re, especially Rh was not sensitive to temperature variation in the growing season. Additionally, Re and Rh at the K_site, as well as Rh at the P_site was negatively correlated with soil moisture, indicating that soil moisture would also play an important role in respirations.
Recent studies have indicated that ecosystem respiration, rather than gross primary production, is often the most critical component determining large-scale spatial and temporal variation in annual net C balance. For example, a study by Vourlitis and Oechel (1999) showed that inter-annual differences in the growing season net CO2 exchange from an arctic tussock tundra ecosystem were almost completely explained by inter-annual differences in ecosystem respiration. Similarly, Valentini et al. (2000) suggested that ecosystem respiration is the driving component of regional differences in net C balance across latitudes in European forests. Moreover, an incomplete understanding of ecosystem respiration has led to the variability of annual grassland net ecosystem carbon dioxide exchange (NEE) estimates (Raich and Potter 1995; Knapp et al. 1998; Wagai et al. 1998). Currently, ecosystem respiration is often separated from NEE flux data through extrapolation of night-time ecosystem respiration (Re) value into daytime (Falge et al. 2002; Xu and Baldocchi 2004; Xu et al. 2004). Despite a number of algorithms being available for the Re partition, all algorithms overestimate or underestimate the Re values (Reichstein et al. 2005). Hence, a chamber-based Re flux measurement is needed not only for the comparison with Re determined from the eddy covariance method, but also for the substitution in cases where no eddy covariance data are available. Moreover, the chamber method could further allow us to separate Re into plant autotrophic respiration and microbial heterotrophic respiration, which could not be obtained from the eddy covariance method. Re measurement and associated component fluxes partition would allow a deeper understanding of ecosystem responses to environmental variability, and contribute to the development of ecosystem carbon cycling models (Running and Coughlan 1988; Aber et al. 1996). So far, although the chamber method can cause a certain disturbance on the soil-plant system, it has been widely used for flux measurement in terrestrial ecosystems worldwide due to its easy operation and low cost (Norman et al. 1992; Dugas et al. 1997; Law et al. 1999, 2001; Dong et al. 2000; Wang et al. 2000, 2003; Angell et al. 2001; Dore et al. 2003; Wang and Wang 2003; Bolstad et al. 2004; Zou et al. 2004; Chimner and Welker 2005; Grogan and Jonasson 2005).
The Tibetan Plateau, which extends over 2.5 million km2, is the youngest and highest plateau in the world. Evidence has indicated that the plateau is experiencing climatic warming (Thompson et al. 1993; Wang and French 1994). Moreover, the Plateau is predicted to undergo even greater increases in surface temperatures in the future (Giorgi et al. 2001). Alpine meadows, including Kobresia humilis meadow, and Potentilla fruticosa scrub meadow, covered about 35% of the plateau area. Previous studies on carbon cycling in these grassland ecosystems have contributed greatly to our understanding of the Tibetan Plateau response to future climate change. However, most studies in this vast Tibetan grassland area were focused on soil respirations (Cao et al. 2001, 2002, 2004; Zhang et al. 2001), and annual NEE (Gu et al. 2003; Kato et al. 2004a, 2004b; Xu et al. 2004, 2005; Zhao et al. 2005a, 2005b). Information on ecosystem respiration and its component fluxes partition is not available. Consequently, there still exist large gaps in our understanding of the processes and mechanisms of carbon dynamics in these grasslands. To fill the gaps and better understand these carbon processes, we have attempted to set different treatments in two alpine meadows to investigate the ecosystem respiration and associated component fluxes. The objectives of this study are as follows: (i) to compare the difference in ecosystem respiration in the two meadows; (ii) to partition ecosystem respiration into plant autotrophic respiration and soil heterotrophic respiration; and (iii) to analyze the effect of temperature and moisture on carbon fluxes.
Results
Diurnal variation of ecosystem and heterotrophic respirations
Both Re and heterotrophic respiration (Rh) rates of two meadow sites varied with air temperatures (Figure 1). Changes in Re diurnal pattern were strongly correlated with changes in air temperature (P < 0.01). By contrast, changes in the Rh diurnal pattern were not as clear as Re, but still significantly correlated with air temperature (P < 0.05). The maximum Re and Rh rates occurred between approximately 13.00 and 15.00 hours, when air temperature reached the maximum, whereas the minimum mostly occurred in deep night between 01.00 and 04.00 hours. With respect to variation amplitude, both Re and Rh at the K. humilis meadow site (K_site) showed relatively larger amplitude than that at the P. fruticosa scrub meadow (P_site). For example, on diurnal flux day in July, the coefficients of variation for Re and Rh at the K_site were 37% and 36%, respectively. The counterparts at the P_site were 26% and 19%, respectively. When a whole day was separated into daytime and night-time, the mean daytime Re rates of the K_site are 1.62 and 1.44 times higher than that in the night-time on the two diurnal flux days, respectively. Meanwhile, the mean daytime Rh rates are 1.72 and 1.50 times higher than that in the night-time, respectively. By contrast, at the P_site, the ratio of mean daytime to night-time Re rates was 1.36, 1.48 respectively, with a ratio of 1.2 for daytime to night-time Rh rates on both diurnal flux days. Additionally, at the P_site, the diurnal flux day maximum air temperature in September was 25.1 °C, much higher than that of 19.7 °C in July. However, the maximum and daily mean Re rates were lower than that in July, while Rh rates were similar on the two diurnal flux days.
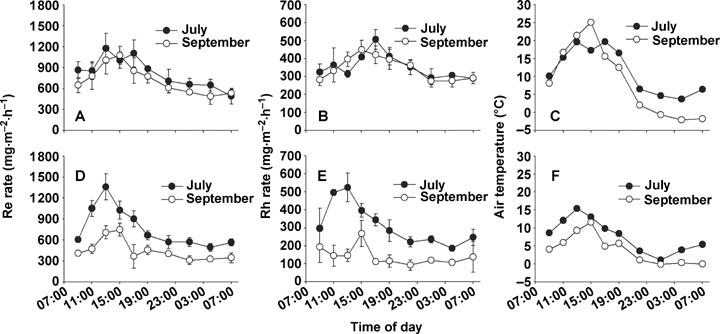
Diurnal variation of ecosystem respiration, heterotrophic respiration, and air temperature in Potentilla fruticosa scrub meadow (A, B, C) and Kobresia humilis meadow (D, E, F).Re, ecosystem respiration; Rh, heterotrophic respiration. Error bars indicate standard deviation.
Monthly variation of ecosystem and heterotrophic respirations
Re rates of the two meadow ecosystems showed a clear monthly variation trend, increasing from mid-growth stages in July to exuberance period in August. When the plants enter the senescence period in September, ecosystem respiration rates declined (Figure 2). The maximum Re rate (917.89 mg CO2· m−2·h−1) occurred on 7 August at the K_site and on 29 July (1 418.19 mg CO2· m−2·h−1) at the P_site, respectively. Differing from Re, Rh rates showed a different variation trend between the two meadow ecosystems. Rh showed a relative clear variation with growth period at the K_site. Whereas, Rh maintained similar emission rates and showed little variation at the P_site (Figure 2).
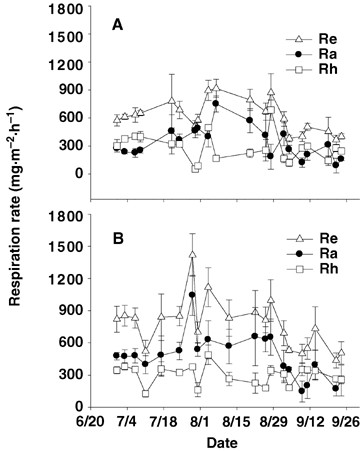
Monthly variation of respiration in Kobresia humilis meadow (A) and Potentilla fruticosa scrub meadow (B).Ra, autotrophic respiration; Re, ecosystem respiration; Rh, heterotrophic respiration. Error bars indicate standard deviation.
Plant autotrophic respiration
Autotrophic respiration (Ra) of the two meadows showed a similar variation trend with Re. It increased from growth period of July to August, then decreased in the plants senescence period in September (Figure 2). During the flux measurement periods, the mean Re of the K_site and the P_site were 614.19 mg CO2·m−2·h−1 and 770.71 mg CO2·m−2·h−1, respectively. Meanwhile, the mean Rh of both meadows was 281.44 mg CO2·m−2·h−1 and 298.95 mg CO2·m−2·h−1, respectively. The mean Ra was 332.75 mg CO2·m−2·h−1 and 471.76 mg CO2·m−2·h−1, respectively. At the K_site, plant autotrophic respiration and soil microbial heterotrophic respiration accounted for 54% and 46% of the ecosystem respiration, respectively. By contrast, At the P_site, the counterparts accounted for 61% and 39%, respectively.
Discussion
Explanation for seasonal pattern of Re
Ecosystem respiration is the sum of Ra and Rh. Thus, Re variability would be caused either by changes in Ra or in Rh, or Ra and Rh simultaneously. In this short-term study, Rh at the K_site showed small variations, while Rh at the P_site showed almost no changes. In addition, Rh contributed 46% to Re at the K_site, while the counterparts at the P_site were only 39%. Hence, the seasonal Re variability at both sites would be caused mainly by changes in Ra, rather than Rh. During the growing season, especially from July to August, alpine plants are favored by plentiful solar radiation, sufficient water availability, and favorable temperatures. Therefore, plant autotrophic respiration from growth and maintenance increased rapidly. Meanwhile, due to the optimal soil temperature and moisture for microbial activities in this period, the heterotrophic respiration of organic carbon decomposition maintained a relatively high level. Consequently, Re showed a rapid increase from July to August. Subsequently, we observed the maximum Re rate on 7 August at the K_site, and on 29 July at the P_site, respectively, corresponding to the optimal plant growth period for the alpine meadows (Kato et al. 2004b). However, in the plants senescence period, despite no significant decline in Rh, due to the reduction of demand in growth and maintenance respiration, Ra reduced, leading to Re decline in September. This is also the reason why the mean and maximum Re rates on the diurnal flux day in September were lower than that in July, despite the temperature on the diurnal flux day in September being higher than that in July.
Re, Rh, Ra differences between two meadow sites
In two subarctic vegetation types, despite the substantial differences in soil temperature, plant biomass as well as soil organic carbon pools between sites, Grogan and Jonasson (2005) reported that there was no overall significant difference in ecosystem respiration rates through the annual cycle between vegetation types. In our study, the mean biomass of the K_site and the P_site from July to September in 2002 was very comparable (Li et al. 2003). At the same sites, Zhao et al. (2005b) also showed a very similar biomass of 1 615.65 g/m2 at the K_site and 1 745.85 g/m2 at the P_site in August 2003. In addition, our previous investigation indicated that both meadows had similar soil organic carbon and total nitrogen content (Table 1). Plant autotrophic respiration was derived from its recently fixed carbon, which was highly associated with biomass. While soil organic carbon served as the substrate of microbial heterotrophic respiration. Due to the similar biomass and soil organic carbon content between the two meadow sites, we had thought that the two sites would have similar Re rates. However, our short-term growing season flux data showed that the two sites differed significantly in Re rates (t = 2.387, P = 0.022). For the monthly Re difference, July and September exerted a larger difference than that in August (Figure 3). When partitioning Re into Rh and Ra, the two sites showed no significant difference in Rh rates (t = 0.451, P = 0.654). Consequently, these Re rate differences between the two sites were mainly due to plant autotrophic respiration rates (t = 2.349, P = 0.024). A study conducted in the same two meadow sites with an eddy covariance (EC) method from 1 July 2003 to 30 June 2004 showed that the annual NEE was 282 and 53 g CO2·m−2·a−1 for the K_site and the P_site, respectively. The authors attributed the discrepancy in NEE to the different soil respiration rates and photosynthetic carbon fixation capacity (Zhao et al. 2005b). Given the similar Rh respiration rates for the two meadows, we surmise this difference would be mainly caused by the differences in plant photosynthetic carbon fixation capacity and autotrophic respiration.
| Characteristics | K. humilis meadow | Scrub meadow | |
|---|---|---|---|
| Dominant species | K. humilis | Potentilla fruticosa | |
| Canopy height (cm) | 25−30 | 50−70 | |
| Soil type | Mat Cry-Gelic Cambisols | Mollic-Gryic Cambisols | |
| Soil pH (0−40 cm) | 8.13 | 7.7 | |
| Soil bulk density (0−40 cm · g−1· cm−3) | 1.01 | 0.82 | |
| 0−10 cm | 5.49 | 5.66 | |
| Soil organic carbon (%) | 10−20 cm | 3.29 | 3.71 |
| 20−30 cm | 2.67 | 3.05 | |
| 30−40 cm | 1.88 | 2.59 | |
| 0−10 cm | 0.50 | 0.46 | |
| Total nitrogen (%) | 10−20 cm | 0.33 | 0.39 |
| 20−30 cm | 0.27 | 0.32 | |
| 30−40 cm | 0.21 | 0.25 | |
| Mean air temperature (°C) | 7.0* | 6.8* | |
| Mean soil surface temperature(°C) | 10.5* | 8.9** | |
| Mean soil temperature at 5 cm depth (°C) | 10.0* | 7.8** | |
| Mean soil moisture (%) | 39.1* | 40.2* |
- Temperature and moisture were characteristics of the flux measurement period. *P < 0.05 using a t test.
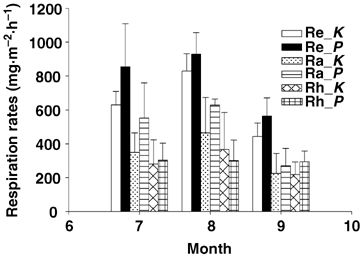
Monthly mean respiration rate in Kobresia humilis meadow (K) and Potentilla fruticosa scrub meadow (P) from July to September.Ra, autotrophic respiration; Re, ecosystem respiration; Rh, heterotrophic respiration. Error bars indicate standard deviation.
Recent findings from soil warming experiments have shown that the response of soil CO2 efflux to one step increase in temperature declined over time (McHale et al. 1998; Luo et al. 2001; Melillo et al. 2002). This decline was generally attributed to the changes of substrate availability. In the present study, as a result of the onset of the plant removal treatment, there was no longer plant litter or residues or even root exudates input into the soil, which would lead to the decline of substrate availability. Due to increasing reductions of substrate availability and unfavorable temperatures for microbial activity, Rh rates should decline gradually. We observed small Rh variations at the K_site. However, Rh maintained a relative stable level and showed little variation at the P_site, which is surprising. Probably, in such a short-term period, the labile soil organic carbon pool would never be exhausted. Hence, the lower response of soil microbial activities to temperature variation in the growing season may account for the results. Nevertheless, when using bare soil CO2 flux measurements to estimate the microbial respiration, Dugas et al. (1999) also reported a relative constant value of 162 mg CO2·m−2·h−1 Rh rate in the growing season.
Effect of temperature on respirations
Temperature is a major control of soil and ecosystem respiration rates (Raich and Schlesinger 1992; Lloyd and Taylor 1994; Raich and Potter 1995; Fang and Moncrieff 2001). In our study, the overall respiration rates showed significant positive correlations with air temperature and soil surface temperature, as well as soil temperature at 5 cm depth (P < 0.05). However, the importance of temperature on respiration rates varied with vegetation types and treatments. For example, air temperature can explain 80% and 43% variability of Re and Rh rates at the K_site, respectively. Whereas at the P_site, air temperature explained 54% and 37% variability of Re and Rh rates, respectively.
In addition, we calculated the classic first-order equation coefficients of A and B for our flux data related to air temperature (respiration rate = Aexp(BT)). Since the coefficient A represents an index that integrated both the chemical quality and the amount of substrate that is available for respiration. Whereas, coefficient B reflects the response of respiration to temperature variation (Grogan and Jonasson 2005). As a result, we found coefficient A value at the P_site was 1.5 times higher than that at the K_site, which means that the P_site has much larger potential substrate supply for respiration than the K_site (Figure 4). With respect to the temperature sensitivity of Re and Rh, we used Q10 instead of coefficient B, since Q10 is also determined by coefficient B. Q10 values were 2.0 and 1.4 for the K_site and the P_site, respectively. The Q10 value of 1.3–3.3 has been reported in previous studies (Raich and Schlesinger 1992; Tjoelker et al. 2001). By contrast, the Q10 value of the P_site was close to the minimum of this range, whereas the Q10 value of the K_site approximately fell into the mid range. The overall Q10 values for the two meadow sites were relatively low. This is partly due to the short-term observation period. In addition, respirations would have different sensitivity to temperature variation in different periods (Xu and Qi 2001; Janssens and Pilegaard 2003). Consequently, the Q10 values in this short term study would not allow us to conclude that respirations in alpine meadows are not sensitive to increasing climate warming.
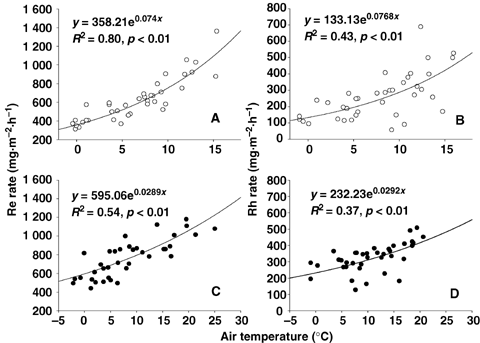
Relationship between respiration rate and air temperature in Kobresia humilis meadow (A, B) and Potentilla fruticosa scrub meadow (C, D).Re, ecosystem respiration; Rh, heterotrophic respiration.
Effect of soil moisture on respirations
Soil moisture is another variable that affects the rate of respiration. For example, Flanagan and Johnson (2005) reported that soil moisture was the dominant environmental factor that controlled seasonal and inter-annual variation of Re in a northern temperate grassland when variation in temperature was held constant. Moreover, numerous studies have indicated that respiration rates were positively related to soil moisture. When soil moisture increased owing to a rain event or wet growing season, they observed a significant increase in respiration rates (Xu and Qi 2001; Reichstein et al. 2002a, 2002b; Xu and Baldocchi 2004; Chimner and Welker 2005). The favorable soil moisture for the soil microbial activity may be responsible for those results. Since both laboratory experiments (Griffiths and Birch 1961; Orchard and Cook 1983) and field measurements (Liu et al. 2002; Rey et al. 2002) have indicated that with the variation of soil moisture, there exists a point at which microbial activity is enhanced or inhibited, leading to increased or decreased microbial respiration.
In our alpine meadow, due to the plentiful precipitation in the growing season, the soil maintained relatively high moisture. For instance, during the flux measurement days, mean soil volumetric water content at the K_site and the P_site were approximately 39.1% and 40.2%, respectively. Therefore, microbial activity may not be affected by soil moisture. On the contrary, our results indicated that Re rates of the K_site and Rh rates of the two meadows showed significant negative correlation to soil moisture (Figure 5). Additionally, when soil moisture reached the maximum due to the rain events of before the flux day, we observed the minimum Rh rate of 54.90 mg CO2·m−2·h−1 and the associated minimum Re rate of 517.72 mg CO2·m−2·h−1 in July at the K_site. We think this result was caused not only by inhibited microbial activity due to the overabundant soil moisture, but also by the pathway block for CO2 transport due to the over soil water content.
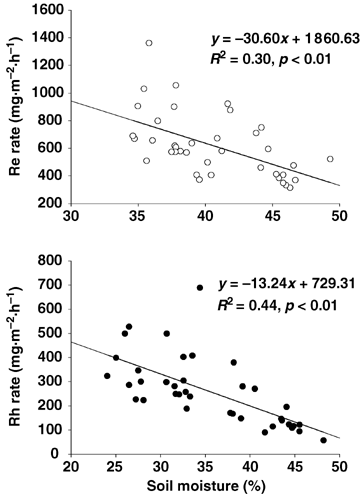
Relationship between respiration rate and soil moisture in Kobresia humilis meadow.Re, ecosystem respiration; Rh, heterotrophic respiration.
Materials and Methods
Site description
The present study was conducted at a Kobresia humilis meadow site (K_site, 37°36′N, 101°20′E, 3 250 m above sea level (a.s.l.)) and a Potentilla fruticosa scrub meadow site (P_site, 37°36′N, 101°18′E, 3 250 m a.s.l.), which were the long term ecological observation sites in Haibei research station of the alpine meadow ecosystem, the Chinese Academy of Sciences. The K_site was dominated by K. humilis, Festuca ouina, Elymus nutans, Gentianna farreri, and Poa sp., with over 95% vegetation coverage. The soil at the K_site is classified as Mat Cry-Gelic Cambisols (Chinese Soil Taxonomy Research Group 1995). The P_site was dominated by Potentilla futicosa, with herbage of K. capillifolia, K. humilis, Saussurea superba and Potentilla nivea interspersed among scrubs. The soil is classified as Mollic-Gryic Cambisols. The mean annual air temperature is –1.7 °C, with a maximum of 27.6 °C and minimum of −37.1 °C. The annual precipitation ranges from 426 mm to 860 mm, 80% of which falls in the growing season from May to September (see also Zhao et al. 2005b for more information on the study sites). The main characteristics of the two meadow sites are shown in Table 1.
Experimental design
On 20 June 2003, ecosystem (Re) and microbial heterotrophic respiration (Rh) treatments were set at the K_site and the P_site, respectively. The sample plots for Re treatments were maintained with natural vegetation coverage, whereas for the Rh treatments, aboveground plants were cut off completely, then the belowground plant live roots were removed at 10 cm soil depth as units from surface to 50 cm. Then root-removed soils were refilled in reverse order of root removal. The area of each sampling plot was designed as 0.25 m2 (50 cm length × 50 cm width). However, a relative larger area than the sample plot needed was made against root encroachment. Additionally, triplicates were randomly set for each treatment.
Gas flux measurements started 10 d after the finish of plant removal treatments, because the soil profile need re-equilibration to minimize the effect of disturbances from root exclusion. Although disturbance could never be completely eliminated, the root removal method was considered available for the measurement of microbial respiration (Rh) (Hanson et al. 2000).
Gas sampling and analysis
Carbon dioxide fluxes were measured using static chamber-gas chromatography (GC) techniques (Wang and Wang 2003) from 30 June to 24 September 2003. The sample chamber was made of thin stainless steel, comprising three parts: top-chamber, mid-chamber and base-chamber, respectively. The top chamber (50 × 50 × 50 cm) was equipped with two fans inside the top, which was driven with a 12-V direct currency to make turbulence in the enclosure. Both the mid-chamber (50 × 50 × 50 cm) and the base-chamber (50 × 50 × 20 cm) have a groove on the upper end. When gas sampling, the grooves were filled with water to avoid gas exchange inside and outside of the chamber. In this study, the mid-chamber was only used for gas sampling in the scrub plots due to its height. Before sampling, the base-chamber was inserted into soil, and during the period of experiments, the base-chamber was not taken out to avoid soil disturbance. Gas sampling was carried out with a 100-mL syringe, at an interval of 0 min, 10 min, 20 min and 30 min, and the sampling frequency was once or twice a week, at times ranging from 09.00 to 11.00 hours. In addition, an intensive flux measurement was conducted at an interval of 2 h in the daytime and 3 h at night-time in July and September to determine the diurnal variation pattern of respirations. Gas samples were stored in the syringes equipped with gas-tight stoppers for a few hours before they were analyzed with the GC installed in the laboratory of the station. The GC configurations for analyzing CO2 and the methods for calculating the flux of CO2 were the same as those described by Wang and Wang (2003).
When gas sampling, simultaneously, ambient air temperature, soil temperature (0 cm, 5 cm), and headspace air temperature of the sample chambers were measured by a portable thermometer (JM624), and soil water content (0–10 cm) was determined by a moisture meter (Time-domain reflectometer, Campbell Scientific, Inc., North Logan, UT, USA).
Ra calculation
We calculated plant autotrophic respiration (Ra) based on the expression as follows: Ra = Re – Rh, where Re is ecosystem respiration, and Rh is microbial heterotrophic respiration.
Statistical analyses
Independent-samples T test was used to compare the differences in mean Re, Rh and Ra rates between the two meadow ecosystems. Linear and exponential regression models were used to describe the relationships between respirations and soil moisture as well as temperature. All statistical analyses were carried out using the SPSS 10.0 software package (SPSS Inc., Chicago, IL, USA).
(Handling editor: Ming Dong)
Acknowledgements
The authors thank Dr Ming-Hua Song and Xing-Liang Xu for their valuable suggestion on the manuscript.




