A new phytoplasma associated with little leaf disease in azalea: multilocus sequence characterization reveals a distinct lineage within the aster yellows phytoplasma group
Abstract
An azalea little leaf (AzLL) disease characterised by abnormally small leaves, yellowing and witches'-broom growth symptoms was observed in suburban Kunming, southwest China. Transmission electron microscopic observations of single-membrane-bound, ovoid to spherical bodies in phloem sieve elements of diseased plants and detection of phytoplasma-characteristic 16S rRNA gene sequence in DNA samples from diseased plants provided evidence linking the disease to infection by a phytoplasma. Results from restriction fragment length polymorphism, phylogenetic and comparative structural analyses of multiple genetic loci containing 16S rRNA, rpsS, rplV, rpsC and secY genes indicated that the AzLL phytoplasma represented a distinct, new 16Sr subgroup lineage, designated as 16SrI-T, in the aster yellows phytoplasma group. The genotyping also revealed that the AzLL phytoplasma represented new rp and secY gene lineages [rp(I)-P and secY(I)-O, respectively]. Phylogenetic analyses of secY and rp gene sequences allowed clearer distinctions between AzLL and closely related strains than did analysis of 16S rDNA.
Introduction
Phytoplasmas are phloem-inhabiting, insect-transmitted cell wall-less bacteria responsible for numerous plant diseases worldwide (Lee et al., 2000; Weintraub & Beanland, 2006; Hogenhout et al., 2008). Possessing small genomes (Marcone et al., 1999; Hogenhout & Šeruga-Musić, 2010) with a unique architecture featuring repetitive genes clustered in sequence-variable mosaics shaped at an early stage of their evolution (Jomantiene & Davis 2006; Jomantiene et al., 2007; Wei et al., 2008a), phytoplasmas have limited metabolic capabilities and rely on their insect and plant hosts for nutrient acquisition (Hogenhout et al., 2008). Achievement of this intimate transkingdom parasitism through coevolution of phytoplasmas with diverse plant and insect hosts likely gave rise to extensive biodiversity among phytoplasmas. Biodiversity of phytoplasmas has long been recognised based on the geographic origins of the diseases they cause, on the identity of specific plant hosts and insect vectors and on symptoms exhibited by diseased plants (Freitag, 1964; Granados & Chapman, 1968; Chiykowski & Sinha, 1989; McCoy et al., 1989). Early insights into genetic interrelationships among phytoplasma strains were obtained from serological and nucleic acid hybridization studies, and phytoplasmas were differentiated into distinct serological groups and genomic strain clusters (Lin & Chen, 1985; Lee & Davis, 1988; McCoy et al., 1989; Kuske et al., 1991; Harrison et al., 1992; Davis & Sinclair, 1998). Knowledge on evolution of the phytoplasma clade and phylogenetic relationships among diverse phytoplasma strains began to accumulate rapidly after the establishment of two major phytoplasma classification schemes.
One scheme is based on phylogenetic analysis of 16S rRNA gene sequences (Kuske & Kirkpatrick, 1992; Namba et al., 1993; Gundersen et al., 1994; Schneider et al., 1995; Seemüller et al., 1998); the other is based on restriction fragment length polymorphism (RFLP) analysis of polymerase chain reaction (PCR)-amplified 16S rRNA gene fragments (Lee et al., 1993, 1998, 2000; Schneider et al., 1993). Although both schemes enable classification of phytoplasma strains into major groups, the RFLP analysis-based scheme has the further advantage of differentiating distinct phytoplasma subgroup lineages within a major group. The power of the RFLP-based classification scheme has been augmented by the recent introduction of virtual RFLP analysis, raising the number of recognised 16Sr groups to 30 (Lee et al., 2000; Wei et al., 2007, 2008b; Zhao et al., 2009a, 2010).
With more and more epidemiological studies being conducted around the world, it has been found that many biologically or ecologically distinct phytoplasma strains possess highly similar 16S rRNA gene sequences and cannot be readily differentiated by analysis of the 16S rRNA gene alone (Lee et al., 2010b). For differentiation of these strains, additional molecular markers have been explored. Among the additional markers, rp, secY, tuf and vmp1 loci have been successfully used for finer differentiation of closely related strains (Lee et al., 2006; Arnaud et al., 2007; Martini et al., 2007; Fialováet al., 2009; Pacifico et al., 2009; Murolo et al., 2010). Application of multilocus analysis holds promise for improved distinctions between closely related strains and enhanced descriptions of diverse phytoplasmas.
Recent studies unveiled a widespread occurrence of phytoplasmal diseases and a remarkable genotypic diversity of phytoplasmas in China (Kuai et al., 2000; Cai, 2007), especially in the southwest province of Yunnan, where 19 phytoplasmal diseases have been reported and phytoplasma strains belonging to no less than five 16Sr groups and 12 subgroup lineages have been identified (Cai, 2007; Cai et al., 2008). The complex physiognomic landscape, dynamic climate pattern and plant and insect species-rich ecosystem of the Yunnan Province are thought to provide superb ecological niches for phytoplasma adaptation, evolution and genetic diversity (Cai, 2007; Cai et al., 2008). In 2009 and 2010, azalea (Rhododendron spp.) plants showing symptoms of little leaf, yellowing and witches'-broom growth were observed in suburban Kunming, the capital city of Yunnan. The disease was designated as azalea little leaf (AzLL) disease. In this communication, we provide evidence to show that the AzLL disease is associated with infection by a previously undescribed phytoplasma, report results from multilocus sequence analysis of the phytoplasma and propose that the AzLL phytoplasma represents a distinct, new lineage in the aster yellows (AY) phytoplasma group.
Materials and methods
Plant samples
Leaf samples were collected from azalea plants showing little leaf, yellowing and witches'-broom growth symptoms in three locations of suburban Kunming, Yunnan Province, southwestern China in 2009 and 2010. Samples from asymptomatic plants in the same region were collected as negative controls.
Transmission electron microscopic observation
Leaf midrib samples of symptomatic and asymptomatic azalea plants were collected, cut into pieces of approximately 2 mm and fixed immediately with 2.5% glutaraldehyde in 0.1 M potassium phosphate buffer (pH 7.2) for 48 h at 4°C. The samples were subsequently postfixed with 2% osmium tetroxide, dehydrated through an ethanol series, infiltrated with propylene oxide and embedded in Epon 812 resin. After an overnight polymerization at 60°C, ultrathin sections were prepared using a microtome (LKB-2188, Bromma, Sweden). The sections were stained with 3% uranyl acetate and lead citrate before being examined under a transmission electron microscope (TEM; JEM-1200EXII, Tokyo, Japan).
DNA extraction and PCR
Total DNA was extracted from 0.5 g leaf tissues of each sample using a modified CTAB-DNeasy procedure as described previously (Cai et al., 2008). Nested PCR assays were performed for amplifications of three phytoplasma genomic segments that contain genes encoding 16S rRNA; ribosomal proteins (rp) S19, L22 and S3; and preprotein translocase SecY subunit, respectively. The primer sets used for the nested PCRs were P1/P7 followed by P1A/16S-SR for 16S rRNA gene amplification (Deng & Hiruki, 1991; Lee et al., 2004), rpF1/rpR1 followed by rp(I)F1/rp(I)R1A for rp gene amplification (Lee et al., 1998; Martini et al., 2007) and L15F1A(I)/MapR1A followed by SecYF1a/SecYR1(I) for secY gene amplification (Lee et al., 2010a). Each reaction mixture, in a total volume of 50 µL, contained 1 µL of the above prepared DNA extract, 25 pmol each of a forward and a reverse primer, 200 µM each of dATP, dCTP, dGTP and dTTP, 5 µL of GeneAmp High Fidelity 10× PCR buffer (with 25 mM MgCl2) and 2.5 units of GeneAmp High Fidelity enzyme mix (Applied Biosystems, Foster City, CA). PCRs were carried out for 35 cycles under the following conditions: denaturation at 95°C for 60 s, annealing at 56°C for 60 s and extension at 72°C for 75 s. Amplicons were analysed by electrophoresis through agarose gel.
Amplicon cloning and sequencing
The phytoplasmal genomic segments amplified above were cloned into plasmid vector pCRII-TOPO (Invitrogen, Carlsbad, CA), and propagated in Escherichia coli. Both strands of cloned phytoplasmal DNA segments were sequenced to achieve at least 4× coverage per base position. DNA sequencing was performed with an ABI PRISM 377 automated DNA sequencer (Applied Biosystems).
Virtual and actual gel restriction fragment length polymorphism analyses and phytoplasma classification
For virtual RFLP analysis, 16S rDNA nucleotide sequences from reference strains of all previously delineated subgroups in the AY phytoplasma group (16SrI) were retrieved from the National Center for Biotechnology Information's nucleotide database. Together with the AzLL phytoplasma 16S rDNA sequences determined in the present study, the retrieved sequences were trimmed to the full F2nR2 region that was bounded by the two conserved nucleotide blocks corresponding to the annealing sites for phytoplasma-universal 16S rDNA primer pair R16F2n/R16R2 (Gundersen & Lee, 1996), and the trimmed sequences were subjected to computer-simulated restriction digestion and virtual gel plotting using iPhyClassifier (Zhao et al., 2009b). The virtual RFLP patterns were automatically compared and a similarity coefficient (F) was calculated for each pair of phytoplasma strains using a Perl program described previously (Wei et al., 2008b). For actual gel-based PCR-RFLP analysis, 16S rDNA F2nR2 fragments generated from nested PCR were subjected to digestions by key restriction enzymes that produced new patterns in virtual RFLP analysis; restriction fragments were separated by electrophoresis on a 3% agarose gel and visualised by ethidium bromide staining. The 16S rDNA RFLP patterns and pattern similarity coefficients were used as a basis for group and subgroup classification of the AzLL phytoplasma according to the criteria established previously (Lee et al., 1998; Wei et al., 2008b). Virtual and actual gel RFLP analyses of rp and secY genes were conducted essentially in the same way as described above for 16S rRNA genes.
Phylogenetic analysis
Phylogenetic trees were constructed with the maximum parsimony method using the PAUP* program. The parsimony analyses were performed with the heuristic search option via random stepwise sequence addition. Uninformative characters were excluded from the analyses. The reliability of the phylogenetic analyses was subjected to a bootstrap test with 1000 replicates. Acholeplasma laidlawii was designated as the out group to root the tree.
Protein structure predictions
AzLL and OYM phytoplasma SecY protein sequences were deduced from corresponding secY gene nucleotide sequences. Three-dimensional structures were predicted using the online SWISS-MODEL program (http://swissmodel.expasy.org/). Predicted three-dimensional structures were visualised and analysed with Swiss PDB-Viewer (Guex & Peitsch, 1997).
Results and discussion
AzLL disease symptoms and evidence of phytoplasma infection
Azalea plants growing in suburban areas of the capital city Kunming of Yunnan Province, China, exhibited abnormal growth patterns that included reduced leaf size (little leaf), shortened internodal length (stunting), excessive branching (witches'-broom-growth) and leaf chlorosis (yellowing) (Fig. 1). Although the symptom combinations and the severity of the symptoms varied from plant to plant, the little leaf symptom was present in all affected plants and was most prominent compared with other symptoms; therefore, the disease was designated as AzLL disease. The disease first occurred in 2009 and then again in 2010. Three locations where the diseased plants were observed belonged to the same municipal district. Because the AzLL disease occurred in a region where multiple phytoplasmal diseases had been reported (Cai, 2007; Cai et al., 2008), and because all symptoms of the AzLL disease were suggestive of phytoplasma infection, tools for diagnosis of phytoplasmal diseases were utilised.

An azalea little leaf (AzLL) phytoplasma-infected azalea plant exhibiting little leaf and yellowing symptoms.
A TEM study revealed the presence of single-membrane-bounded, ovoid to spherical bodies in phloem sieve elements of leaf vein and stem samples from diseased azalea plants (Fig. 2), but not in the samples from asymptomatic control plants. These membrane-bound bodies were concentrated near sieve plates, and were within the size range of phytoplasma cells (200 to 1000 nm in diameter), a further indication of phytoplasmal infection.
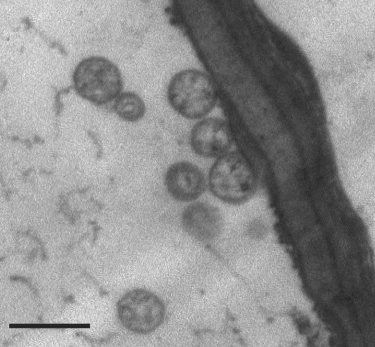
Transmission electron micrograph of phytoplasma cells within a sieve tube of a diseased azalea plant. Each phytoplasma cell is surrounded by an electron-dense cytoplasmic membrane. The scale bar represents 0.5 µm.
DNA amplicons of approximately 1.5 kb were obtained in nested PCR that were primed by phytoplasma-universal rDNA primer pairs P1/P7 and P1A/16S-SR using template DNA extracted from diseased azalea plants. DNAs amplified from five individual samples in separate independent nested PCRs were cloned and sequenced, and found to be identical 16S rRNA gene sequences; a representative sequence was deposited (GenBank no HQ285917). The sequence contained a signature motif, 5′-caagaybatkatgtktagcyggdct-3′ (IRPCM, 2004), characteristic of phytoplasma 16S rRNA genes. Amplification and nucleotide sequencing of near-full-length 16S rRNA gene provided convincing evidence connecting AzLL disease and phytoplasmal infection.
AzLL phytoplasma represents a new subgroup in the aster yellows phytoplasma group
The AzLL phytoplasma's near-full-length 16S rRNA gene shares 99.5% sequence identity with that of ‘Candidatus Phytoplasma asteris' reference strain Oenothera phytoplasma 86-7 (OAY, GenBank accession M30790); therefore, AzLL phytoplasma is a ‘Ca. Phytoplasma asteris'-related strain. Virtual RFLP analysis of 16S rDNA confirmed this relationship and showed that AzLL belongs to the AY phytoplasma group (group 16SrI), the largest and most diverse phytoplasma group known (Marcone et al., 2000, Lee et al., 2004).
Prior to this study, phytoplasmas in the AY group had been differentiated into no less than 17 subgroups based on RFLP analysis of 16S rDNA sequences (Lee et al., 2004; Jomantiene et al., 2011). In the present study, a virtual RFLP analysis was performed on the AzLL phytoplasma 16SrDNA F2nR2 fragment with a defined set of 17 enzymes routinely used for identification and classification of phytoplasmas [AluI, BamHI, BfaI, BstUI (ThaI), DraI, EcoRI, HaeIII, HhaI, HinfI, HpaI, HpaII, KpnI, Sau3AI (MboI), MseI, RsaI, SspI and TaqI; Lee et al., 1998]. The analysis resulted in a unique restriction profile (Fig. 3A), which differed from those of representative strains of all previously delineated AY subgroups. The similarity coefficients between the collective 16S rDNA F2nR2 RFLP profile of AzLL phytoplasma and those of the representative strains of the previously delineated subgroups were ≤92.5%. This value is significantly lower than the threshold for recognition of a new subgroup (97%; Lee et al., 1998; Wei et al., 2008b), supporting recognition of a new 16S rDNA RFLP subgroup in the AY group. RFLP patterns from in silico digestions of two restriction enzymes, BstUI and RsaI, effectively distinguished AzLL phytoplasma from strains belonging to all previously delineated subgroups (Fig. 3B and Fig. 3C). An actual gel-based RFLP analysis, using the two distinguishing enzymes, generated restriction profiles (Fig. 3D) identical to those produced by virtual RFLP analysis (Fig. 3B and Fig. 3C), validating the results of the virtual RFLP analysis and further justifying recognition of the AzLL phytoplasma as the representative of a new 16SrI subgroup, hereby designated as 16SrI-T.
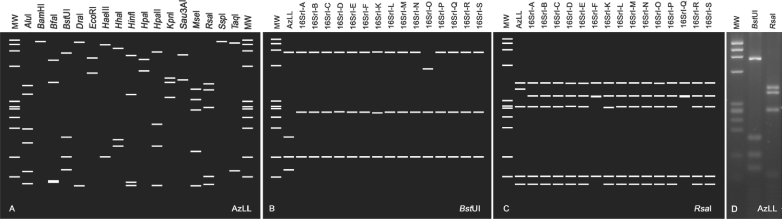
Virtual and actual gel RFLP analyses of AzLL phytoplasma16S rDNA F2nR2 fragment. (A) Virtual RFLP patterns from in silico digestions of AzLL phytoplasma F2nR2 fragment by a defined set of 17 restriction enzymes listed in the Results and discussion section. (B and C) Restriction enzymes that produce unique restriction patterns distinguishing AzLL phytoplasma from representative strains of previously delineated 16SrI subgroups. (D) PCR-RFLP patterns from laboratory enzymatic digestions of AzLL F2nR2 amplicon by BstUI and RsaI. MW: ϕX174DNA HaeIII digest, RFLP: restriction fragment length polymorphism, AzLL: azalea little leaf.
Additional evidence of AzLL phytoplasma as representative of a distinct, new lineage
Previous studies indicated that rp and secY genes carry valuable genetic markers and can be used, in conjunction with 16S rRNA genes, to identify and distinguish closely related, but distinct phytoplasma strains and lineages (Lee et al., 1998, 2006, 2010a; Martini et al., 2007; Jomantiene et al., 2011). To further characterise the AzLL phytoplasma, genomic segments that contain genes encoding preprotein translocase SecY subunit (secY) and ribosomal proteins S19 (rpsS), L22 (rplV) and S3 (rpsC) were cloned and sequenced.
Results from virtual RFLP analysis of the 1.2 kb AzLL phytoplasma rpsS-rplV-rpsC locus (GenBank accession no. HQ285918) revealed new BfaI and Sau3AI RFLP patterns (Fig. 4A and Fig. 4B) having recognition sites that were absent from this locus in representative strains of all previously established rp(I) subgroups, indicating the AzLL phytoplasma represented a distinct rp subgroup. To validate the new BfaI and Sau3AI RFLP patterns, conventional RFLP analysis was performed; results from this actual gel electrophoresis confirmed the new patterns produced from the virtual RFLP analysis (Fig. 4C). In accordance with the criteria and naming convention established for recognition of new rp subgroups (Lee et al., 1998), we hereby designate AzLL phytoplasma as the representative of a new rp subgroup, rp(I)-P.
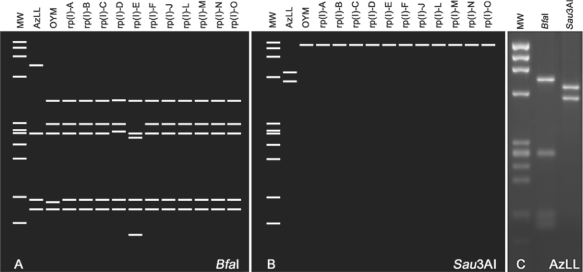
Virtual and actual gel RFLP patterns from analyses of AzLL phytoplasma rpsS-rplV-rpsC locus. (A and B) Unique AzLL phytoplasma subgroup rp(I)-P patterns derived from digestion by BfaI and Sau3AI, respectively. (C) BfaI and Sau3AI restriction patterns derived from actual gel-based PCR-RFLP analysis. MW: ϕX174DNA HaeIII digest, RFLP: restriction fragment length polymorphism, AzLL: azalea little leaf.
Distinction between AzLL phytoplasma and strains representative of other AY group phytoplasmas was further evidenced by the results from comparative analysis of the genomic locus bearing the secY gene. Nested PCR amplification primed by primer pairs L15F1A(I)/MapR1A and SecYF1a/SecYR1(I) resulted in an amplicon of 1776 bp, which was subsequently cloned and sequenced. The nucleotide sequence data confirmed that the genomic segment covered a partial rplO gene, a complete secY gene and a partial adk gene (GenBank accession no. HQ285919). For comparative analyses, the 1776 bp sequence was trimmed to a 1358 bp segment bounded by the annealing sites for primer pair AYsecYF1/AYsecYR1 in accordance with Lee et al. (2006, 2010a). On the basis of percentage sequence identity and RFLP pattern similarity coefficients derived from comparative analyses of the secY locus, the AzLL phytoplasma was most distantly related to AYWB (94.3% sequence identity and 0.63 RFLP pattern similarity coefficient) and most closely related to OnP1 (99.0% sequence identity and 0.95 RFLP pattern similarity coefficient) and OYM (98.9% sequence identity and 0.90 RFLP pattern similarity coefficient), among AY phytoplasmas studied thus far. RFLP analysis using only one restriction enzyme, TaqI, was sufficient to distinguish AzLL phytoplasma from representatives of all other secY(I) subgroups (Fig. 5). According to previously established criterion (Lee et al., 2006), AzLL represents a new secY lineage, secY(I)-O. It is pertinent to note that the deduced AzLL SecY amino acid sequence differed from the SecY sequence of OYM, a closely related AY phytoplasma strain, by nine amino acid residues, with most of the altered amino acid residues being located in the amphipathic regions of the proteins and four of them causing differences in charge or polarity of the affected positions (Fig. 6). As SecY is the main transmembrane subunit of the bacterial protein secretory pathway, such amino acid substitutions may have functional implications, signifying the lineage difference between AzLL and other AY phytoplasmas.
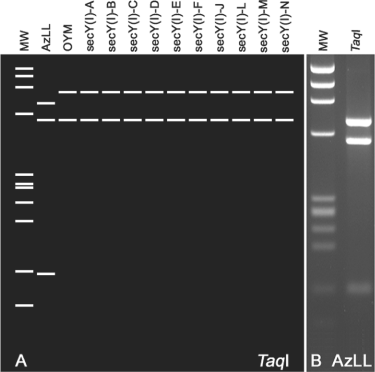
Virtual and actual gel RFLP patterns from analyses of AzLL phytoplasma secY gene. (A) Unique AzLL phytoplasma secY(I) patterns produced for TaqI digestion. (B) Actual gel-based RFLP analysis confirms the virtual gel pattern. MW: ϕX174DNA HaeIII digest, RFLP: restriction fragment length polymorphism, AzLL: azalea little leaf.

Structural comparison between AzLL and OYM SecY proteins. Three-dimensional structures were modelled based on the sequence alignment with Bacillus subtilis SecY (Pdb3Dl3H). Predicted structures of AzLL SecY (left) and OYM SecY (right) are shown side by side. Amino acid residues that differ in two strains are labelled. The number following each amino acid name indicates the position of the amino acid. Most of the altered amino acid residues are located at surface loops of the proteins. AzLL: azalea little leaf, OYM: onion yellows mild.
Relationship between the azalea little leaf phytoplasma and new 16SrI strains from East Asia
Over the past decade, new phytoplasmas associated with plant diseases worldwide have been discovered at an increasingly rapid pace, and recent studies have unveiled remarkable genetic diversity and lineage-specific evolution among phytoplasmas (Lee et al., 2000; Davis et al., 2003, 2005; Cai et al., 2008). Although many phytoplasmas can be differentiated based on 16S rRNA gene nucleotide sequences alone, some require analysis of other genetic loci (Marcone et al., 2000; Andersen et al., 2006; Arnaud et al., 2007; Pacifico et al., 2009; Lee et al., 2010b). Results from multilocus analysis have revealed that phytoplasma strains belonging to a given 16Sr subgroup may represent separate rp, secY, tuf, vmp1 or other gene lineages (Lee et al., 2006; Arnaud et al., 2007; Martini et al., 2007; Fialováet al., 2009; Pacifico et al., 2009; Murolo et al., 2010); on the other hand, strains belonging to one rp or secY gene lineage may be affiliated with different 16SrI subgroups (Lee et al., 2006; Martini et al., 2007; Jomantiene et al., 2011).
In the present study, we found that the AzLL phytoplasma rpsS-rplV-rpsC and secY loci share high levels of sequence identity with the corresponding loci of several new strains from East Asia. These East Asian strains include chinaberry witches'-broom phytoplasma strains CbWB-Hainan and CbWB-Wsh from China; mulberry dwarf phytoplasma strains MD-TS (or MD-TA), MD-NY and MD-XT from China; mulberry dwarf phytoplasma strain MD-Gyeongbuk from Korea; amaranth virescence phytoplasma AV from China; goldenrain phytoplasma strain GRP from Korea and mulberry yellow dwarf phytoplasma strain MYD-AnH from China (Table 1).
| Strain, Associated Disease and Origin | RFLP Subgroup Classification | |||||
|---|---|---|---|---|---|---|
| 16SrI | GenBank No. | rp(I) | GenBank No. | secY(I) | GenBank No. | |
| AYWB, aster yellows witches'-broom, USA | 16SrI-A | CP000061 | UC | CP000061 | UC | CP000061 |
| BB, tomato big bud, USA | 16SrI-A | AY180955 | rp(I)-A | AY183686 | secY(I)-A | AY803178 |
| GD1, gray dogwood stunt, USA | 16SrI-A | DQ112021 | rp(I)-M | AY264864 | secY(I)-M | AY803171 |
| AV2192, aster yellows, Germany | 16SrI-B | AY180957 | rp(I)-B | AY183708 | secY(I)-B | AY803167 |
| AY1, Maryland aster yellows, USA | 16SrI-B | AF322644 | rp(I)-B | NA | secY(I)-B | AY803177 |
| HyPH, hydrangea phyllody, Italy | 16SrI-B | AY265207 | rp(I)-K | NA | secY(I)-B | AY803173 |
| MBS, maize bushy stunt, Mexico | 16SrI-B | AF487779 | rp(I)-L | AY264858 | secY(I)-L | AY803175 |
| OAY, oenothera aster yellows, USA | 16SrI-B | M30790 | rp(I)-B | M74770 | UC | NA |
| OYM, onion yellows mild, Japan | 16SrI-B | AP006628 | UC | AP006628 | UC | AP006628 |
| PRIVC, primrose virescence, Germany | 16SrI-B | AY265210 | rp(I)-B | AY264854 | secY(I)-B | AY803176 |
| CPh, clover phyllody, Canada | 16SrI-C | AF222065 | rp(I)-C | AY264862 | secY(I)-C | AY803179 |
| PaWB, paulownia witches'-broom, Taiwan | 16SrI-D | AY265206 | rp(I)-D | GQ375570 | secY(I)-D | AY803184 |
| BBS3, blueberry stunt, USA | 16SrI-E | AY265213 | rp(I)-E | AY264863 | secY(I)-E | AY803169 |
| ACLR-AY, apricot chlorotic leaf roll, Spain | 16SrI-F | AY265211 | rp(I)-N | AY264866 | secY(I)-N | AY803166 |
| STRAWB2, strawberry multiplier, USA | 16SrI-K | U96616 | rp(I)-J | U96617 | secY(I)-J | AY803180 |
| OnP2, onion proliferation, Lithuania | 16SrI-L | GU223209 | rp(I)-B | GU228514 | secY(I)-B | GU228516 |
| AVUT, aster yellows, Germany | 16SrI-M | AY265209 | rp(I)-B | AY264855 | secY(I)-B | AY803167 |
| IOWB, ipomoea obscura witches'-broom, Taiwan | 16SrI-N | AY265205 | rp(I)-F | AY264859 | secY(I)-F | AY803182 |
| 98UW166B, aster yellows, USA | 16SrI-O | AF268405 | UC | NA | UC | NA |
| AYIP, aster yellows, Croatia | 16SrI-P | AF503568 | UC | NA | UC | NA |
| CherLL, cherry little leaf, Lithuania | 16SrI-Q | AY034089 | UC | NA | UC | NA |
| ChBL, cherry bunchy leaf , Lithuania | 16SrI-R | HM067754 | rp(I)-O | HM067756 | UC | NA |
| LcLL, lilac little leaf, Lithuania | 16SrI-S | HM067755 | rp(I)-C | HM067757 | UC | NA |
| AzLL, azalea little leaf, China | 16SrI-T | HQ285917 | rp(I)-P | HQ285918 | secY(I)-O | HQ285919 |
| AV-Shandong, infecting amaranth, China | (16SrI-B) | FJ853155 | UC | FJ853156 | UC | NA |
| CbWB-Wsh, chinaberry witches'-broom, China | (16SrI-B) | DQ630726 | UC | DQ648460 | UC | GU390884 |
| CbWB-Y3/Hainan, chinaberry witches'-broom, China | (16SrI-B) | EF990733 | UC | EU348781 | UC | FJ159696 |
| GRP goldenrain aster yellows, South Korea | (16SrI-pn) | EU430729 | UC | FJ705831 | UC | NA |
| MD-Gyeongbuk, mulberry dwarf, South Korea | UC | NA | UC | AB569973 | UC | NA |
| MD-Ko, mulberry dwarf, South Korea | (16SrI-B) | AY075038 | UC | NA | UC | NA |
| MD-NY, mulberry dwarf, China | (16SrI-B) | EU999736 | UC | FJ154858 | UC | GU968583 |
| MD-TS/TA, mulberry dwarf, China | (16SrI-B) | FJ844439 | UC | FJ844440 | UC | GU968584 |
| MD-XT, mulberry dwarf, China | (16SrI-pn) | FJ844442 | UC | FJ844443 | UC | GU968585 |
| MYD-AnH, mulberry yellow dwarf, China | (16SrI-B) | GQ249410 | UC | GQ373255 | UC | NA |
| PP-Chandigarh, infecting periwinkle, India | (16SrI-B) | FN257485 | UC | FN263249 | UC | FM991880 |
| PP-Himachal, infecting periwinkle, India | (16SrI-B) | FN257484 | UC | FN263247 | UC | FM991879 |
| PVP-Egypt, Egyptian periwinkle virescence, Egypt | (16SrI-pn) | EF546439 | UC | FN263248 | UC | FM991881 |
- UC: unclassified; NA not available; pn: potentially new subgroup.
- Unless otherwise specified, subgroup classification information is based on the following references: Lee et al., 1998, 2000, 2006, 2010a; and Martini et al., 2007. Classification data for strains CherLL, ChBL, and LcLL are from Jomantiene et al., 2011, respectively. Data for strain AzLL reported in this communication are in bold.
- Thirteen newly identified Asian AY strains are listed below the broken horizontal line. Subgroup classification of these strains remains undocumented. Data in parentheses are tentative classification based on results of virtual RFLP analysis 16S rRNA gene sequences using iPhyClassifier (Zhao et al., 2009b).
Interestingly, most of the new East Asian strains belong to subgroup 16SrI-B on the basis of RFLP analysis of 16S rDNA sequences. Yet, these strains shared higher levels of nucleotide sequence identity of rpsS-rplV-rpsC and secY loci with AzLL (16SrI-T) than with well-known Asian strain OYM (a member of subgroup 16SrI-B), and exhibited collective RFLP patterns of these loci that were more comparable to those of AzLL than to those of OYM. For example, all of the new East Asian strains share the unique BfaI restriction pattern that is characteristic of the AzLL rp lineage [rp(I)-P] (Fig. 7A) and the unique TaqI restriction pattern characteristic of the AzLL secY lineage [secY(I)-O] (Fig. 7B).
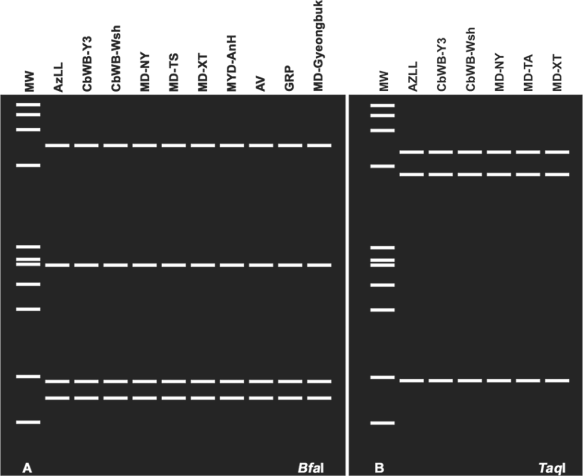
RFLP pattern similarities among AzLL phytoplasma and related East Asian strains. (A) Unique rp(I)-P BfaI pattern shared by AzLL and related East Asian strains. (B) Unique secY(I)-O TaqI pattern shared by AzLL and related East Asian strains. MW: ϕX174DNA HaeIII digest. Refer to Table 1 for strain and gene sequence information, RFLP: restriction fragment length polymorphism, AzLL: azalea little leaf.
Relationships between AzLL and strains classified in subgroup 16SrI-B were further assessed by phylogenetic analysis of the 16S rRNA gene, the rpsS-rplV-rpsC locus and the secY gene. Phylogenetic relationships inferred from these analyses (Fig. 8) were in agreement with the results from RFLP analyses of these genetic loci and were consistent with the concept that AzLL represents a new 16SrI, rp(I) and secY(I) lineage. In the 16S rDNA-based tree, the high degree of nucleotide sequence conservation among 16 rRNA genes largely blurred distinctions among closely related subgroups in group 16SrI, but in spite of the low resolving power of 16S rDNA, strain AzLL was distinguished from subgroup 16SrI-B strains and the other strains studied (Fig. 8A). The rp-based tree indicated that the rpsS-rplV-rpsC locus in AzLL and other strains from China shared a recent ancestral nucleotide sequence, distinct from that of strains originating in other regions (Fig. 8B). Similarly, the secY genes of AzLL and other strains from China appeared in the same subclade, indicating that these genes also shared a common recent ancestral nucleotide sequence, distinct from that of strains originating in other regions (Fig. 8C).
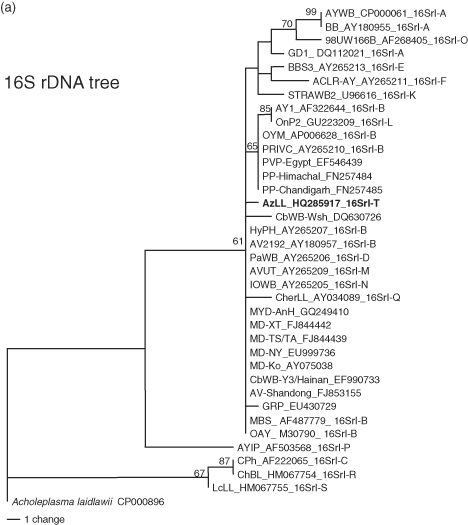
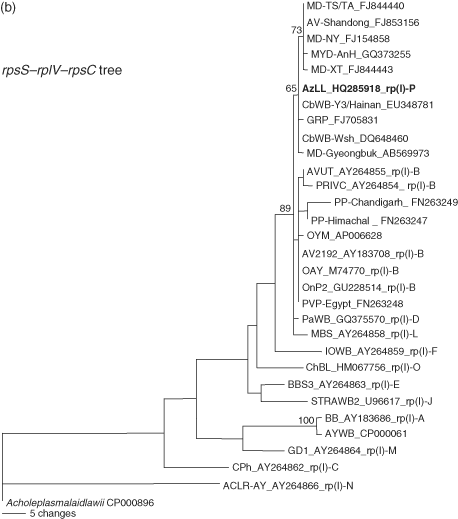
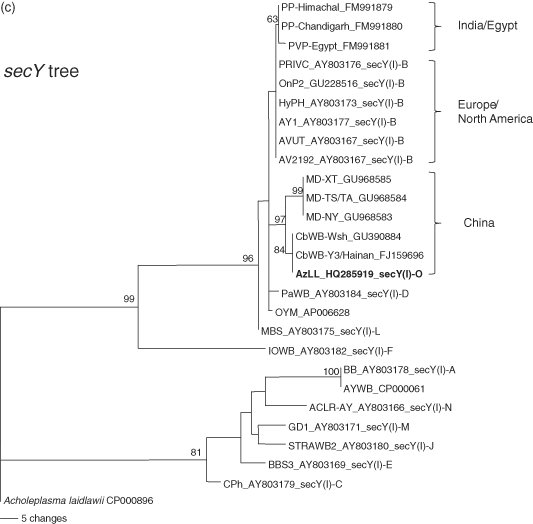
Phylogenetic trees inferred from maximum parsimony analysis of 16S rRNA, rp, and secY gene sequences. The phytoplasma strains used in the phylogenetic tree construction include representative members of previously delineated 16SrI, rp(I), and secY(I) subgroups and East Asian strains closely related to AzLL phytoplasma (Table 1). AzLL phytoplasma are highlighted in bold. Numbers at nodes indicate percent bootstrap values above 50 (1000 replicates). Note that rp (B) and secY (C) gene sequences appear to have higher resolving power than 16S rRNA gene (A). AzLL: azalea little leaf.
Multilocus analysis of closely related phytoplasma strains in the AY group employing differentially conserved nucleotide sequences, such as those of 16S rDNA, rp and secY loci, presents significant advantages over single gene analysis. In particular, differences in the resolving power of such loci enable distinctions among phytoplasmas that share different levels of relatedness (Lee et al., 2010b). Thus, while 16S rRNA gene sequences are valuable for distinguishing clearly divergent strains at species and higher taxonomic levels, the less highly conserved rp and secY gene sequences offer greater potential for distinguishing closely related lineages that may or may not represent evolutionary divergence at the level of species. Multilocus analysis in this study revealed the association of a new disease with a previously unknown phytoplasma, characterised three genetic loci of the new phytoplasma lineage and identified genetic markers of geographic strain origins. Multilocus characterization, such as that in the present study, forms a step towards fuller description of newly found phytoplasmas and provides the kind of gene lineage information that should be useful for eventual delineation and description of new species in a yet-to-be-adopted formal system of phytoplasma taxonomy, phasing out the current provisional Candidatus status.
Acknowledgement
This study was supported by the U.S. Department of Agriculture, Agricultural Research Service (Project number 1275-22000-246-00) and the National Natural Science Foundation of China (Grant number 31060239).




