Multiple gene analyses reveal extensive genetic diversity among ‘Candidatus Phytoplasma mali’ populations
Abstract
This study focused on evaluating the genetic diversity among ‘Candidatus Phytoplasma mali’ (‘Ca. P. mali’) populations in orchards of north-western Italy, where apple proliferation (AP) disease is widespread and induces severe economic losses. ‘Ca. P. mali’ was detected through restriction fragment length polymorphism (RFLP) analysis of PCR-amplified 16S rDNA in 101 of 114 samples examined. Collective RFLP patterns, obtained by restriction analyses of four amplified genomic segments (16S/23S rDNA, PR-1, PR-2 and PR-3 non-ribosomal region, ribosomal protein genes rplV-rpsC and secY gene), revealed the presence of 12 distinct genetic lineages among 60 selected representative ‘Ca. P. mali’ isolates, underscoring an unexpected high degree of genetic heterogeneity among AP phytoplasma populations in north-western Italy. Prevalence of distinct genetic lineages in diverse geographic regions opens new interesting avenues for studying the epidemiology of AP disease. Furthermore, lineage-specific molecular markers identified in this work could be useful for investigating the biological life cycle of ‘Ca. P. mali’.
Introduction
Phytoplasmas are phloem-restricted, cell wall-less prokaryotic parasites belonging to the class Mollicutes (Lee et al., 2000). They are associated with diseases affecting hundreds of plant species and are transmitted by phloem-sucking insects (Weintraub & Beanland, 2006). As phytoplasmas cannot be successfully cultivated in cell-free media, their identification and differentiation is achieved mainly by DNA-based techniques. Actual (Lee et al., 1998) and virtual (Wei et al., 2007) restriction fragment length polymorphism (RFLP) analyses of 16S rDNA allowed delineation of at least 30 phytoplasma groups. ‘Candidatus Phytoplasma mali’ (‘Ca. P. mali’) is the aetiological agent of apple proliferation (AP), a quarantine disease widespread in the most important apple-growing regions in Europe, where it causes severe production losses and considerable economic damages. According to the 16S rDNA RFLP-based classification scheme, ‘Ca. P. mali’ belongs to the group 16SrX (subgroup 16SrX-A) and is closely related to ‘Candidatus Phytoplasma pyri’ (‘Ca. P. pyri’) (subgroup 16SrX-C) and ‘Candidatus Phytoplasma prunorum’ (‘Ca. P. prunorum’) (subgroup 16SrX-F), the causal agents of pear decline (PD) and European stone fruit yellows (ESFY) diseases, respectively (Seemüller & Schneider, 2004). In nature, ‘Ca. P. mali’ is transmitted from infected plants to healthy ones by insects of the family Psyllidae. In Italy, Cacopsylla melanoneura Förster is the main vector of ‘Ca. P. mali’ in the north-western regions (Tedeschi & Alma, 2007), while Cacopsylla picta Förster (known as the main vector of ‘Ca. P. mali’ in Germany) (Jarausch et al., 2007) transmits the pathogen in the north-eastern regions (Carraro et al., 2001). Moreover, additional vectors and natural plant hosts have been reported (Tedeschi & Alma 2006; Tedeschi et al., 2009). The biological complexity of AP disease has stimulated research on molecular markers of ‘Ca. P. mali’ genetic diversity. Analysis of the PR-1, PR-2 and PR-3 non-ribosomal region proved the existence of at least three ‘Ca. P. mali’ genotypes (AT-1, AT-2 and AP15) differently distributed in orchards in southwestern Germany and north-eastern Italy (Cainelli et al., 2004; Jarausch et al., 2004). Further work based on molecular characterisation of rplV-rpsC genes identified at least four ‘Ca. P. mali’ genotypes according to geographical and, in some cases, also with epidemic distribution in north-eastern Italy, Hungary and Serbia (Martini et al., 2008; Paltrinieri et al., 2010). Recently, analyses of ribosomal (16S/23S rDNA, rplV-rpsC) and non-ribosomal (aceF, secY, pnp, imp, hflB) genes allowed finer differentiation of ‘Ca. P. mali’ strains and revealed their presence in diverse geographic areas (Danet et al., 2007; Schneider & Seemüller, 2009; Casati et al., 2010). Moreover, results from chromosome enzymatic cleavage and Southern blot hybridisation assays indicated a significant genetic variability among ‘Ca. P. mali’ isolates exhibiting different virulence levels (Seemüller & Schneider, 2007). To investigate the genetic diversity among ‘Ca. P. mali’ populations in north-Italian orchards, in this work a PCR–RFLP-based multilocus sequence analysis (MLSA) was performed on four distinct chromosome segments including 16S/23S rDNA, PR-1, PR-2 and PR-3 non-ribosomal region, ribosomal protein genes rplV-rpsC and secY gene. Multiple distinct genetic lineages were described. The findings expand our knowledge about complex structures of ‘Ca. P. mali’ populations in Italy, underscoring the value of molecular markers for studying the ecology of AP disease.
Materials and methods
Sample collection, nucleic acid preparation and detection of ‘Ca. P. mali’
Leaf samples from 99 apple trees showing typical symptoms of AP disease were collected from 2003 to 2007 in orchards of Lombardia, Piemonte and Valle d’Aosta regions, north-western Italy. Seventy five individuals of C. melanoneura were captured by beat-tray method (Horton, 1999) in orchards of Valle d’Aosta; adults were identified under the stereomicroscope.
Total plant DNA was extracted from 0.5 g of leaf mid-veins using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Extraction of insect DNA was performed on 15 batches with each having 5 adult psyllid individuals, as previously described (Marzachìet al., 1998).
Detection of ‘Ca. P. mali’ was carried out by means of amplification of 16S-23S rDNA, in nested PCRs primed by phytoplasma-universal primer pair P1/P7 (Deng & Hiruki, 1991), followed by 16SrX group-specific primer pair R16F1(X)/R16R1(X) [F1/R1(X)] (Lee et al., 1995), and subsequent SspI-RFLP assay as previously described (Lee et al., 1998). ‘Ca. P. mali’ strain AT (subgroup 16SrX-A), ‘Ca. P. pyri’ strain PD (subgroup 16SrX-C), ‘Ca. P. prunorum’ strain LP (subgroup 16SrX-F), ‘Ca. Phytoplasma asteris' strain SAY (subgroup 16SrI-B) and ‘Ca. Phytoplasma ulmi’ strain EY1 (subgroup 16SrV-A) were employed as reference controls. Reference phytoplasma strains were maintained in plants of Madagascar periwinkle [Catharanthus roseus (L.) G. Don] in greenhouse. DNA extracted from healthy periwinkles, maintained in greenhouse, and reaction mixtures devoid of DNA were used as negative controls.
Sequencing and characterisation of 16SrX phytoplasma secY gene
Universal degenerate primer pairs were designed for amplifying phytoplasma secY gene from ‘Ca. P. mali’, ‘Ca. P. pyri’ and ‘Ca. P. prunorum’ (group 16SrX). Previously published secY gene sequences of phytoplasmas belonging to genetically diverse groups 16SrI (Oshima et al., 2004; Lee et al., 2006), 16SrV (Lee et al., 2004; Arnaud et al., 2007), 16SrX (Kube et al., 2008) and 16SrXII (Tran-Nguyen et al., 2008) were retrieved from GenBank and aligned using the software ClustalW2 (http://www.ebi.ac.uk/tools/clustalw2/). The differentially conserved regions in the alignment were utilised for designing 12 universal degenerate primers; 35 primer pair combinations were used in PCRs for the amplification of partial sequences of secY gene from phytoplasmal reference strains (listed in Table 1). PCRs were performed, in an automated thermal cycler (PCR Mastercycler Gradient, Eppendorf), using 1 µL of DNA template in 25 µL of reaction mixture containing 2.5 µL of 10× PCR buffer, 2 mM of MgCl2, 200 µM of each dNTP, 1 µM of each primer and 0.625 U of Platinum®Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA). PCR conditions were initial denaturation for 4 min at 94°C followed by 35 cycles of denaturation for 3 min at 94°C, annealing for 2 min at 42°C and extension for 3 min at 72°C and a final extension for 7 min at 72°C. To design 16SrX-specific primer pairs, PCR products (1242 bp) amplified by universal primer pair fSecY1/rSecY1 (Table 2) from phytoplasma reference strains of group 16SrX (AT, AP15, LP and PD) were purified by means of the QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions, cloned in plasmid vector pCR2.1-TOPO (Invitrogen) and propagated in Escherichia coli as described (Schuman, 1994). Both strands of cloned inserts were sequenced by using primer pairs M13for and M13rev. DNA sequencing was performed by a commercial sequencing service (Primm, Milan, Italy). Nucleotide sequences were analysed by the software FinchTV 1.4 (http://www.geospiza.com), assembled by employing the Contig Assembling program of the sequence analysis software BIOEDIT 7.05 (http://www.mbio.ncsu.edu/bioEdit/bioedit.html) and deposited in GenBank at accession numbers HM237289 (AT), HM237294 (AP15), HM237295 (LP) and HM237296 (PD). Nucleotide sequences were compiled in FASTA format, aligned using the software ClustalW2 and searched for 16SrX group-specific primer pairs through the software Primer Select (DNAstar, Madison, WI, USA). Their specificity was verified by PCRs on DNAs from periwinkles infected by phytoplasmas belonging to group 16SrX and to other groups (listed in Table 1). Furthermore, secY gene sequences were searched for ‘Ca. P. mali’-specific single nucleotide polymorphisms (SNPs). Localisation of SNPs in recognition sites for restriction enzymes was determined by virtual RFLP analyses using the software pDRAW32 (http://www.acaclone.com/) and verified by actual in vitro RFLP assays.
| Phytoplasma Strain | Subgroup | PCRa | ||
|---|---|---|---|---|
| fSecY1/rSecY1 | fATY2/rATY2 | |||
| CY | Chrysanthemum yellows | 16SrI-B | + | − |
| KV | Clover phyllody | 16SrI-C | + | − |
| KV-I | Clover phyllody | 16SrI-C | + | − |
| SAY | Western aster yellows | 16SrI-B | + | − |
| SUNHP | Sunhemp witche's-broom | 16SrII-A | + | − |
| GVX | Western X disease | 16SrIII-A | + | − |
| PYRL | Peach yellows leaf roll | 16SrIII-A | + | − |
| VAC | Vaccinium witche's-broom | 16SrIII-B | + | − |
| EY1 | Elm yellows | 16SrV-A | + | − |
| ALY | Alder yellows | 16SrV-C | + | − |
| FD70 | Flavescence dorée | 16SrV-C | + | − |
| RUS | Rubus stunt | 16SrV-E | + | − |
| ULW | Elm witche's-broom | 16SrV-A | + | − |
| BLL | Brinjal little leaf | 16SrVI-A | + | − |
| BLTVA | Periwinkle virescence | 16SrVI-A | + | − |
| TBB | Tomato big bud | 16SrVI-A | + | − |
| ASHY | Ash yellows | 16SrVII-A | + | − |
| AT | Apple proliferation | 16SrX-A | + | + |
| AP15 | Apple proliferation | 16SrX-A | + | + |
| PD | Pear decline | 16SrX-C | + | + |
| LP | Plum neptonecrosis | 16SrX-F | + | + |
| BVK | Leafhopper-borne | 16SrXI-A | + | − |
| STOL | Stolbur | 16SrXII-A | + | − |
- aThe symbol + indicates positive PCR reaction; the symbol − indicates negative PCR reaction.
| Genome segment | PCR-Amplificationa | RFLP Assayb | ||
|---|---|---|---|---|
| Primer | References | Enzyme | References | |
| 16S/23S rDNA | P1 5′-AAGAGTTTGATCCTGGCTCAGGAT-3′ (d) | Deng & Hiruki (1991) | HpaII, HpyCH4V, FauI | Casati et al. (2010) |
| P7 5′-CGTCCTTCATCGGCTCTT-3′ (d) | ||||
| P1A 5′-ACGCTGGCGGCGCGCCTAATAC-3′ (n) | Lee et al. (2004) | |||
| P7A 5′-CCTTCATCGGCTCTTAGTGC-3′ (n) | ||||
| PR-1/PR-2/PR-3 | AP13 5′-CTACAGATTTCACACATTGG-3′ (d) | Jarausch et al. (1994) | HincII, PagI | Jarausch et al. (2000) |
| AP10 5′-TTTTCACAACGTATTCCGCC-3′ (d) | ||||
| AP14 5′-CAGATTTCACACATTGGTTAT-3′ (n) | Casati et al. (2010) | |||
| AP15 5′-ATTTTTGTTGTTTTCTACCCAT-3′ (n) | ||||
| rplV-rpsC | rpL2F3 5′-WCCTTGGGGYAAAAAAGCTC-3′ (d) | Martini et al. (2007) | AluI | Martini et al. (2008) |
| rp(I)R1A 5′-GTTCTTTTTGGCATTAACAT-3′ (d) | ||||
| rpAP15f 5′-AGTGCTGAAGCTAATTTGG-3′ (n) | Martini et al. (2008) | |||
| rpAP15r 5′-TGCTTTTTATAGCAAAAGGTT-3′ (n) | ||||
| secY | fSecY1 5′-AATHTTTTTHACYTTATTYATTATT-3′ (d) | This work | MseI, AluI, HpyCH4V | This work |
| rSecY1 5′-ACAATAATWARMARACTDGTYCC-3′ (d) | ||||
| fATY2 5′-TAGGACGTAGTATACAAATCC-3′ (n) | This work | |||
| rATY2 5′-GGTCCCCCTATTTTAAATTCC-3′ (n) | ||||
- Ca. P. mali, Candidatus Phytoplasma mali; RFLP, restriction fragment length polymorphism; MLSA, multilocus sequence analysis.
- a(d) Primer used in direct PCRs, (n) primer used in nested PCRs.
- bDigestions were performed on nested PCR products.
Characterisation of ‘Ca. P. mali’ isolates through PCR–RFLP-based multilocus sequence analysis
Molecular characterisation of 60 ‘Ca. P. mali’ isolates, identified in this study and selected as representative of distinct geographic regions, was performed by PCR–RFLP-based assays of four phytoplasmal genomic portions, including 16S/23S rDNA, PR-1, PR-2 and PR-3 non-ribosomal region, ribosomal protein genes rplV and rpsC and secY gene. German ‘Ca. P. mali’ strains AT and AP15, maintained in periwinkle, were analysed as reference strains. Nested PCR reaction mixtures (total volume of 25 µL) for amplification of secY gene contained 2.5 µL of 10× PCR buffer, 2 mM of MgCl2, 200 µM of each dNTP, 0.48 µM of each primer and 0.625 U of Taq polymerase (Invitrogen). Nested PCRs for secY gene amplification were performed at the following conditions: initial denaturation for 4 min at 94°C followed by 35 cycles of denaturation for 45 s at 94°C, annealing for 2 min at 55°C and extension for 30 s at 72°C and final extension for 7 min at 72°C. PCR–RFLP analyses of the genomic segments are summarised in Table 2.
Phylogenetic analysis
Nucleotide sequences of secY genes, amplified from ‘Ca. P. mali’ reference strains AT (accession no. HM237289) and AP15 (accession no. HM237294), from representative ‘Ca. P. mali’ isolates V147 (accession no. HM237290), V247 (accession no. HM237291), T3 (accession no. HM237292) and T10 (accession no. HM237293), ‘Ca. P. pyri’ strain PD (accession no. HM237296), ‘Ca. P. prunorum’ strain LP (accession no. HM237295) and from previously described phytoplasma strains of group 16SrI, 16SrV and 16SrXII, were employed for phylogenetic analyses. Phytoplasma secY gene sequences were aligned using ClustalW2; in order to eliminate poorly aligned positions, alignments were trimmed through the software GBlocks 0.91b (http://molevol.ibmb.csic.es/Gblocks.html). Minimum evolution analyses were performed using the neighbour-joining method and boot-strap replicated 1000 times with the software MEGA 4.0 (http://www.megasoftware.net/). Spiroplasma kunkelii served as outgroup of the trees.
Results and discussion
Identification of ‘Ca. P. mali’ in north-western Italy
The 16SrX-specific primer pair F1/R1(X) produced amplicons from DNA from 97 of 99 (98%) plant samples and from 4 of 15 (27%) insect batches examined (data not shown). DNAs from EY1 (16SrV-A)-infected, SAY (16SrI-B)-infected and healthy periwinkle plants, and water devoid of DNA template yielded no observable amplification. All amplicons showed SspI-RFLP patterns that were indistinguishable from one another and from the SspI pattern characteristic of the reference strain AT (Lee et al., 1998) (data not shown), indicating that the isolates detected in diseased apple plants and insects belonged to the species ‘Ca. P. mali’. High percentage of positive samples confirmed the strong association between specific AP disease symptoms and infection by ‘Ca. P. mali’; moreover, apple samples were collected in middle-old age orchards (plants from 10 to 20 years old), where trees were probably infected before the application of mandatory measures for the control of ‘Ca. P. mali’ spreading. On the other hand, the presence of negative samples could be connected with the low titre of phytoplasmas in symptomatic apple tree tissues (Smart et al., 1996).
Analyses of secY gene sequences among 16SrX phytoplasmal group
In a recent work, it was demonstrated that RFLP and phylogenetic analyses of secY gene sequences permitted finer differentiation of closely related phytoplasma strains (Lee et al., 2006, 2010). In this study, we analysed secY gene sequences of 16SrX phytoplasma strains and we utilised sequence information for designing RFLP assays for discriminating isolates within 16SrX-A subgroup. Degenerate universal primer pairs fSecY1/rSecY1, designed for amplifying partial secY gene sequences from all species in the provisional genus ‘Ca. Phytoplasma’, primed amplification of DNA from templates derived from phytoplasma strains of diverse 16Sr taxonomic subgroups (Table 1). 16SrX group-specific primer pairs fATY2/rATY2 permitted amplification of secY gene partial sequence only from 16SrX phytoplasma strains AT, AP15, PD and LP (Table 1). Nested PCRs primed by primer pairs fSecY1/rSecY1 followed by fATY2/rATY2 amplified secY gene from all the ‘Ca. P. mali’ isolates identified in this study (data not shown). PCR products from isolates V147, V247, T10 and V145 were sequenced and aligned with those from 16SrX phytoplasma isolates maintained in periwinkle (AT, AP15, PD and LP). Such isolates shared secY gene sequence identity of 89.6–100%, consistent with the data previously published by Danet et al. (2007). Italian ‘Ca. P. mali’ isolates (V145, V147, T10 and V247) shared 99.7–100% sequence identity among themselves and 96.2–96.3% sequence identity with German reference strain AT; ‘Ca. P. mali’ isolates shared 89.6–94.3% sequence identity with phytoplasma strains PD and LP. Phylogenetic analyses confirmed that Italian and German ‘Ca. P. mali’ isolates clustered together in a subclade distinguished from strains PD and LP (data not shown). Compared to the strain AT, the Italian ‘Ca. P. mali’ isolates had a 17-bp deletion in the secY gene (from nucleotide position 408 to 424; Fig. 1). The deletion caused a shift in open reading frame (ORF) and the original ORF was restored by a second deletion at position 431. Similarly, strains PD and LP had a deletion of 21 bp (from position 408 to 428) and 24 bp (from position 408 to 431), respectively compared to German strain AT (Fig. 1A and Fig. 1B). Moreover, secY gene sequences of Italian ‘Ca. P. mali’ isolates were distinguished from the German strain AT by 17 additional SNPs and from strains PD and LP by 14 additional SNPs (Table S1, Supporting information and Fig. 1B). These data were in line with the previous findings by Danet et al. (2007), but more accurately described secY gene variability between ‘Ca. P. mali’ isolates and the German reference strain AT. Intriguingly, secY SNPs that distinguished Italian and German AT phytoplasma strains were non-synonymous coding SNPs responsible for numerous amino acid substitutions in SecY protein sequences (Table S1 and Fig. 1C). Changes in SecY amino acid sequences may modify the function of SecY and consequently alter the substrate specificity and/or protein transport kinetics of the Sec translocation system. It was recently reported that (a) antigenic membrane proteins are exported to cellular membrane by Sec system (Kakizawa et al., 2004); (b) specific binding of phytoplasmal antigenic membrane proteins and insect cytoskeleton microfilaments determined the insect's capability in transmitting phytoplasmas (Suzuki et al., 2006); and (c) the main insect vectors of ‘Ca. P. mali’ are C. picta in Germany and north-eastern Italy (Jarausch et al., 2007; Mayer et al., 2009) and C. melanoneura in north-western Italy (Tedeschi et al., 2009). In light of these evidence, it would be interesting to learn whether differences in SecY protein sequences could be a factor in determining the different biological cycle of German and Italian ‘Ca. P. mali’ isolates.
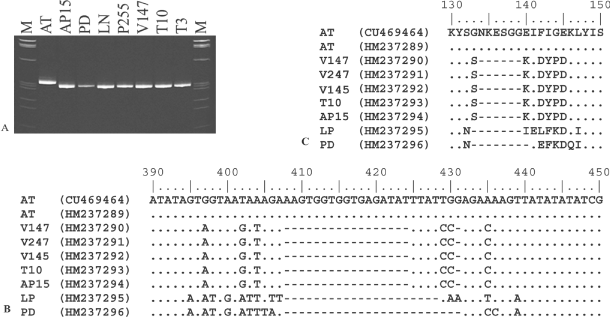
Deletions in secY gene and SecY protein sequences. (A) SecY gene PCR products amplified by means of primer pairs fATY9/rATY12 (335bp) and separated by electrophoresis on 10% polyacrylamide gel [M: molecular marker Φx 174 digested by HaeIII (Invitrogen)]; (B) SecY gene sequence alignment from nucleotide positions 390 (from the ATG start codon) to 450; (C) SecY protein sequence alignment from amino acid positions 130 to 150 (translated from nucleotide sequences in Fig. 2B). AT, ‘Candidatus Phytoplasma mali’ strain AT; AP15, ‘Ca. P. mali’ strain AP15; V147, ‘Ca. P. mali’ strain V147; V247, ‘Ca. P. mali’ strain V247; V145, ‘Ca. P. mali’ strain V145; T10, ‘Ca. P. mali’ strain T10; LP, ‘Candidatus Phytoplasma prunorum’ strain LP; Pear decline, ‘Candidatus Phytoplasma pyri’ strain PD.
Single nucleotide polymorphisms and virtual RFLP analyses on secY gene sequences identified additional molecular markers that could easily differentiate Italian ‘Ca. P. mali’ isolates from other strains. Enzymatic digestion of fATY2/rATY2 amplicons by MseI, AluI and HpyCH4V produced distinguishing restriction profiles (data not shown).
Molecular typing of ‘Ca. P. mali’ isolates
To date, MLSA was applied for investigating the genetic diversity in various bacterial taxa (Naser et al., 2006; Mignard & Flandrois, 2008; Pascual et al., 2010). Recently, MLSA was utilised for studying the complex relationships within 16SrV grapevine-infecting phytoplasmas and confirmed the presence of three genetically close distinct flavescence dorée phytoplasma clusters (Arnaud et al., 2007). Moreover, a previous preliminary study utilized an MLSA approach based on analyses of four non-ribosomal genes (secY, aceF, imp and pnp) for typing fruit tree phytoplasmas of group 16SrX (Danet et al., 2007) and confirmed the distinction between ‘Ca. P. mali’ reference strains AT and AP15 (Jarausch et al., 1994). To gain an insight into the genetic diversity among ‘Ca. P. mali’ populations in north-western Italy, in this study a PCR–RFLP-based MLSA was performed on four distinct chromosome segments: two ribosomal (16S/23S rDNA and rplV-rpsC genes) and two extra-ribosomal (PR-1, PR-2, PR-3 region and secY gene). Collective RFLP patterns, obtained by multiple gene sequence analyses, revealed the presence of 12 distinct ‘Ca. P. mali’ genetic lineages, from number 1 to 12, among 60 selected representative Italian isolates. All the 12 lineages were distinct from the lineage (number 13) represented by the German ‘Ca. P. mali’ reference strain AT (Tables 3 and 4, Fig. 2). Surprisingly, results of RFLP-based analyses suggested that ribosomal gene sequences (16S/23S rDNA and rplV-rpsC genes) were more informative than non-ribosomal gene sequences (PR1, PR2, PR3 non-ribosomal region and secY gene) in distinguishing ‘Ca. P. mali’ isolates. In fact, four and six restriction patterns were defined based on 16S/23S rDNA and rplV-rpsC amplicons, respectively, while three and two restriction patterns were evidenced based on PR1, PR2, PR3 non-ribosomal region and secY gene (Table 3 and Fig. 2). In comparison with previous works (Martini et al., 2008; Casati et al., 2010), new ‘Ca. P. mali’ RFLP patterns were identified in this study: the pattern 16SrX-A4 in 16S/23S rDNA and the patterns rpX-E and rpX-F in rplV-rpsC genes. On the other hand, based on RFLP analysis of secY amplicons, all Italian ‘Ca. P. mali’ isolates were identical to each other and to the AP15 reference strain, and differed only from the German reference strain AT.
| Lineagea | Isolateb | PR-1, PR-2 and PR-3 | 16S/23S rDNA | rplV-rpsC | secY | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HincII | PagI | Profile | HpaII | HpyCH4V | SmuI | Profile | AluI | Profile | M., A., H. | Profile | ||
| 1 | V147 | A | A | AT-1 | A | A | A | 16SrX-A2 | C | rpX-C | A | secY(X)-A |
| 2 | V145 | A | A | AT-1 | A | A | B | 16SrX-A1 | E | rpX-E | A | secY(X)-A |
| 3 | V247 | A | A | AT-1 | A | A | A | 16SrX-A2 | D | rpX-D | A | secY(X)-A |
| 4 | V240 | A | A | AT-1 | A | A | B | 16SrX-A1 | A | rpX-A | A | secY(X)-A |
| 5 | V246 | A | A | AT-1 | A | A | A | 16SrX-A2 | A | rpX-A | A | secY(X)-A |
| 6 | P270 | A | A | AT-1 | A | A | A | 16SrX-A2 | E | rpX-E | A | secY(X)-A |
| 7 | M31 | A | A | AT-1 | B | A | A | 16SrX-A3 | B | rpX-B | A | secY(X)-A |
| 8 | P264 | A | A | AT-1 | A | A | B | 16SrX-A1 | C | rpX-C | A | secY(X)-A |
| 9 | P267 | A | A | AT-1 | A | A | B | 16SrX-A1 | D | rpX-D | A | secY(X)-A |
| 10 | T9 | B | A | AP-15 | B | B | A | 16SrX-A4 | A | rpX-A | A | secY(X)-A |
| 11 | T10 | A | B | AT-2 | B | A | A | 16SrX-A3 | F | rpX-F | A | secY(X)-A |
| 12 | T16 | B | A | AP-15 | B | A | A | 16SrX-A3 | A | rpX-A | A | secY(X)-A |
| 13 | AT | A | A | AT-1 | B | A | A | 16SrX-A3 | B | rpX-B | B | secY(X)-B |
- Ca. P. mali, Candidatus Phytoplasma mali; RFLP, restriction fragment length polymorphism; M. MseI; A., AluI; H., HpyCH4V.
- aDetermined on the basis of unique collective RFLP patterns.
- bRepresentative ‘Ca. P. mali’ isolate.
| Origin | Host | Genetic Lineage | ‘Ca. P. mali’ Isolatesa | Number of Isolates |
|---|---|---|---|---|
| Lombardia | Malus×domestica Borkh. | 1 | V147; B32; B33; B42; B43; B44; B47; B48; V149; V154; V244; M5; M6 | 13 |
| Malus×domestica Borkh. | 2 | V145; V241; V251; M7; M8; M22; M24; M26; M30 | 9 | |
| Malus×domestica Borkh. | 3 | V247; V148 | 2 | |
| Malus×domestica Borkh. | 4 | V240 | 1 | |
| Malus×domestica Borkh. | 5 | V246; V242; V54 | 3 | |
| Malus×domestica Borkh. | 6 | V167; M28 | 2 | |
| Malus×domestica Borkh. | 7 | M31 | 1 | |
| Piemonte | Malus×domestica Borkh. | 1 | T6; T7; T8; T11 | 4 |
| Malus×domestica Borkh. | 2 | P257; P276; P277; P278 | 4 | |
| Malus×domestica Borkh. | 3 | P254; P255; P259; P262; P263; P268; P269; P274; P275 | 9 | |
| Malus×domestica Borkh. | 6 | P270 | 1 | |
| Malus×domestica Borkh. | 8 | P264 | 1 | |
| Malus×domestica Borkh. | 9 | P267 | 1 | |
| Malus×domestica Borkh. | 10 | T9 | 1 | |
| Malus×domestica Borkh. | 11 | T10 | 1 | |
| Valle d’Aosta | Malus×domestica Borkh. | 1 | T4 | 1 |
| Malus×domestica Borkh. | 2 | T2; T3 | 2 | |
| Cacopsylla melanoneura | 2 | T14; T19; T20 | 3 | |
| C. melanoneura | 12 | T16 | 1 | |
| Germany | Catharanthus roseus | 10 | AP15 | 1 |
| C. roseus | 13 | AT | 1 |
- Ca. P. mali, Candidatus Phytoplasma mali.
- aRepresentative isolates of diverse ‘Ca. P. mali’ genetic lineages (Table 3) are in bold.
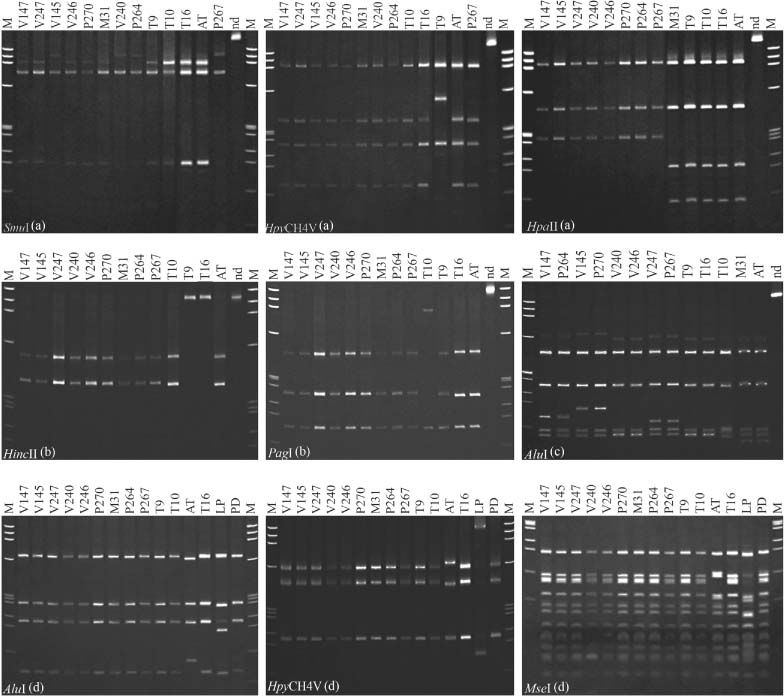
Restriction fragment length polymorphism patterns discriminating ‘Candidatus Phytoplasma mali’ genetic lineages. (A) Profiles from digestions of P1A/P7A PCR products (16S/23S rDNA); (B) profiles from digestions of AP14/AP15 PCR products (PR-1, PR-2 and PR-3 non-ribosomal region); (C) profiles from digestions of rpAP15f/rpAP15r PCR products (rplV and rpsC genes); (D) profiles from digestions of fATY2/rATY2 PCR products (secY gene). Phytoplasma acronyms are listed in Tables 1 and 3 [M, molecular marker Φx 174 digested by HaeIII (Invitrogen); nd, PCR product not digested]. Electrophoretic analyses were carried out on 6% polyacrylamide gel.
Genetic diversity and ecology of ‘Ca. P. mali’ in Italy
Extensive genetic diversity, revealed in this study through MLSA and evidenced by the description of 12 ‘Ca. P. mali’ genetic lineages in orchards of north-western Italian regions (Table 3), opened new perspectives for the investigation of ecology and epidemiology of AP disease. In fact, prevalence and distribution of the ‘Ca. P. mali’ genetic lineages differed in the geographic areas examined here (Table 4). Genetic lineages 1 (20 isolates/60), 2 (18 isolates/60) and 3 (11 isolates/60) included 82% of the ‘Ca. P. mali’ isolates examined, but lineage 1 was prevalent in Lombardia (13 isolates/31), lineage 2 in Valle d’Aosta (5 isolates/7) and lineage 3 in Piemonte (9 isolates/22) (Table 4). Furthermore, it is important to note that certain genetic lineages were present only in specific regions; for example, lineages 4 and 6 were identified only in Lombardia orchards, lineages 7–10 were found only in Piemonte and lineage 12 was identified only in Valle d’Aosta. Variation of genetic diversity among phytoplasma populations in association with specific ecological niches was reported for other phytoplasmas in previous work and a hypothesis was proposed that strain population diversity may be influenced by diverse ecological relationships that alter the population composition through strain selection (Cai et al., 2008; Quaglino et al., 2009). In the case of AP, the existence of diverse insect vectors and host plants in different geographic areas could reinforce this idea, opening the possibilities for further investigation with the aim to extend the knowledge about the distribution and frequencies of ‘Ca. P. mali’ genetic lineages in Italy and other European countries.
In conclusion, MLSA based on PCR–RFLP assays confirmed its usefulness in describing accurately the genetic diversity among closely related phytoplasma strains and/or isolates, as described in previous work (Duduk et al., 2009). In this study, a broad genetic heterogeneity (12 genetic lineages) was underscored among ‘Ca. P. mali’ populations in north-western Italy, opening a new intriguing scenario for future researches aimed to investigate the possible association of lineage-specific molecular markers and phytoplasma biological properties. Findings from this study encourage further researches (a) to extend the molecular characterization of ‘Ca. P. mali’ to strains/isolates from additional geographical areas and (b) to include more genes that are potentially involved in phytoplasma–host interaction mechanisms to MLSA. Research in that direction could improve the knowledge of ‘Ca. P. mali’ ecologies.
Acknowledgements
The work has been supported by the Regional Administration of Lombardia. Project: Ricerche sugli scopazzi del melo (apple proliferation) in Lombardia (APROLOMB).




