Viruses in soils: morphological diversity and abundance in the rhizosphere
Abstract
Soil viruses are potentially of great importance as they may influence the ecology and evolution of soil biological communities through both an ability to transfer genes from host to host and as a potential cause of microbial mortality. Despite this importance, the area of soil virology is understudied. Here, we report the isolation and preliminary characterisation of viruses from soils in the Dundee area of Scotland. Different virus morphotypes including tailed, polyhedral (spherical), rod shaped, filamentous and bacilliform particles were detected in the soil samples. An apparent predominance of small spherical and filamentous bacteriophages was observed, whereas tailed bacteriophages were significantly less abundant. In this report, we also present observations and characterisation of viruses from different soil functional domains surrounding wheat roots: rhizosheath, rhizosphere and bulk soil. In spite of the differences in abundance of bacterial communities in these domains, no significant variations in viral population structure in terms of morphology and abundance were found. Typically, there were approximately 1.1–1.2 × 109 virions g−1 dry weight, implicating remarkable differences in virus-to-bacteria ratios in domains close to roots, rhizosphere and rhizosheath (approximately 0.27) and in bulk soil (approximately 4.68).
Introduction
Viruses infect organisms belonging to all the primary domains of life (Bacteria, Archaea and Eukarya) and exist wherever life is found. However, the central focus of virus research has traditionally concerned human, animal and plant viruses because of their prominent role as pathogens (for reviews, see Van der Want & Dijkstra, 2006; Acheson, 2007). In addition, some prokaryotic viruses or bacteriophages have also been well studied as convenient model systems for molecular biology and tools for phage therapy (for reviews, see Kutter et al., 2005; Summers, 2005). Environmental virology is also now fast becoming an important area of research and has already produced significant achievements, in particular, in the field of marine ecosystems (Wommack & Colwell, 2000; Suttle, 2005, 2007). However, soil viruses are less well studied.
A global number of about 2000 virus species has recently been recognised (Fauquet et al., 2005), but this is likely to be a gross underestimate because of the as yet unidentified ‘species’ from unsampled or poorly investigated habitats (Breitbart & Rohwer, 2005; Fierer et al., 2007). Virus particles are abundant in air (Verreault et al., 2008), water (Wommack & Colwell, 2000; Suttle, 2005, 2007), marine sediments (Danovaro et al., 2001), extreme thermal environments (Rice et al., 2001), biofilms (Brüssow & Kutter, 2005), soils (Ashelford et al., 2003; Williamson et al., 2005, 2007) and other environments. Moreover, recent metagenomic analyses have demonstrated hitherto unknown diverse assemblages of viruses in different environments (Breitbart et al., 2002, 2004; Breitbart & Rohwer, 2005; Fierer et al., 2007; Dinsdale et al., 2008). Fierer et al. (2007) reported that although DNA sequences for bacteriophages were present in soils from different ecosystems, the majority of the 4577 sequences analysed showed no similarity to previously described sequences.
Viruses of soil bacteria, like all the other bacteriophages (Kutter et al., 2005; Campbell, 2006), can be virulent (lysing host bacteria) or temperate (existing in a repressed state as a prophage) (Ashelford et al., 1999b; Williamson et al., 2007; Ghosh et al., 2008). In many cases, the prophage DNA actually integrates into and replicates along with the host chromosome. Such a lysogenic state may continue for many generations until the prophage is activated either spontaneously or by some physical or chemical factors in a process called prophage induction (Campbell, 2006). During induction and consequent viral replication through the lytic cycle (Kutter et al., 2005), bacteriophages can incorporate bacterial genes and transfer them from one bacterium to another (transduction), mediating horizontal gene transfer (for review, see Barkay & Smets, 2005). An indication of the potential reservoir of genetic diversity in soil viruses and its potential to manipulate the host metabolism is provided by the finding that bacteriophages in soil carry many different ‘specialisation genes’ not required by the virus. For example, 130 genes (of a possible 157) encoding motility and chemotaxis proteins were found in soil bacteriophage genomes, despite their inability to be motile (Dinsdale et al., 2008). Like marine bacteriophages (Wommack & Colwell, 2000; Suttle, 2005, 2007), bacteriophages in soils may also have the direct capacity to regulate population size, structure and activity of the bacterial communities, for example through lysis. It has been reported that bacteriophages can affect the colonisation ability (Stephens et al., 1987), survival and biocontrol activity (Keel et al., 2002) of fluorescent Pseudomonas spp. in natural soils. Bacteriophages can also significantly reduce soil populations of some rhizobia strains (Hashem & Angle, 1990). Thus, as potential causes of mortality and horizontal gene transfer, soil viruses are of great importance.
However, very little is known about the structure and functions of autochthonous soil viruses and their interactions with host populations. Early studies have used cultivable soil microbe hosts or PCR techniques involving already recognised specific primers and have therefore mainly targeted specific virus groups rather than entire virus populations (Ashelford et al., 1999a,b, 2000; Monpoeho et al., 2001). We are aware of only a few studies describing direct examination of virus populations from soils. Using transmission electron microscopy (TEM), Ashelford et al. (2003) found substantial populations of tailed bacteriophages in Oxford and Cardiff soils. In addition to tailed bacteriophages, Williamson et al. (2005) also analysed other virus morphotypes such as spherical, elongated and filamentous virus-like particles from Delaware soils. More recent metagenomic analyses of double-stranded (ds)DNA-containing bacteriophage types (Fierer et al., 2007) showed that only some of these bacteriophages were similar in nucleotide sequences to bacteriophages that infect the soil bacteria Actinoplanes, Mycobacterium, Myxococcus and Streptomyces as well as the halophilic archaeon Haloarcula, whereas the vast majority of the viral sequences found in soils were novel showing no similarity to any known sequences.
The objective of this work was to characterise morphological diversity and abundance of viruses isolated from soils in the Dundee area of Scotland and from functionally different regions of a soil on which a wheat crop was growing.
Microbial communities are heterogeneously distributed in soils with the presence of roots having a major effect on the numbers and types found at specific locations (Foster, 1986). In many graminaceous plants, domains constituting the rhizosheath, rhizosphere and bulk soil have been demonstrated to have different physical and chemical properties and different biological communities and activities (Gregory, 2006). Rhizosphere and rhizosheath, the narrow regions of soil surrounding mature and young roots, respectively, are directly influenced by extensive populations of the associated soil micro-organisms (bacteria and fungi), which have a critical impact in the maintenance of plant health and soil quality (Walker et al., 2003; Gregory, 2006). As a first step towards understanding the role of viruses infecting soil micro-organisms in these processes, we characterised morphological diversity and abundance of viruses in rhizosphere and rhizosheath versus bulk soil.
Materials and methods
Initial soil samples were taken from a Dystric-Fluvic Cambisol (classification of Food and Agriculture Organization, FAO) soil, with a sandy loam surface texture, during the fallow period of a soil disturbance experiment, in which barley had been grown for the previous 3 years, on the Scottish Crop Research Institute (SCRI) field site near Dundee (56 27′16″N, 03 04′55″W).
Rhizosphere, rhizosheath and bulk soil samples were collected from the upper 10 cm of a separate field at SCRI, Invergowrie, Dundee, with the same soil type, on 23 June and 27 June 2006. Three wheat (Triticum aestivum) plants (at the early grain filling stage of crop growth) were sampled at each time point, and the soil was separated into three categories and bulked to form a single sample of each compartment at each time point. The plants were gently shaken, and loose soil was collected as bulk soil. Soil aggregates and particles held by the root system were removed with more vigorous shaking and by forceps and collected as rhizosphere soil, while soil particles attached to the basal regions of nodal roots as an intact sheath were removed by forceps and collected as rhizosheath.
Soil samples (50–100 g) were homogenised with an equal volume of 0.067 M Na/K Sorensen’s phosphate buffer (pH 7.4) in a blender (3 min at 22 000 r.p.m.) at 4°C, and the homogenate was filtered through muslin. The solids collected in the muslin were then rehomogenised with a further 0.5 volumes of buffer and filtered through muslin. In the initial experiments, these soil suspensions were centrifuged at low speed (5 min at 8000 g) to sediment soil particles, filtered through a 0.2 μm Nalgene Fast PES Filter Unit (Nalge Nunc International, Rochester, NY, USA) to remove bacteria and small soil particles essentially as described earlier by Ashelford et al. (2003) and Williamson et al. (2005) and then examined by TEM as below.
For further purification of soil viruses, the initial extracts filtered through muslin were combined and lysozyme was added to a final concentration of 200 μg mL−1. This was stirred at room temperature for 20 min to allow digestion of bacterial cell walls to occur. Chloroform, 10% (v/v), was then added to the extracts and mixed well. The extracts were centrifuged at 8000 g for 10 min. The aqueous phase of the supernatant was collected and filtered first through glass wool and then through a 0.2 μm Nalgene Fast PES Filter Unit (Nalge Nunc International). The filtrate was then spun in an R50.2Ti rotor in an Optima™ L-80 XP ultracentrifuge at 105 000 g for 2 h through a 25% sucrose cushion. The resulting pellets were resuspended in a small volume (0.01 × original volume) of 0.033 M Na/K Sorensen’s phosphate buffer (pH 7.4) at 4°C. The resuspended extracts were spun in Eppendorf tubes in a bench-top microcentrifuge at 2000 g for 5 min, and the supernatant was collected. After removing the supernatant from the initial extraction, soil pellets were resuspended in fresh eluent and the extraction procedure was repeated three more times.
TEM analysis of virus particles was performed essentially as described by Danovaro et al. (2001). Carbon-coated copper grids were floated for 5 min on 10 μL drops of samples on wax slides. Grids were then removed from the drops, and excess sample was drained from the grids using filter paper. Then, 10 μL drops of 1% (w/v) phosphotungstic acid (pH 7.0) were put on the grids and left for 30 s and then drained from the grids using filter paper. Grids were examined in a Jeol 100S electron microscope at 80 kV. Virus particles were enumerated in 8–10 fields per grid, and 800–1000 individual particles were examined from each replicate of each soil compartment. Average virus counts were based on a grand mean of three replicates (experiments) and expressed as means ± standard deviations. In preliminary experiments using tobacco mosaic virus sample as concentration standard, this procedure has been shown to allow the absorbance of 20% of total virus particles. Similarly, using different dilutions of our soil virus samples and allowing them to dry onto the grids, we calculated that 20% of the total virus particles were sticking to the grids using our method. For example, whereas total direct count of virus particles carried out by drying 10 μL drop of virus preparation on a grid (at dilution of soil sample 1:100) was 1.10 × 109 virions g−1 dry weight (for bulk soil), the floating method described above showed 0.22 × 109 virions g−1 dry weight. This trend was consistent over different soil samples. Moreover, it was also consistent for all particular virus morphotypes (for example, bulk soil sample showed direct counts of tailed bacteriophages of 4.4 × 107 and 0.87 × 107 virions g−1 dry weight by drying and floating methods, respectively). This confirms that the floating method is representative of total extractable virus particles, although it visualises only 20% of them, and therefore, the abundance values presented in this paper were extrapolated to correct for the deficit.
Total direct bacterial counts were carried out essentially as described by Williamson et al. (2005). Ten-millilitre portions of 10−4 soil extract dilutions were filtered through 0.22-μm black polycarbonate filters (Millipore, Billerica, MA, USA) and stained with SYBR Gold (Invitrogen, Carlsbad, CA, USA). Bacteria were enumerated under epifluorescence microscope in 40 fields per filter. Average bacterial counts were based on a grand mean of three replicates (experiments) and expressed as means ± standard deviations.
Principal component analysis and ANOVA significance test to assess morphological virus population structure were performed in GENSTAT version 9.2 (VSNi, Hemel Hempstead, UK).
Results
Isolation of viruses from soil
In the initial experiments, viruses were isolated from soil in the fallow period of an intensive barley rotation using a purification procedure essentially similar to those described by Ashelford et al. (2003) and Williamson et al. (2005). Soil was sampled from plots under conventional ploughing and zero tillage regimes. The isolation procedure that included two consecutive extractions (see below), low-speed centrifugation and filtration through a 0.2-μm-pore-size membrane filter to reduce debris allowed us to clearly detect tailed bacteriophage particles in TEM. However, other types of virus particles could not be recorded unequivocally as they were hard to distinguish against the remaining debris. Similar observations were reported by Ashelford et al. (2003) who focused therefore only on the counts of tailed bacteriophage particles. To expand our investigation to other virus morphotypes, we applied additional purification steps such as treatment of samples with chloroform and high-speed centrifugation through sucrose cushion to decrease background debris. We also treated initial soil samples with lysozyme to lyse all the bacteria and hence release virus particles that did not lyse bacteria themselves. Although such treatments might destroy some virus particles and hence lead to underestimates of their abundance, they significantly reduced background and allow the detection of virus morphotypes undetectable before these treatments.
To determine how many extractions were required to extract virus particles, soil samples were sequentially extracted four times, and we found that approximately 75% of the virus particles were eluted in the first extraction, 20% were eluted in the second extraction and 5% were eluted in the third extraction. The fourth extraction did not result in further increase in numbers of extractable virus particles. Therefore, the isolation procedure described in this work included two consecutive extractions, which were enough to extract the vast majority of virus particles (approximately 95%).
Morphotypes of viruses isolated from soils under differential tillage
Although TEM may underestimate counts of virus particles from soils as suggested earlier (Ashelford et al., 2003; Williamson et al., 2003), we used this method to ensure that actual virus particles were being observed and counted. TEM analysis of the virus preparations showed that virtually identical morphological types of viruses were isolated from the different soil samples tested (see below). Moreover, the vast majority of the isolated virus particles were morphologically similar to known virus taxonomic groups (Ackermann, 2005, 2007; Fauquet et al., 2005). The isolated virus particles were tailed, spherical, rod shaped, filamentous or bacilliform.
Tailed bacteriophage particles
All known tailed bacteriophages contain dsDNA and belong to the order Caudovirales, which consists of three families: Myoviridae (long rigid contractile tails), Siphoviridae (long flexible non-contractile tails) and Podoviridae (short contractile tails) (Ackermann, 2005, 2007; Fauquet et al., 2005). Tailed viruses (bacteriophages) isolated from the soil samples were of various sizes and morphologies (Fig. 1a, I–IV). Particles with rigid tails were morphologically similar to those found in viruses belonging to the family Myoviridae with either slightly elongated or spherical (Fig. 1a, I) heads of between 55 and 90 nm diameter with tails of between 75 and 150 nm length. Some of Enterobacteria and Pseudomonas phages belong to this virus family (Ackermann, 2005, 2007; Fauquet et al., 2005). Particles with spherical (50–75 nm in diameter) or elongated (from 85 × 55 to 105 × 45 nm) heads and long flexible non-contractile tails (from 120 to 225 nm in length) morphologically resembled bacteriophages of the family Siphoviridae (Fig. 1a, II–III). Viruses affiliated with the Enterobacteria, Bacillus, Mycobacterium or Streptomyces exhibit these characteristics. Bacteriophages with icosahedral heads (55–75 nm in diameter) and short tails (15–20 nm in length), morphologically similar to Enterobacteria or Bacillus bacteriophages of the family Podoviridae, were isolated as well (Fig. 1a, IV). The distribution of virus morphotypes indicated that the majority of tailed bacteriophages found in the soil samples belong to the family Podoviridae (approximately 50% of total tailed bacteriophages). The families Myoviridae and Siphoviridae were represented by approximately similar numbers of tailed bacteriophages (approximately 25% of total tailed bacteriophages in each family). These results are in a good agreement with previously published findings showing the presence of different types of tailed bacteriophages (belonging to the order Caudovirales) in soils of other geographic areas (Ashelford et al., 2003; Williamson et al., 2005; Fierer et al., 2007).
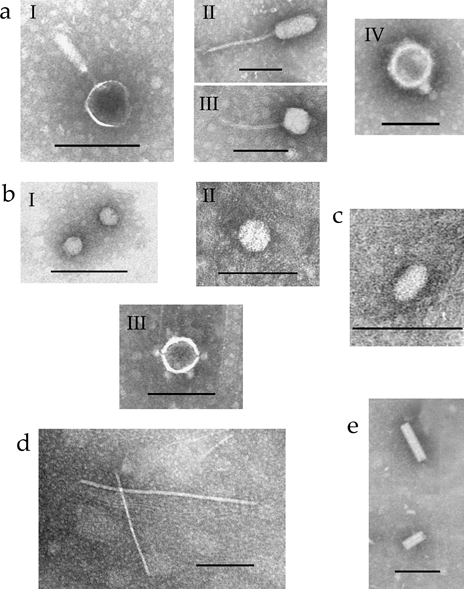
Transmission electron microscopy images of viruses isolated from Dundee soils. (a) Tailed bacteriophages similar to Myoviridae (I), Siphoviridae (II and III) and Podoviridae (IV) bacteriophage particles. (b) Small spherical (I) and large spherical viruses with (III) and without (II) spikes. (c) Bacilliform virus. (d) Filamentous virus. (e) Rod-shaped virus. Bars, 100 nm.
Spherical viruses
Spherical viruses of various sizes were also isolated from the soil samples (Fig. 1b). Although TEM-based identification of such virus particles is difficult, small spherical particles could be similar in size (approximately 25 nm in diameter; Fig. 1b, I) to single-stranded (ss) RNA-containing bacteriophages of the family Leviviridae (affiliated with the Enterobacteria or Pseudomonas) or small plant viruses (Ackermann, 2005; Fauquet et al., 2005).
The larger spherical particles with a diameter of approximately 40 nm (Fig. 1b, II) could be similar in size with dsRNA-containing virus particles of the families Partitiviridae, Chrysoviridae and Totiviridae, some members of which infect fungi, including fungal plant pathogens such as Rhizoctonia solani, Fusarium solani and Ustilago maydis (Fauquet et al., 2005). Thus, it is conceivable that the spherical particles found in this work may be similar to some or all of these virus groups. In addition, spherical particles (with a diameter of approximately 60 nm) with apical spikes were also detected (Fig. 1b, III). These particles may resemble dsDNA-containing virions formed by members of the family Tectiviridae (Fauquet et al., 2005). Our preliminary studies of viruses from cultured soil bacteria have confirmed that particles with this morphology are found in natural infections of some soil bacteria (data not shown). The presence of spherical types of virus particles was also reported for Delaware soils (Williamson et al., 2005).
Filamentous particles
Filamentous particles isolated from soils (Fig. 1d) showed considerable variation in both diameter and length. Some may correspond to the virus particles of the Inovirus genus with diameters approximately 7 nm and lengths between 700 and 2000 nm. Viruses of this genus contain circular ssDNA within flexible filamentous virions (Ackermann, 2005; Fauquet et al., 2005). The presence of this type of virus particles was also reported for Delaware soils (Williamson et al., 2005).
Rod shaped
A rod-shaped type of virus architecture was represented by particles that resemble rigid rod-shaped viruses with a central canal (Fig. 1e). These virus particles were 50–90 nm in length and 14–16 nm in diameter. Particles with similar morphology are found in the genus Plectrovirus belonging to the family Inoviridae (Fauquet et al., 2005). This genus includes ssDNA-containing bacteriophages infecting Acholeplasma spp. and Spiroplasma spp., some of which are plant pathogens. However, it also cannot be completely ruled out that these particles represent fragments of some rod-shaped plant viruses or bacteriophage tails.
Bacilliform particles
Finally, bacilliform particles were also isolated from Dundee soils. These particles were approximately 20 × 50 nm (Fig. 1c) and resembled the ssRNA-containing Mushroom bacilliform virus belonging to the family Barnaviridae (Fauquet et al., 2005).
Distribution of virus morphotypes in different soil domains surrounding wheat roots
All the virus morphotypes revealed in the initial experiment collected from fallow soils and described above were also found in virus preparations isolated from rhizosheath, rhizosphere and bulk soil surrounding wheat roots. The virus morphotype most frequently occurring in all the three soil domains was represented by small spherical particles (Fig. 2).
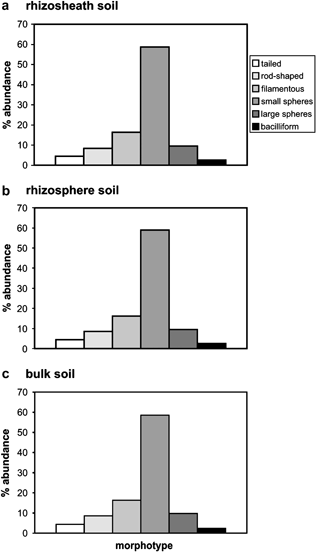
Relative abundance of viral morphologies from different soil domains: rhizosheath (a), rhizosphere (b) and soil bulk (c) surrounding wheat roots. Categories include tailed, rod-shaped, filamentous, bacilliform and small spherical and larger spherical virus particles. Histograms are based on measurements of 800–1000 particles per sample. Analysis was based on duplicate field samples. Morphological types are described in Fig. 1.
Filamentous virus particles were another prominent virus category found in similar proportions in the rhizosheath, rhizosphere and bulk soil (Fig. 2). All other morphotypes such as tailed, rod shaped, large spherical and bacilliform particles were also observed in all soil domains around wheat roots. The frequency of their presence in different domains was also similar (Fig. 2). Moreover, a combination of multivariate and ANOVA analysis of the distribution of virus morphotypes observed by TEM did not indicate any significant differences between the virus populations from any of the soil domains, although bulk soil may present a more variable distribution pattern than the other compartments.
These results demonstrate a remarkable diversity of virus morphotypes in the rhizosheath, rhizosphere and bulk soil with no clear differences between their virus populations. In all the three soil domains, an apparent predominance of small spherical and filamentous bacteriophages was observed.
Bacterial and viral abundance in different soil domains surrounding wheat roots
Total direct counts of bacteria carried out by epifluorescence microscopy (Fig. 3) showed no significant difference between rhizosphere [(4.3 ± 0.4) × 109 cells g−1 dry weight] and rhizosheath [(4.1 ± 0.3) × 109 cells g−1 dry weight]. However, the bacterial abundance in bulk soil was significantly lower [(0.25 ± 0.04) × 109 cells g−1 dry weight; Fig. 3] (P < 0.01).
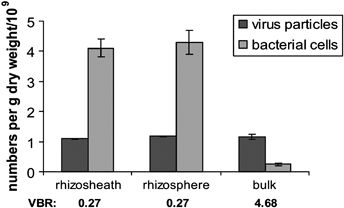
Abundance of viruses and bacteria g−1 dry weight in fallow soil and different soil domains: rhizosheath, rhizosphere and soil bulk surrounding wheat roots. VBR values are indicated. Analysis was based on duplicate soil samples except for the fallow soils that had three replicates. Bars represent standard deviations.
Quantitative analysis of virus populations was performed using TEM. Although this method may lead to more underestimates than some others, such as epifluorescence microscopy, its clear advantage is a capacity to detect regular-shaped virus particles (as the vast majority of viruses have a regular structure). Moreover, assuming that virus counts were similarly underestimated, the comparative analysis of virus abundance in different soil zones should not be affected. In contrast to microbial communities, the abundance of virus particles was similar in all the three soil domains: rhizosphere, rhizosheath and bulk soil. Typically, there were approximately 1.1–1.2 × 109 virions g−1 dry weight (1.18 ± 0.014 × 109 virions g−1 dry weight for rhizosphere, 1.09 ± 0.014 × 109 virions g−1 dry weight for rhizosheath and 1.17 ± 0.085 × 109 virions g−1 dry weight for bulk soil) (Fig. 3). These observations clearly indicate that although viruses were extremely abundant in all soils tested, the virus-to-bacteria ratios (VBRs) were much greater in bulk soil (approximately 4.68) than in rhizosphere and rhizosheath (approximately 0.27) (Fig. 3).
Discussion
This work describes morphological characterisation of viruses isolated from arable soils around Dundee including different soil domains surrounding wheat roots. The majority of virus particles identified in this work were similar to previously known viral morphotypes represented by tailed, polyhedral (spherical), rod-shaped, filamentous and bacilliform virions and were detected in soils previously (Ashelford et al., 2003; Williamson et al., 2005). The preliminary classification of these viruses based on morphological similarities with the already recognised virus taxonomic groups will be more precisely defined in the future by conducting metagenomic analysis of viral nucleic acids, as has been conducted by LeRoy et al. (2008) for bacteriophages isolated from Seine River sediment. Although precise host ranges for all these viruses remain to be identified, these viruses can presumably infect soil and plant-parasitic bacteria and fungi belonging to the taxonomic groups mentioned above. Tailed, filamentous and rod-shaped viruses similar to those described in this work were also shown to infect Archaea, which have been shown to be relatively abundant in many soil samples (Stedman et al., 2006).
Quantitative analysis of virus particles from soils strictly depends on the methods of virus extraction, purification and enumeration. The more vigorous purification procedures may lead to losses and underestimates of virus particle numbers but when used in combination with TEM will reduce errors related to detection of contaminant false (non-viral) particles. Using a mild extraction procedure, Ashelford et al. (2003) were able to count only tailed bacteriophage particles or their heads. To unequivocally identify other types of virus particles, we extended our purification scheme as described above. We also used lysozyme to release and detect virus particles from infected but unlysed bacteria. Such viruses may also play an essential role in the population dynamics of soil microbial communities, and hence, it is relevant to include them in the soil virus population. Thus, although our estimates may not be absolutely accurate, we assume that similar treatments of different soil samples (including purification and enumeration procedures) allow their comparative analysis.
The presence of tailed bacteriophages in the rhizosphere was reported earlier (Campbell et al., 1995; Ashelford et al., 1999a, 2000). In this work, we demonstrate that virus populations of different soil domains surrounding wheat roots (rhizosphere, rhizosheath and bulk soil) were similar in morphological distribution. Moreover, they were, surprisingly, not only dominated by small spherical and filamentous virus particles but also contained virions of other morphotypes (Fig. 2). Although the numbers of tailed bacteriophage particles revealed in this work (approximately 4.8 × 107 virions g−1 dry weight) were in a good agreement with the abundance of tailed bacteriophages (or their heads) previously determined in rhizosphere and bulk soils in Oxford and Cardiff (Ashelford et al., 2003), they comprised only approximately 4% of total virus populations described in this work. Such a morphological distribution of virus populations is quite different from that observed among 5500 known phage isolates where tailed morphotypes significantly prevailed (Ackermann, 2007). Tailed bacteriophages were also found to dominate in most of the Delaware soils tested (Williamson et al., 2005). Such differences in morphological distributions of virus populations may be explained by biogeographic peculiarities of soils and their bacterial communities accommodating different virus morphotypes. However, it cannot be completely ruled out that the extraction procedures used in this work could have resulted in a breakage of some bacteriophage tails, miscategorising virus particles into the spherical morphotype.
Virus particles were extremely abundant in all the three soil domains. Moreover, the abundance of virus particles was similar in all of them (approximately 1.1–1.2 × 109 virions g−1 dry weight) (Fig. 3). Although these data were obtained with TEM, they are consistent with previously published data on epifluorescence microscopy of virus particles from Delaware soils (1.31–4.17 × 109 virions g−1 dry weight in forest soils and 0.87–1.1 × 109 virions g−1 dry weight in agricultural soils; Williamson et al., 2005).
In contrast to the commonly observed increased populations of bacteria in rhizosphere and rhizosheath compared with soil bulk, for example by Foster (1986) and Kandeler et al. (2002), which were also confirmed in our work (approximately 4 × 108 cells g−1 dry weight in rhizosphere and rhizosheath versus approximately 0.25 × 108 cells g−1 dry weight in bulk soil), we found that virus abundance did not increase in the soil domains surrounding wheat roots. This result indicates remarkable differences in VBRs in domains close to roots, rhizosphere and rhizosheath (approximately 0.27) and in bulk soil (approximately 4.68). No definite reason for the apparently contrasting behaviours of virus particles and other microbial populations leading to very low VBRs in response to roots can be advanced. It is tempting to suggest that physical, chemical or biochemical conditions existing near roots cause a decline in infectivity of viruses (yield of virus particles per single bacterium) and in this way regulate microbial populations. Another possibility is that bacteria specifically invading rhizosphere and rhizosheath are less susceptible to viruses, and amounts of virus particles are determined by bacteria common to different soil domains. Taking into account that the high VBRs in soil bulk compared with rhizosphere and rhizosheath owe more to lower bacterial abundance than to high viral abundance, this difference can also be explained by difficulties in extraction of bacteria from bulk soil. Other sources of errors originated from two measurements (virus particles and bacteria) during virus and bacteria isolation (extraction), and counting may also affect the VBR values (Williamson et al., 2007).
Typically, the VBR of marine waters is about 10 (Wommack & Colwell, 2000), while VBR of marine sediments has been closer to 1 (Danovaro et al., 2001). The VBRs reported for the forest soils in Delaware were approximately 4.6–12 (Williamson et al., 2005, 2007). However, the VBRs for Delaware agricultural soils have been remarkably higher (approximately 3000 at less rigorous bacteria extraction procedure and approximately 460 at more rigorous extraction procedure), possibly because of difficulties of extraction of bacteria from these soils as suggested by the authors (Williamson et al., 2005, 2007). The VBR determined in our work for bulk soil was similar to those reported for the forest soils in Delaware.
High abundance of virus particles in soils reported here as well as in previous publications (Ashelford et al., 2003; Williamson et al., 2005) suggests that at least the majority of virus particles represented by all observed morphotypes are autochthonous soil viruses, which were produced in situ. However, it cannot be completely ruled out that some viruses were exogenous and transported to soils, for example, with water.
It is worth noting that in spite of similarities in morphology and abundance of virus populations in different soil domains surrounding wheat roots, the populations may still be diverse in the precise composition of virus species. The challenges for the future are to isolate the viruses described here and analyse their genomic nucleic acids and to resolve whether plant roots and their associated microbial communities play any significant role in the composition and dynamics of soil viral populations using culture-based and physiological experiments. The metagenomic analysis will also be applied to help analyse virus diversity in the different soil domains surrounding plant roots.
Acknowledgements
The work was supported by Scottish Government Rural and Environment Research and Analysis Directorate. We wish to thank Kathryn Wright for her valuable assistance with artwork.




