POSITIVE CONTRAST MAGNETIC RESONANCE BURSOGRAPHY FOR ASSESSMENT OF THE NAVICULAR BURSA AND SURROUNDING SOFT TISSUES
Abstract presented at the American Association of Equine Practitioner's Annual Convention, December 2008 and American College of Veterinary Surgeons Annual Convention, October 2008 and the Annual Meeting of the American College of Veterinary Radiology, November 2007.
Abstract
Magnetic resonance (MR) imaging is often performed to determine the cause of palmar heel pain. We evaluated how distension of the navicular bursa affected the MR appearance of the navicular bursa and associated structures. An MR evaluation was performed on normal cadaver limbs and cadaver limbs from horses with lameness localized to the foot. The normal navicular bursae were injected with 2, 4, or 6 ml of solution. The bursae of the feet from lame horses were injected with 4 or 6 ml, and the MR study was repeated. All bursae were examined grossly to verify the presence or absence of adhesions. Clinical patients that had initial MRI abnormalities suggesting adhesions were also evaluated. Distension of the proximal recess of the normal navicular bursa, proximal to the collateral sesamoidean ligament was achieved with 2 ml. Separation of the collateral sesamoidian ligament from the deep digital flexor tendon (DDFT) was achieved with 4 ml. The separation of the navicular bone from the DDFT and distal sesamoidian impar ligament required 6 ml. Adhesions were more clearly defined in the bursa of the two pathologic cadaver limbs following distension. MR bursography used on clinical patients allowed the determination of the presence or absence of adhesions. In these horses, this determination could not have been definitively made without this technique. MR bursography is useful in horses where the presence of adhesions cannot be clearly defined by MRI.
Introduction
Palmar heel pain, a common cause of equine lameness, can have many different etiologies. The prognosis depends on the severity of injury and the structures involved. Injury to the deep digital flexor tendon (DDFT) and surrounding structures such as the distal sesamoidian impar ligament (DSIL), collateral sesamoidean ligament, also called the navicular suspensory ligament, and navicular bone, as well as adhesions between these structures, are all potential causes of palmar heel pain.1–3 Adhesions carry a poor to grave prognosis for return to soundness.4 Of 34 limbs of horses with palmar heel pain, seven (21%) had evidence adhesions between the DDFT and DSIL in magnetic resonance (MR) images.5 In another group of similar horses, 44% had flexor cortex erosion of the navicular bone, and 66% of those horses had adhesions between the navicular bone and the DDFT.3 Thus, detection of adhesions is necessary for an accurate prognosis.
MRI is the modality of choice for assessing soft tissue structures of the equine digit. However, the ability to detect adhesions is impacted by the anatomy of the foot and the close proximity of structures at risk.3,6,7 This is especially true when there is minimal fluid in the navicular bursa.3 In MR images, the region of the navicular bursa has a thin-band of intermediate signal dorsal to the DDFT.8 The normal DDFT is separated from the palmar distal surface of the collateral sesamoidian ligament by the proximal recess of the navicular bursa. The distal recess of the navicular bursa separates the palmar surface of the DSIL from the dorsal margin of the DDFT.9 Fluid in the navicular bursa allows clear delineation of the dorsal margin of the DDFT in MR images.
Adhesions are suspected when the normal fluid signal of the navicular bursa cannot be visualized and the navicular bursa contains tissue that appears continuous with the collateral sesamoidian ligament, DSIL and/or DDFT. Adhesions are also suspected when defects identified in the flexor surface of the navicular bone contain tissue that extends to the DDFT.6,10 The heterogeneity of the DSIL makes subtle abnormalities in tissue signal difficult to evaluate. Adhesions are considered when fluid in the distal recess of the navicular bursa cannot be identified between the DSIL and DDFT.5 However, synovial proliferation and/or synovial hypertrophy in the navicular bursa adjacent to surrounding structures can be difficult to differentiate from adhesion formation. Synovial proliferation and/or hypertrophy can have similar signal intensity to the DDFT, collateral sesamoidian ligament, and/or DSIL or may not be separated from these structures by fluid. This lack of separation can make the synovial proliferation and/or synovial hypertrophy appear continuous with the DDFT, collateral sesamoidian ligament and/or DSIL, similar to adhesions. Furthermore, the close proximity and similar signal intensity of the navicular bone flexor surface to the DDFT can obscure the presence of adhesions in horses lacking substantial defects in the navicular bone flexor surface and/or the DDFT. These problems are similar to those encountered in MRI of the human temporomandibular joint.11
When histologic findings were compared with MR findings where flexor surface defects were present, the sensitivity of MRI for detection of all histologic abnormalities of the flexor surface of the navicular bone was 83%; but the specificity was only 65%.10 Considering the low specificity, it is possible that distension of the navicular bursa may improve the specificity of MR imaging for certain lesions. Synovial proliferation and/or hypertrophy will be displaced by distension of the navicular bursa, whereas adhesions will not. Navicular bursa distension allows for the differentiation of synovial proliferation and/or hypertrophy from adhesions. In addition, parasagittal splitting of the DDFT, dorsal margin tearing of the DDFT, or defects in the navicular bone flexor surface not visible initially could be identified following positive contrast MR bursography. Detection of DDFT injury is important for giving an accurate prognosis. Only 28% of horses with a primary DDF tendonitis returned to full athletic function without recurrent lameness. Furthermore, 95% of horses with DDF tendonitis combined with navicular bone and/or bursa injury suffered from recurrent or persistent lameness.3
Our purpose was to use positive contrast bursography to identify the limits of the navicular bursa, to distinguish synovial proliferation from adhesions and to identify pathologic change in the bursa and adjacent structures that was not clearly defined without fluid distention of the bursa. We hypothesized there would be a specific volume of fluid which would distend the navicular bursa and delineate the adjacent structures thereby more clearly defining normal structures from pathologic changes associated with the navicular bursa and surrounding structures.
Materials and Methods
There were two parts to our work; a cadaver study and a clinical study. For the cadaver study, 20 forelimbs of horses with no known history of palmar heel pain and six forelimbs from horses with lameness localized to the front feet were imaged using a 1.0 T Pegasus MR system from ONI Medical Systems.* Proton density turbo spin echo and short tau inversion recovery (STIR) sequences were used. Sagittal, transverse, and dorsal plane images (3 mm) were obtained. These sequences were selected for soft tissue contrast and for determining the presence of fluid (Table 1). Following the initial MR imaging, the navicular bursa was injected with a 1:1 ratio of 0.9% saline: Hypaque-76® (Diatrizoate Meglumine and Diatrizoate Sodium)† in varying volumes using a 20 G, 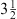 in. spinal needle inserted on the palmar aspect of the limb, midway between the heel bulbs, approximately 1 cm proximal to the coronary band. The needle was directed parallel to the sole of the hoof and advanced approximately midway through the foot, until bone was encountered. Correct needle placement was verified with digital radiography, then the allocated volume of contrast medium was injected into the navicular bursa. An additional radiograph was made to confirm of the presence of contrast medium in the navicular bursa. The MR protocol was then repeated. Five forelimbs were injected with 2 ml, five with 4 ml, five with 5 ml, and five with 6 ml.
in. spinal needle inserted on the palmar aspect of the limb, midway between the heel bulbs, approximately 1 cm proximal to the coronary band. The needle was directed parallel to the sole of the hoof and advanced approximately midway through the foot, until bone was encountered. Correct needle placement was verified with digital radiography, then the allocated volume of contrast medium was injected into the navicular bursa. An additional radiograph was made to confirm of the presence of contrast medium in the navicular bursa. The MR protocol was then repeated. Five forelimbs were injected with 2 ml, five with 4 ml, five with 5 ml, and five with 6 ml.
| Sequence | TR (ms) | TE (ms) | NEX | FOV (cm) | Flip Angle (°) | ST (mm) | Gap (mm) | Matrix |
|---|---|---|---|---|---|---|---|---|
| PD TSE | 3000 | 30 | 2 | 15 | 90 | 3 | 0 | 320 × 192 |
| STIR | 5300 | 14 | 2 | 15 | 90 | 3 | 0 | 288 × 192 |
- TR, time of repetition; TE, time of echo; NEX, number of excitations; FOV, field of view; ST, slice thickness; PD, proton density; TSE, turbo spin; STIR, short tau inversion recovery.
Of the six cadaver limbs with lameness localized to the front feet, three were injected with 4 ml, one with 4.5 ml, one with 5 ml, and one with 6 ml.
All limbs were imaged within 6 h of euthanasia. After imaging, a bandsaw was used to remove the heel bulbs and the sole. The soft tissues on the palmar aspect were dissected and the DDFT was retracted palmarly until the navicular bursa was reached.
The material assessed in the clinical study was comprised of the front feet of 16 horses presenting with palmar heel pain that had undergone positive contrast MR bursography as of 2005. Age ranged from 2 to 15 years. There were eight Quarter horses, six Warmbloods, one Thoroughbred, and one Paint horse. Bursography was used to distinguish between adhesions and synovial proliferation and/or hypertrophy, and to identify other sources of pathologic change in adjacent structures. Precontrast MR images were evaluated to determine if navicular bursa distension was indicated, based on the suspicion of navicular bone flexor surface abnormalities, or the presence of adhesions. In addition, bursography was performed in horses where the precontrast findings were not consistent with the degree of lameness. Synovial proliferation in the navicular bursa was determined to not be adhered to the DDFT if a clear area of signal intensity consistent with the presence of fluid was present bordering the dorsal margin of the DDFT following distension of the navicular bursa.
Clinical patients received a palmar digital nerve block before distention of the navicular bursa. The same technique described above for needle placement and confirmation of the needle position was used for the clinical patients. The volume of fluid selected for the clinical patients was based on the findings of the initial study and the level of the navicular bursa that was being investigated. Therefore, attempting to separate the navicular bone from the DDFT required more volume than if separation of the tendon was require at the proximal recess. If excessive distension caused concern of navicular bursa rupture, the fluid could be withdrawn after imaging. Bursography added an additional 15–20 min of anesthesia time.
Results
The navicular bursa was normal grossly in all cadaver limbs from the sound horses. In these limbs, distension of the proximal navicular bursa recess, proximal to the collateral sesamoidian ligament attachment on the navicular bone, was achieved with 2 ml of fluid. Separation of the collateral sesamoidian ligament from the DDFT at the level of the insertion on the navicular bone required 4 ml of fluid. Separation of the DDFT from the navicular bone and the DSIL required 5–6 ml of fluid (Fig. 1).
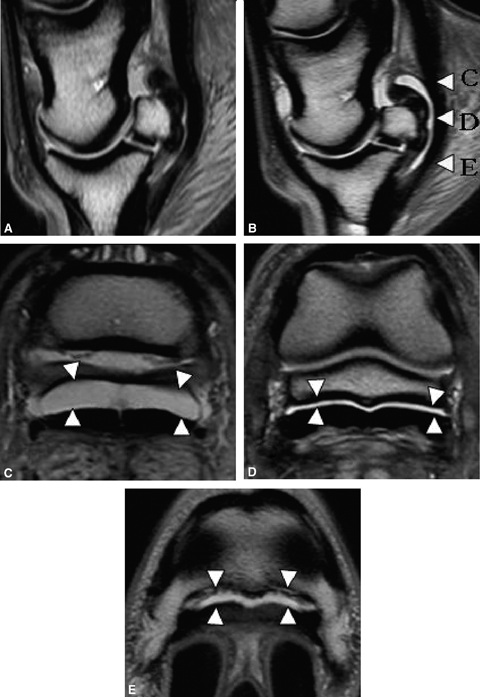
Sagittal proton density images: normal bursa pre (A) and post (B) 6 ml injection. Letters (C, D, and E) indicate the level where the transverse proton density images were obtained. Note the fluid in the navicular bursa separating the deep digital flexor tendon from the CSL (C), the navicular bone (D) and the distal sesamoidian impar ligament(E) (arrowheads).
The navicular bursa in the cadaver limbs from two of the six lame horses had severe abnormalities. Focal fluid accumulation with associated trabecular bone loss was observed in the navicular bone. Additionally, there was bone loss that extended through the flexor surface, with extensive tissue in the navicular bursa between the DDFT and the navicular bone. The abnormal tissue was concentrated in the regions of bone loss on the navicular bone flexor surface. The severity and distribution of the abnormalities were similar in both of these limbs. In one navicular bursa, injection of 4 ml of contrast medium resulted in fluid signal, which more clearly defined adhesions between the DDFT and the adjacent structures (collateral sesamoidian ligament, navicular bursa, and DSIL) (2, 3). Thickening and fibrosis, as well as reduced capacity of the navicular bursa, was also observed (Fig. 2). In this navicular bursa, fluid was present further distally in the navicular bursa when compared with a nonpathologic limb injected with the same volume. In the other navicular bursa, injection of 6 ml of contrast medium was met with significant resistance followed by extravasation of contrast medium into tissues palmar to the DDFT tendon. In these two pathologic cadaver limbs, the initial MR images were characterized by abnormal tissue in the navicular bursa. After navicular bursa distension, this tissue in the navicular bursa was not displaced, consistent with adhesions, which were confirmed with dissection.
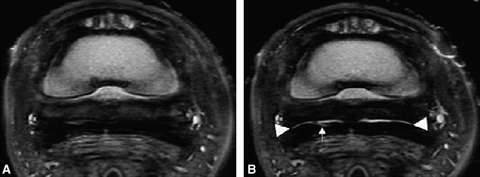
Transverse proton density images: pathologic bursa pre (A) and post (B) 4 ml injection. (A) No fluid is present in the navicular bursa predistension. (B) Following distension of the bursa, a very thin layer of fluid is visible (arrowheads) dorsal to the deep digital flexor tendon, except for at midline. Although fluid can be identified in the bursa and there are not extensive adhesions, the bursa appears restricted, likely due to chronic inflammation and fibrosis. There are focal adhesions on midline. A linear lesion in the medial lobe of the deep digital flexor tendon is more clearly delineated following distension of the navicular bursa (arrow).
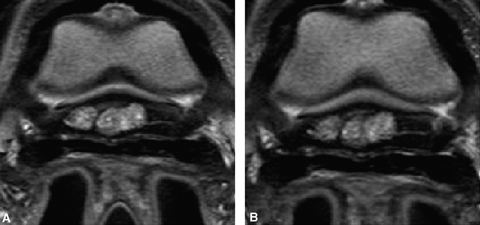
Transverse proton density images: pathologic bursa pre (A) and post (B) maximal distension, which was 4 ml. There is no separation between the deep digital flexor tendon and navicular bone (A). The bursa remains unchanged, confirming the presence of adhesions and scarring of the bursa (B).
In the remaining four cadaver limbs, adhesions were identified with bursography and could be differentiated from areas of synovial proliferation. In two limbs, DDFT fiber disruption that extended to the dorsal surface of the tendon was identified following distension of the navicular bursa. On the initial study, this area of abnormality had increased signal intensity on proton density images without a corresponding signal increase on STIR images, which could be interpreted as degenerative injury and/or scarring as opposed to fiber disruption. Following distension of the navicular bursa, fluid extended into the margins of the tear resulting in abnormal signal intensity on proton density and STIR images.
The summary of our clinical patients is as follows, organized such that the prebursography findings of a particular area of interest are listed, followed by the postbursography findings. In prebursography MR images of the 16 clinical patients, dorsal margin fraying and fibrillation was found at the level of the proximal navicular bursa recess in 15, at the level of the navicular bone in nine, and at the level of the distal navicular bursa recess in four. Core lesions of the DDFT were identified at the level of the proximal navicular bursa predistension in two horses. Linear parasagittal intermediate signal intensity in the DDFT on proton density (PD) images was found at the level of the proximal recess of the navicular bursa in nine horses, at the level of the navicular bone in seven horses and at the level of the distal recess of the navicular bursa in three horses. Linear parasagittal intermediate signal intensity on PD and STIR images of the DDFT was found at the level of the proximal recess of the navicular bursa in five horses, at the level of the navicular bone in three horses and at the level of the distal recess of the navicular bursa in one horse. Linear parasagittal high signal intensity on PD and STIR images in the DDFT was found at the level of the proximal recess of the navicular bursa in one horse and at the level of the distal recess of the navicular bursa in one horse.
After bursography, linear parasagittal high signal intensity in the DDFT on PD and STIR images was identified at the level of the proximal recess of the navicular bursa in six horses, at the level of the navicular bone in nine horses and at the level of the distal recess of the navicular bursa in two horses, indicating a split in the tendon and communication with the navicular bursa. Widening of the linear parasagittal splitting in the DDFT was identified postbursography at the level of the proximal recess of the navicular bursa in five horses, at the level of the navicular bone in three horses and at the level of the distal recess of the navicular bursa in four horses, indicating communication with the navicular bursa. Extension of high signal intensity on PD and STIR images between the DDFT and the distal digital annular ligament was identified at the level of the proximal recess of the navicular bursa in two horses and at the level of the navicular bone in three horses, indicating a complete parasagittal split in the DDFT.
Dorsal margin tearing of the DDFT predistension was identified at the level of the proximal recess of the navicular bursa in one horse, at the level of the navicular bone in one horse and at the level of the distal recess of the navicular bursa in two horses. Dorsal margin tearing of the DDFT was identified postdistension at the level of the proximal recess of the navicular bursa in one horse (Fig. 4), at the level of the navicular bone in two horses and at the level of the distal recess of the navicular bursa in two horses.
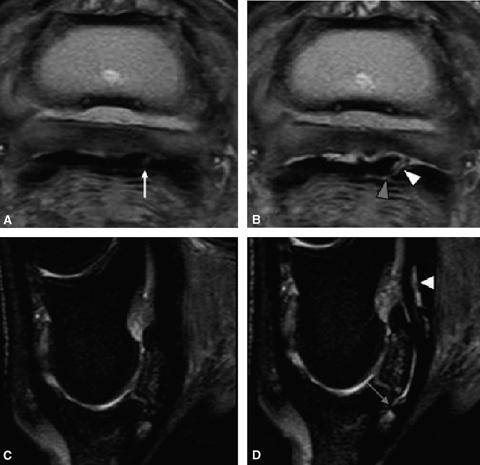
(A) and (B) are transverse proton density images, and (C) and (D) are sagittal STIR images. (A) A parasagittal lesion is present in the lateral lobe of the deep digital flexor tendon (DDFT) before distension of the bursa (arrow). Following distension of the navicular bursa (B), it is evident that this is a complete parasagittal tear that communicates with the navicular bursa (white arrowhead). Fluid has entered the tear defining the margins and fluid can be identified extending through the tear between the deep digital annular ligament and the DDFT (gray arrowhead). In addition there is separation of the DDFT from the CSL demonstrating no adhesions to this lesion and revealing potential surgical access of this lesion through bursoscopy. (C) Before distension, there is no abnormal signal intensity within the DDFT (D) Following distension, there is increased signal intensity present within the DDFT, indicating communication with the navicular bursa.
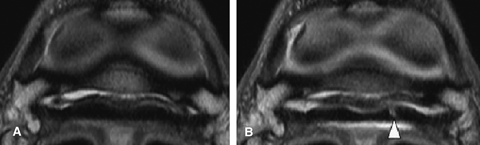
Synovial proliferation within the navicular bursa was identified at the level of the proximal recess of the navicular bursa in 15 horses, at the level of the navicular bone in five horses and at the level of the distal recess of the navicular bursa in eight horses. Synovial proliferation was not displaced at the level of the proximal recess of the navicular bursa in 10 horses (Fig. 5), at the level of the navicular bone in three horses and at the level of the distal recess of the navicular bursa in seven horses, indicating the presence of adhesions. Synovial proliferation was not displaced at the level of the proximal recess of the navicular bursa in five horses, at the level of the navicular bone in two horses (Fig. 6) and at the level of the distal recess of the navicular bursa in three horses, ruling out the suspicion of adhesions. A flexor surface defect of the navicular bone was detected predistension in one horse. Flexor surface defects of the navicular bone not identified on the initial study were identified in three horses postdistension. Distension ruled out potential flexor surface defects of the navicular bone in two horses. In one horse, a parasagittal split in the tendon was identified in the medial lobe of the DDFT at the level of the navicular bone that extended to the insertion on the distal phalanx following distension of the navicular bursa (Fig. 7), which was not visible predistension.
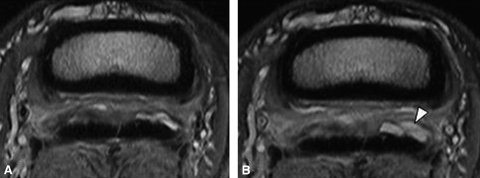
Transverse proton density images: pre (A) and post (B) 4 ml injection. The deep digital flexor tendon has multiple abnormalities. There is abnormal tissue present in the proximal recess of the navicular bursa, primarily located dorsal to the medial lobe of the deep digital flexor tendon (DDFT) (A). Following distension of the bursa, the tissue did not move away from the dorsal margin of the DDFT confirming the presence of adhesions. Increased fluid can be seen in the lateral aspect of the bursa following distension (arrowhead) (B).
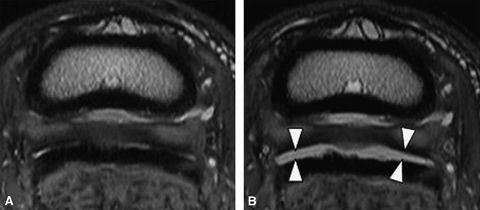
Transverse proton density images: pre (A) and post (B) 6 ml injection. (A) The dorsal margin of the medial lobe of the deep digital flexor tendon (DDFT) is irregular with abnormal signal intensity and is focally enlarged. In multiple areas fluid cannot be identified dorsal to the deep digital flexor tendon. These findings are suspicious for adhesions between the navicular bursa, DDFT, and CSL. (B) Following distension the navicular bursa is completely separated from the DDFT and the CSL, indicating no adhesions between these structures. Arrowheads indicate fluid distension of the bursa.
Transverse proton density images: pre (A) and post (B) bursal distension. A parasagittal tendon tear can be identified in the medial lobe of the deep digital flexor tendon (DDFT) (arrowhead) at the level of the navicular bone. The tear extended distally to the DDFT insertion of the distal phalanx. Abnormal signal intensity was not present in the tendon before distension of the navicular bursa.
Discussion
In the normal navicular bursa, fluid signal intensity is continuous along the dorsal surface of the DDFT within the proximal and distal recesses. Fluid in the navicular bursa can sometimes be detected at the level of the navicular bone. The presence of the fluid signal intensity depends on the size of the synovial fossa of the navicular bone, as well as the position of the foot and the amount of fluid present in the navicular bursa. Adhesions have been described as soft tissue accumulation adjacent to the collateral sesamoidian ligament or DDFT resulting in loss of visualization of the normal fluid space6; however, synovial proliferation and/or hypertrophy can also have this appearance. Tissues with similar signal intensities can appear continuous or adhered when they are in close proximity to each other.11 The protocol used in this study was selected based on the sequences currently used in our musculoskeletal protocol with the intent to optimize soft tissue–fluid contrast. This could have also been achieved using T2-weighted FSE images. However, bursographyis performed following an initial, complete MR examination. Therefore, an abbreviated MR protocol is selected based on tissue contrast and time. Although gradient echo images have higher spatial resolution, they have reduced soft tissue contrast when compared with proton density images and poor differentiation between fluid and tissue when compared with proton density and STIR images.
Based on our results, distension of the navicular bursa can be useful in horses where the presence of adhesions is not clearly defined or when pathologic change consistent with the degree of lameness is not identified. The volume of contrast medium required is dependent on the area of interest and the severity of the abnormalities. Less volume is needed to distend and separate the proximal aspect of the navicular bursa and the associated soft tissue structures such as the collateral sesamoidian ligament and DDFT. A greater volume is needed to distend and separate the distal aspect of the navicular bursa and the associated soft tissue structures such as the DSIL and the DDFT. These volumes would be applicable for differentiating synovial proliferation and/or hypertrophy from adhesions in horses with mild scarring of the synovial membrane of the navicular bursa. In our experience, attempting to distend a severely pathologic navicular bursa with 6 ml of contrast medium may result in fluid leakage back into the needle tract or rupture of the navicular bursa. Fibrosis of the synovial membrane and adhesions may reduce the capacity of the navicular bursa to hold this volume. It has been reported that movement of the arthroscope and instruments within affected bursae during bursoscopy is restricted, which also seem to indicate reduced capacity.12
In cadaver limbs with severe abnormalities, 4 ml of contrast medium was adequate to separate the DDFT from the navicular bone and DSIL. As opposed to distending with a finite volume of contrast medium, horses may require injection of contrast medium until moderate to severe resistance is encountered, at which point maximum distension is achieved for that navicular bursa. The decreased volume required to achieve moderate resistance correlates with the degree of pathologic change in the bursa. Contrast medium pooling was identified palmar to the DDFT, however, a thin linear area of contrast medium suggesting a needle track was not identified in the DDFT or digital cushion on radiography or MRI. The MR exam is performed immediately following bursal distension, and further leakage or tissue reaction over time would not yet be present. Examination of this area at a different time period following bursal distension may allow identification of the needle track.
It has been suggested that approximately 10 ml of contrast medium was needed for complete separation of all structures within the navicular bursa. However, limbs were previously frozen and this may have caused the bursa to be less pliable, therefore requiring an increased volume to attain the same separation. Furthermore, 11 of the 22 bursae were ruptured in the process of distension, indicating excessive volume was introduced into the navicular bursa.13 In contrast, we had two navicular bursae rupture out of seven, which were distended with 6 ml. In addition, no bursa were ruptured with 5 ml.
The clinical patients that have undergone MR bursography (n=16) have yielded additional findings. In several horses, tissue in the navicular bursa that appeared adhered to various structures was displaced easily with a small amount of fluid. Additionally, communication of tendon tears with the navicular bursa was an important finding (Fig. 4). It demonstrated the opportunity for surgical lesions in the proximal recess of the navicular bursa with bursoscopy. In the two clinical patients presented with tendon fiber disruption identified following bursal distension, the tendon lesions had increased signal intensity on proton density images with a slight or no concurrent signal increase on T2-weighted and STIR images. This signal pattern is consistent with mild to moderate degeneration and is not necessarily associated with a tendon tear. This appearance can be present at the initial stages of tendon injury as detected on MR images. In addition, lesions can retain this appearance for extended periods of time, up to years following healing of fiber pattern disruption. Distension of the navicular bursa demonstrated the severity of the tendon lesion more accurately in these horses. Additional findings that were not previously identified until the bursa was distended included detection of small flexor surface erosions that were obscured due to the close proximity of the dorsal margin of the DDFT and other soft tissue lesions. Furthermore, a greater number of horses than expected with DDFT lesions extending to the dorsal surface of the tendon did not have adhesions to the navicular bursa. The post bursal distension studies provided clinically relevant information in horses where the initial examination did not represent the degree of tendon injury accurately or in horses where the findings did not correlate with the degree of lameness. In horses with severe or extensive tendon degeneration on the initial study, this technique will not likely have any impact on patient management.
MR bursography allows for treatment as well as further clarification of the disease process to be achieved simultaneously. Injection of the navicular bursa with corticosteroids, sodium hyaluronate, and amikacin has been used for horses with chronic palmar heel pain that are unresponsive to corrective shoeing and systemic nonsteroidal antiinflammatory medication.14 Distension of the bursa with corticosteroids may be associated with a slight risk of temporary inhibition of healing,15 however; we feel that injection with hyaluronic acid and a low dose of short acting steroid benefits the horse by reducing pain. Additionally, the horse will be rested for a long period of time if a tendon lesion is detected. Therefore, the presence of corticosteriods is most likely not detrimental due to the therapy being combined with rest. The incorporation of navicular bursa injection with patients undergoing MR for palmar heel pain may be beneficial. Evaluation of the initial MR images was used to determine if treatment of the navicular bursa was indicated.
MR bursography has been performed in a limited number of horses when imaged in a standing MR system.‡ Separation of the DDFT from the collateral sesamoidian ligament and DSIL can be achieved; however, adequate separation for evaluation of the navicular bone flexor surface has not been achieved. This is likely due to the pressure between the DDFT and navicular bone with the limb in a weight bearing position.