COMPARISON OF SONOGRAPHIC FEATURES OF BENIGN AND NEOPLASTIC DEEP LYMPH NODES IN DOGS
Preliminary results from this study were presented as oral abstract at the 2009 annual meeting of the ACVR, Memphis, TN.
Abstract
The differentiation of benign vs. neoplastic lymph nodes impacts patient management. Specific sonographic features are typically considered when assessing lymph nodes in dogs. However, the usefulness of these criteria in distinguishing benign vs. malignant lymph nodes remains largely unknown, especially for deep lymph nodes. Our aim was to compare sonographic features in benign and neoplastic deep lymph nodes with the hope of identifying predictive criteria. Thirty-one deep lymph nodes (16 mesenteric, 10 medial iliac, three hepatic, one sternal, and one cranial mediastinal) in 31 dogs were examined prospectively with B-mode and Color flow Doppler. Lymph nodes were aspirated using ultrasound-guidance and final diagnosis were established based on cytologic and/or histopathologic interpretation. Prevalence of each sonographic feature and combinations of two features was calculated for each group and compared using a χ2-test or Student's t-test for unequal variances. Ten lymph nodes were benign (hyperplastic and/or inflammatory) and 21 were neoplastic. All were hypoechoic, except for one neoplastic lymph node. Maximal short-axis diameter (P=0.0006) and long-axis diameter (P=0.01), and SA/LA ratio (P=0.008) were increased significantly for neoplastic (2.8, 5.5 cm, and 0.50, respectively) vs. benign (1.2, 3.8 cm, and 0.34, respectively) lymph nodes. The prevalence of other features was similar between groups. Doppler evaluation was possible in 77% of lymph nodes, but there was no significant difference between groups. When any two ultrasound features were combined, the only difference between benign and neoplastic lymph nodes was for the combination of contour regularity and appearance of the perinodal fat (P=0.03).
Introduction
With technologic progress, deep abdominal and thoracic lymph nodes are more easily detectable with ultrasonography and their features have been described for certain diseases.1–3 Being able to differentiate benign diseases such as hyperplasia, inflammation from neoplastic lymph nodes is important for initial diagnosis and for establishing tumor stage and prognosis. Although cytologic sampling is helpful in differentiating lymph node diseases, these techniques are limited in some animals due to lymph node location.
Several ultrasonographic features have been associated with lymph node malignancy. Compared with normal lymph nodes, neoplastic lymph nodes tend to be large and round, irregular in shape, hypoechoic, sharply delineated, and heterogeneous, and are associated with reduced hilar tissue definition, and altered vascularization.1–6 Deep acoustic enhancement has also been associated with malignancy.2,3 On the other hand, the differentiation between neoplastic and benign lymph node diseases is more challenging due to overlap of ultrasonographic characteristics.2–4 In addition, only a few studies have been reported specifically for deep lymph nodes in dogs, and these were retrospective.1,4
Our purpose was to assess several sonographic features prospectively in benign and neoplastic deep lymph nodes in dogs, and to compare their prevalence in these groups. We hypothesized that some of these features considered individually or in combination would predict the presence or absence of malignancy.
Materials and Methods
Thirty-one dogs examined between December 2006 and September 2009 were evaluated. These dogs underwent ultrasound-guided aspiration and/or biopsy with cytologic and/or histopathologic analysis of abdominal or thoracic lymph nodes, as requested by the attending veterinarian.
Two dogs had samples from two lymph nodes, but only one randomly chosen lymph node was included in the analysis. In both dogs, the second lymph node sampled was the controlateral medial iliac lymph node in which the sonographic features and final diagnosis were identical to the first lymph node.
All ultrasound exams were performed with ultrasound equipment* using a 5–8 MHz electronic microconvex transducer. The following parameters were scored by the attending board-certified veterinary radiologist at the end of the examination: lymph node maximum short-axis diameter and long-axis diameter (mm), echogenicity (normal, reduced, or mixed) (Fig. 1A and B), deep acoustic enhancement (absent or present), parenchymal uniformity (homogeneous or heterogeneous), shape (normal, elongated, or rounded) (Fig. 1A and B), contour (definition: well-defined or ill-defined; and regularity: regular, irregular, or lobular) (Fig. 2A and B), cavitations (well-defined hypoechoic or anechoic areas with or without mobile particles; present or absent), hilar tissue definition (normal or reduced), and the appearance of the perinodal fat (normal, hyperechoic, or hypoechoic) (Fig. 2A and B). The short-axis diameter to long-axis diameter (SA/LA) ratio was also calculated.
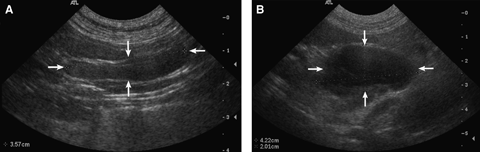
(A) Benign (hyperplastic) mesenteric lymph node. The lymph node (arrows) is elongated and homogeneous, and measures 0.7 cm short-axis diameter (SA) × 3.57 cm long-axis diameter (LA), for a ratio of 0.20. (B) Neoplastic (lymphoma) mesenteric lymph node. The lymph node (arrows) is rounded and mildly heterogeneous, and measures 4.71 cm SA × 6.75 cm LA, for a SA/LA ratio of 0.70.
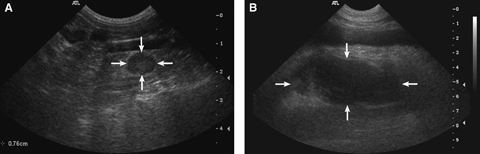
(A) Regular benign (inflammatory) mesenteric lymph node (arrows). Note the normal perinodal fat. (B) Irregular neoplastic (histiocytic sarcoma) right medial iliac lymph node (arrows). Note the hyperechoic perinodal fat.
Color flow Doppler was used to evaluate lymph node vasculature with pulse repetition frequency kept low to maximize vessel detection. The presence and distribution of vascular flow as well as the number of vessels detected were noted. When the Doppler signal was sufficient, distribution was classified into one of three groups: hilar, peripheral, or mixed.2,3 In case of absence of flow on Color Doppler, Power Doppler was used for increased sensitivity.
For each dog, lymph node scoring was completed before ultrasound-guided cytologic sampling or biopsy/necropsy, which occurred immediately or within 72 h following the ultrasound examination. Five dogs were euthanized. Cytology and histopathology were both performed in four of these dogs, and correlated in all. In the fifth dog, only histopathology was performed. Cytologic or histologic samples were reviewed by a board-certified clinical pathologist or by a board-certified pathologist, respectively. Based on the cytologic and/or histopathologic results, the lymph nodes were categorized into benign (hyperplastic nodes and lymphadenitis) and neoplastic groups.
The prevalence of each ultrasound feature was calculated within each group and compared between groups using a χ2-test or Student's t-test for unequal variances.
The mean maximal height, maximal length, and ratio specifically for mesenteric lymph nodes was also calculated within each group and compared between groups using the same tests as above. All ultrasound features except for Doppler features were combined in pairs and prevalence of these combinations were also compared using a χ2-test. Significance was defined as P<0.05.
Results
Twenty-one dogs had neoplastic lymph nodes; 10 with lymphoma, five with histiocytic sarcoma and six with metastasis. Of the metastatic lymph nodes there were one undifferentiated sarcoma, three carcinomas, and two mast cell tumors. Ten dogs had benign lymph nodes; six were hyperplastic and four were lymphadenitis. There were no cytologically/histologically normal lymph nodes.
Dogs with benign lymph nodes had a mean age of 5.5 ± 3.7 years old and mean weight of 22 ± 12.9 kg, vs. a mean age of 8.1 ± 3.3 years old and mean weight of 35.8 ± 13.5 kg, for dogs with neoplasia. The neoplasia group was composed of four Labrador Retrievers, four Golden Retrievers, three Rottweilers, two Bernese Mountain Dogs, and one each of eight other breeds. The benign group comprised 10 dogs from 10 different breeds. In the neoplasia group, fine-needle aspiration cytology was used alone for diagnosis in nine mesenteric, two right medial iliac, two left medial iliac, two hepatic, one sternal, and one cranial mediastinal lymph nodes. Histopathologic evaluation was performed in a right medial iliac lymph node in another dog and both techniques were performed in two right medial iliac and one mesenteric lymph nodes. Lymph nodes in the benign group included five mesenteric, two right medial iliac, one left medial iliac, and one hepatic, which were sampled by fine-needle aspiration, and one mesenteric lymph node, which underwent fine-needle aspiration cytology and histopathology.
Significant differences between benign and malignant lymph nodes were only seen in the maximal short-axis diameter (P=0.0006), maximal long-axis diameter (P=0.01), and SA/LA ratio (P=0.008) (Table 1). Means for these features were greater in neoplastic lymph nodes (Fig. 1A and B). All lymph nodes were hypoechoic except for one neoplastic lymph node. There was no significant difference of other features between groups (Fig. 3A and B). Irregular and lobular lymph node criteria were grouped together and the prevalence recalculated and compared with regular lymph nodes between groups. No significant difference in prevalence was found between neoplastic and benign lymph nodes.
| Ultrasound Features | Neoplastic LN(n=21) | Benign LN(n=10) | P-Values |
|---|---|---|---|
| Max. short-axis diameter (cm) | 2.8 ± 1.7* | 1.2 ± 0.4* | 0.0006 |
| Max. long-axis diameter (cm) | 5.5 ± 2.4* | 3.8 ± 1.0* | 0.01 |
| Ratio SA/LA | 0.50 ± 0.17* | 0.34 ± 0.13* | 0.008 |
| Normal | 1 (4.8%) | 0 (0%) | 1 |
| Hypoechoic | 20 (95.2%) | 10 (100%) | |
| Deep acoustic enhancement | |||
| Present | 4 (19.1%) | 1 (10.0%) | 0.66 |
| Absent | 17 (80.9%) | 9 (90.0%) | |
| Uniformity | |||
| Homogeneous | 8 (38.1%) | 4 (40.0%) | 1 |
| Heterogeneous | 13 (61.9%) | 6 (60.0%) | |
| Hilar tissue definition | |||
| Present | 8 (38.1%) | 4 (40.0%) | 1 |
| Absent | 13 (61.9%) | 6 (60.0%) | |
| Shape | |||
| Elongated | 2 (9.5%) | 3 (30.0%) | 0.30 |
| Rounded | 19 (90.5%) | 7 (70.0%) | |
| Contour | |||
| Definition | |||
| Well-defined | 17 (80.9%) | 9 (90.0%) | 0.65 |
| Ill-defined | 4 (19.1%) | 1 (10.0%) | |
| Regularity | |||
| Regular | 7 (33.3%) | 5 (50.0%) | 0.45 |
| Irregular† | 14 (66.7%) | 5 (50.0%) | |
| Cavitations | |||
| Present | 2 (9.5%) | 3 (30.0%) | 0.30 |
| Absent | 19 (90.5%) | 7 (70.0%) | |
| Adjacent mesentery | |||
| Normal | 8 (38.1%) | 6 (60.0%) | 0.44 |
| Hyperechoic | 13 (61.9%) | 4 (40.0%) | |
- * Significant difference between groups (P<0.05).
- † The irregular category includes irregular and/or lobulated lymph nodes.
- LN, lymph node; n, number of lymph nodes; Max, maximum; SA/LA, short-axis diameter to long-axis diameter ratio.
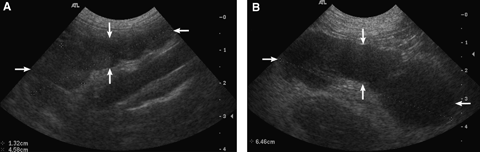
(A) Benign (reactive) mesenteric lymph node (arrows). (B) Neoplastic (lymphoma) mesenteric lymph node (arrows). Notice the similar aspect of the two lymph nodes.
When only benign and neoplastic mesenteric lymph node measurements were compared, there was a significant difference between maximal short-axis (P=0.01) and long-axis diameter (P=0.03) but not in SA/LA ratio (P=0.22).
Owing to excessive respiratory motion and/or excessive depth, Doppler evaluation (Table 2) was possible on only 24 (77%) lymph nodes (six benign [60%] and 18 neoplastic [86%]). No blood flow was recorded in two benign or seven neoplastic lymph nodes. No statistically significant difference was found for any Doppler criteria and lymph node category.
| Ultrasound Features | Neoplastic LN | Benign LN | P-Values |
|---|---|---|---|
| Vascular flow | |||
| Present | 11 (61.1%) | 4 (66.7%) | 0.81 |
| Absent | 7 (38.9%) | 2 (33.3%) | |
| Distribution | |||
| Hilar | 7 (63.7%) | 2 (50.0%) | 0.23 |
| Peripheral | 0 (0%) | 1 (25.0%) | |
| Mixed | 4 (36.3%) | 1 (25.0%) | |
| Number of vessels | |||
| 0 | 7 (38.9%) | 2 (33.3%) | 0.96 |
| 1–4 | 6 (33.3%) | 2 (33.3%) | |
| >5 | 5 (27.8%) | 2 (33.3%) | |
- LN, lymph node.
When any two B-mode ultrasound features were combined, the only statistical difference between neoplastic and benign lymph nodes was for the combination of contour regularity and appearance of the perinodal fat (P=0.03). Specifically, 50% of benign lymph nodes were regular and associated with a normal perinodal fat (Fig. 2A) vs. 10% of neoplastic lymph nodes. 38% of neoplastic lymph nodes were irregular and associated with a hyperechoic perinodal fat (Fig. 2B) vs. 40% of benign lymph nodes. The rest of the neoplastic lymph nodes were irregular with normal perinodal fat (28%) or regular with hyperechoic perinodal fat (24%). There was no significant difference between the two groups for any other combinations of B-mode features.
Discussion
We found that only maximal short-axis diameter and long-axis diameter and SA/LA ratio was statistically different between benign and malignant lymph nodes. That SA/LA increased is consistent with short-axis diameter increasing to a greater degree. These results are consistent with others pertaining to both superficial2 and deep (medial iliac) lymph nodes.1 In those studies, neoplastic lymph nodes were enlarged and round whereas normal, benign, and lymphoma remission lymph nodes were smaller and more elongated. Nonetheless, the range of size for neoplastic lymph nodes in our study, as evidenced by the larger standard deviation, was great and overlapped that of benign lymph nodes, as reported previously for superficial metastatic lymph nodes.2
Most lymph nodes studied herein were hypoechoic, regardless of disease group. Normal and benign lymph nodes are expected to be isoechoic to mildly hypoechoic relative to the perinodal fat, whereas neoplastic lymph nodes are hypoechoic.2,3,7–9 While our results are in agreement with these studies, they reinforce the fact that the echogenicity is not a useful indicator of lymph node malignancy. Interestingly, one lymph node in the neoplastic group was isoechoic to perinodal fat, and diagnosed with sarcoma. We did not grade echogenicity as this parameter is confounded by operator, patient, and depth variables.
Heterogeneity was associated with neoplasia in two studies,1,4 which differed from our results. Similarly, in humans, both homogeneity and heterogeneity have been associated with neoplasia.10,11 One explanation is that the appearance of neoplastic lymph nodes varies according to the type and location of the primary tumor. This is supported by the fact that the proportion of different types of tumors varies between studies. Importantly, lymphomatous lymph nodes were more prevalent in our study (48%) than in another (30%).4 Nonetheless, a consensus does not even exist with regard to lymphomatous lymph nodes. For example, superficial lymphomatous lymph nodes have been described as homogeneous6 whereas deep lymphomatous lymph nodes have been described as heterogeneous.1 In our study, 60% of lymphomatous lymph nodes were heterogeneous.
Using higher probe frequency with increased axial spatial resolution might have allowed more accurate lymph node characterization and perhaps heterogeneity would have been identified more often, although this was not possible in most dogs because of tissue depth. We also emphasize that several imaging parameters, particularly those involving postprocessing, can vary greatly and affect echotexture.
Up to 80% of lymphomatous superficial lymph nodes may have reduced hilar tissue definition.6 However, others state that approximately 40% of superficial lymph nodes, neoplastic or not, do not have clear hilar tissue definition and that the occurrence of a hyperechoic hilus was not significantly different between neoplastic and benign lymph nodes.2,3 In our study, nearly 60% of both benign and neoplastic lymph nodes were characterized as having reduced hilar tissue definition. This suggests that the hilus is often affected in both benign and neoplastic diseases, or that the hilus is not assessed clearly with B-mode ultrasonography. Also, lymph node uniformity assessment is compromised with increasing insonation depth; in our study, hilar definition was present in 67% of dogs weighing <30 kg vs. 6% of dogs weighing >30 kg. Regardless, the usefulness of hilar tissue definition is questionable.
Neoplastic lymph nodes have peripheral or mixed vascularization with a greater number of vessels.2,3,6 Our Doppler results were inconclusive as Doppler examination was impossible on some nonsedated dogs. The deep location of the lymph nodes also makes Doppler examination more difficult. This difficulty is similar to another study where Doppler examination was possible on 66% of all lymph nodes and 87% of reactive and neoplastic lymph nodes.5 We did not notice that Doppler signal was decreased by the presence of hyperechoic perinodal fat, as might be expected.
Combining features aids in the differentiation of benign and neoplastic lymph nodes.2 Three features were useful when combined: lymph node size, vascular flow distribution, and pulsatility index. In our study, we found combination of features useful only when we combined contour regularity and appearance of the perinodal fat: a regular lymph node with normal perinodal fat is unlikely to be neoplastic. The difficulty in performing Doppler examination on several of our dogs and the inability to test combinations of more than two features due to the small sample size limited the evaluation of feature combination.
In humans, little information is available regarding deep lymph nodes, as visualization is complicated by the gastrointestinal gas.12 The most commonly identified sonographic criteria of malignancy include a size >1 cm, a hypoechoic appearance, sharply demarcated borders, and a round contour.10,11,13–15 A missing hilus sign is mentioned occasionally.14 While these features are rarely present simultaneously, using combinations of signs can increase the accuracy of detecting malignancy.10,11,13–15 Moreover, the use of Color and Power Doppler, and of contrast-enhanced ultrasonography, allows better characterization of lymph node vascularization, and also better differentiation between benign and neoplastic lymph nodes.13,14
As for animals, the lack of complete reliability of sonographic features or combination of features has been described for humans.10,11,13 Hence, cytology remains a routine test in humans to determine if a lymph node is neoplastic. Computed tomography, magnetic resonance imaging, and positron-emission tomography are also used. However, even if these modalities are superior to transcutaneous ultrasound in detecting abnormal lymph nodes, they do not offer clear gain over endoscopic-ultrasonography, which has the advantage of allowing visualization and tissue sampling.16–19
Regardless of the small sample size, the similar prevalence of most features in both groups suggests that a much larger sample size would be necessary to find any other statistical difference. In addition, the uneven distribution between benign and malignant lymph nodes decreased the statistical power.
Lymph node size is directly related to the weight of the patient.7,20 In our study, dogs with neoplastic lymph nodes were significantly heavier than dogs with benign lymph nodes. This could have influenced the difference in lymph node size between groups. Moreover, normal lymph nodes of different anatomic regions have different size and conformation. For example, mesenteric lymph nodes are longer than other abdominal lymph nodes.1,7,8 Therefore, not considering lymph node region could have altered our results regarding the short-axis diameter, long-axis diameter, and ratio. However, the sample size prevented consideration of lymph node location according to their anatomic region and the number of lymph nodes from each anatomic region was similar between the two groups: 48% malignant mesenteric nodes vs. 60% benign mesenteric nodes. Nonetheless, when mesenteric lymph nodes were considered alone, the LA/SA ratio was no longer significantly different between groups.
We used cytology as the gold standard. Ultrasound-guided biopsies are not always possible anatomically, and may be too hazardous when the lymph node is deep or small. It may also be difficult to acquire several samples of the lymph node in those instances. Cytology is used regularly as a clinical standard. A very good correlation between cytologic and histopathologic evaluation and/or clinical follow-up has been found in humans and dogs.14,21,22 In the four dogs in which both cytologic and histopathologic evaluation were performed, the results were concordant.
Other sonographic tools such as pulsed-wave Doppler and harmonic ultrasonography may have been useful in distinguishing benign from neoplastic lymph nodes. Resistivity and pulsatility indices are increased in neoplastic lymph nodes and cut-off values are reported.2,5 However, given the difficulty in obtaining a good Doppler signal in some dogs, the use of pulsed-wave Doppler may not have been conclusive. In other work, contrast harmonic ultrasonography improved the identification of the angioarchitecture in superficial lymphomatous lymph nodes in dogs compared with Power Doppler ultrasound.6 However, the use of contrast harmonic ultrasonography in other categories of lymph nodes and its capacity to differentiate between these categories are lacking and contrast harmonic ultrasound is of more limited use in deep structures.