USE OF SURGICAL HEMOCLIPS IN RADIATION TREATMENT PLANNING
Presented at the American College of Veterinary Radiology Annual Conference, Chicago, IL, 2005.
This study was supported by the Sprecher Institute for Comparative Cancer Research.
Abstract
The goal of this prospective study was to determine the effect of hemoclip use on the size of radiation treatment fields based on a 3-cm margin around a surgical incision alone (field setup 1) vs. a 3-cm margin around the surgical incision plus hemoclips (field setup 2). Forty-seven dogs that underwent surgical resection of a total of 55 soft tissue masses had surgical hemoclips placed at the time of surgery and orthogonal radiographs made immediately postoperatively. Radiation treatment field simulation was done and field areas measured. Additional determinations included number of hemoclips outside of the radiation treatment field based on a margin around the incision alone, hemoclip distance from the incision, and association between incision length and greatest distance of hemoclips from the incision. There was a significant difference in radiation treatment field size using information regarding the location of hemoclips in conjunction with the surgical scar compared with the surgical scar alone for truncal (P=0.0003) vs. extremity tumors (P=0.087). In simulating radiation treatment fields hemoclips were located outside of field setup 1 for the majority of tumors (79%) resected from the trunk but only in a minority of tumors (10.7%) resected from extremity sites. The findings from this study suggest that surgical hemoclips have potential utility in simulation of radiation treatment fields in the postoperative setting.
Introduction
Increased attention to all aspects of radiation therapy, including delineation of the tumor or postoperative surgical bed, radiation treatment planning, patient positioning, and treatment delivery, has resulted in a more thorough understanding of successful treatment planning.1–4 However, these efforts cannot overcome the uncertainty in radiation treatment field setup that arises when the location of residual disease cannot be accurately delineated. Many animals are irradiated postoperatively, and the previous tumor extent and surgical field are unknown or poorly defined. As a result, radiation treatment fields are sometimes based on a specified margin (e.g., 3 cm) around a surgical scar.
Hemoclips inserted at surgery to delineate the limits of resection can be useful for design of radiation fields. A smaller margin of normal tissue may be irradiated if surgically placed clips are present after lumpectomy for human breast cancer, and hemoclips are important for adequately defining radiation treatment fields to include all residual tumor.5–9 If the surgical scar alone was used for breast irradiation without consideration of the position of hemoclips, geographic misses would have occurred in 53–68% of women.10,11 Furthermore, wide disparity has been noted between the location of the surgical scar and hemoclips, indicating that treating a region based on a surgical scar may result in irradiation of excess normal tissue. For example, in breast radiation boost after lumpectomy fields set up based on the surgical scar included tissue that did not need to be irradiated and portions of the tumor bed were occasionally excluded.12
Hemoclips have also been used clinically to identify areas at high risk for recurrence in women with breast cancer.6 Other validated applications include endoscopically placed mucosal clips in humans with esophageal tumors.13 Importantly, increased morbidity has not been associated with surgical hemoclip placement or hemoclip presence in the irradiated field.6,13
Computed tomography (CT)-based, computer-generated treatment planning is usually done preoperatively but can also be of value postoperatively, especially if hemoclips were placed at the time of tumor excision. Hemoclips may also have utility in manual radiation treatment planning.4 The purpose of this study was to evaluate the potential impact of hemoclips placed at surgery to delineate the surgical bed on treatment simulation for radiation therapy in comparison with radiation treatment simulation based on the surgical scar alone.
Materials and Methods
Forty-seven dogs that underwent surgical resection of a soft tissue mass between May 2004 and October 2005 were studied prospectively. Dogs were excluded from this study if they had a skin grafting or flap procedure performed to close the surgical defect. Owner consent was obtained and the study was done with institutional Institutional Animal Care and Use Committee approval. In a subset of dogs, more than one mass was excised, resulting in a total of 55 potential radiation treatment sites.
Dogs that underwent surgery for a soft tissue mass were entered in the study and therefore included dogs that were not candidates for postoperative radiation therapy; this was judged acceptable due to the simulation nature of this study. There were a variety of pure breed and mixed breed dogs, ranging in weight from 5.7 to 49 kg (median=31.5 kg). Sites of resection included extremity (n=28), trunk (n=24), and head (n=3). A peripheral lymph node was excised in seven dogs and represented a subset of extremity site (n=5 dogs), trunk (n=1 dog), or head (n=1 dog) sites. All masses that were resected were submitted for histopathologic evaluation, including surgical margin assessment, although all biopsies were not reviewed by the same pathologist. A variety of tumor types were encountered but are not enumerated here as this had no bearing on the results or conclusions.
All dogs had hemoclips* placed at the time of surgery to outline the surgical bed. The surgeons were instructed to place a minimum of five hemoclips at the dorsal, ventral, cranial, caudal, and deep aspects of the surgical bed, and to place the hemoclips in fascia or muscle rather than in skin or superficial subcutaneous tissues. Immediately postoperatively under general anesthesia, orthogonal radiographs of the surgical site(s) and surrounding normal tissues were obtained. A 0.8-mm diameter wire marker† was placed along the incision to allow radiographic visualization of the location of the surgical scar. A circular metal marker was placed at the level of the surgical incision or midplane along the incision, and was used to determine the radiographic magnification factor. Radiation treatment field simulation for manual setup of radiation treatment fields entailed determination of a radiation field based on the incision alone with a 3-cm margin around the incision (field setup 1), and then repeated using a 3-cm margin around the incision plus hemoclip location (field setup 2). The field area for each setup was measured using a planimeter‡ with a correction made for the radiographic magnification factor; this procedure was repeated once to obtain two measurements of the field area for each of the two setups. All field area determinations were done by one author (M.C.M.). Determinations included the magnification factor, number of hemoclips, incision length, radiation treatment field area, percentage change in treatment field area between field setups 1 and 2, the number and relative percentage of hemoclips located outside of field setup 1, and hemoclip distance from the incision. A statistical program§ was used for statistical analysis. All data are presented as means±standard deviation of the mean, and median. Student's unpaired t-test was used to find the significance between two groups. Linear regression analysis was used to determine the degree of correlation between the length of the incision and the greatest distance of hemoclips from the incision; correlation was expressed as r2. A P-value of <0.05 was considered significant.
Results
The number of hemoclips placed ranged from three to 40 (mean=10, median=8). Two dogs had less than five hemoclips placed (three hemoclips in one dog, four hemoclips in one dog) although it was requested that a minimum of five hemoclips be placed. A total of eight surgeons were involved in this study, including four faculty surgeons and four surgery residents. The area for field setup 1 ranged from 31.1 to 202.6 cm2 (mean=89.3±SD 45.0, median=84.1). The field area for field setup 2 ranged from 39.5 to 342.2 cm2 (mean=125.3±SD 76.4, median=101.4). The percentage increase in field size between field setup 1 and 2 ranged from 1.3% to 93.7% (mean=35.0%±SD 22.4%, median=32.4%). In comparing field size for field setup 1 with setup 2 there was a significant difference when all sites were included (P=0.003). The same comparison but only with extremity sites was not significantly different (P=0.087), while with truncal tumors the difference was significant (P=0.0003).
Field setup 1 resulted in hemoclips being outside of the radiation treatment field in 22/55 (40%) sites. The number of hemoclips outside of treatment field setup 1 ranged from one to eight, and as a relative percentage of the total number of hemoclips ranged from 8% to 50%. Hemoclips were located outside of field setup 1 for 3/28 (10.7%) of extremity sites (range=1–2 hemoclips; median=1 hemoclip), 19/24 (79%) of truncal sites (range=1–8 hemoclips; median=3 hemoclips), and 0/3 (0%) head sites. The hemoclip distance from the incision for the extremity sites (n=28 sites) ranged from 0 to 3.8 cm with three sites having hemoclips located >3 cm from the incision. The hemoclip distance from the incision for truncal sites (n=24 sites) ranged from 0 to 8.0 cm, with 19 sites having hemoclips located >3 cm from the incision, 12 sites had hemoclips >4 cm from the incision, eight sites with hemoclips >5 cm from the incision, and five sites with hemoclips >6 cm from the incision (1, 2). There was a significant association between incision length and greatest distance of hemoclips from the incision when evaluating all sites (r2=0.4, P<0.0001). For an incision length of <9.55 cm all hemoclips were within 4 cm of the incision. When the incision length was >9.55 cm the maximum hemoclip distance was more variable.
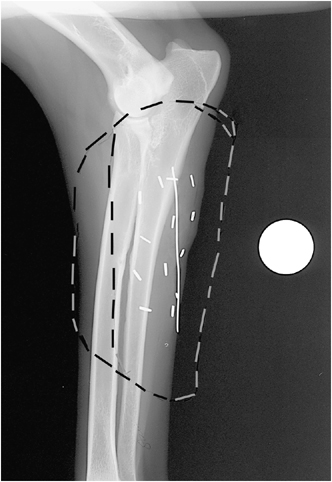
Lateral radiograph of left antebrachium of a German shepherd after surgical resection of a hemangiopericytoma with hemoclips outlining the tumor bed (n=13), wire over the incision (7.54 cm in length), and circular metal marker for determination of the magnification factor. Marks on the image outline the radiation field for Field setups 1 and 2.
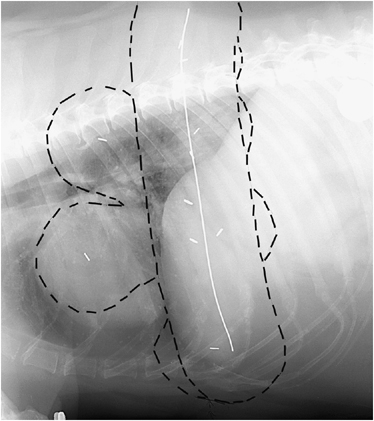
Right lateral thoracic radiograph of a Labrador retriever after surgical resection of an intermediate grade mast cell tumor with hemoclips outlining the tumor bed (n=10), wire over the incision (21.7 cm in length), and circular metal marker for determination of the magnification factor. Marks on the image outline the radiation field for Field setups 1 and 2.
There were five dogs with extremity tumors where it would not be possible to exclude a strip of skin to spare the lymphatics if the radiation treatment field was set up based on a 3-cm margin around the incision, and in an additional 11 dogs it would not be possible to spare a strip of skin if the field was setup based on a 3-cm margin around the incision and hemoclips.
Discussion
Hemoclips were considered important for simulating radiation treatment fields for the majority of tumors (79%) resected from the trunk. There was a significant difference in radiation treatment field size considering hemoclip information only for truncal tumors. Hemoclips did not result in significant field size alterations for lymph node excision sites, or for masses on the head, and hemoclips had an effect on field size only in a small subset of extremity tumors (10.7%). The limited number of dogs with masses on the head reduces the possibility of any conclusion regarding this site.
Hemoclip placement at extremity sites is useful for determining whether it is feasible to spare a strip of skin. There were five dogs with extremity tumors where it would not be possible to spare a strip of skin if the radiation treatment field was set up based on a 3-cm margin around the incision, and this increased to an additional 11 dogs if the field was setup based on a 3-cm margin around the hemoclips and the incision.
Historically, imaging of postoperative mast cell tumor sites was done in only two of seven studies, with radiographs and CT being used; margins ranged from 2 to 3 cm.14–20 Treatment planning was by hand calculation in five of seven studies and based on CT in a subset of patients in the other two. Imaging of postoperative soft tissue sarcoma sites was done in only two of five studies and included radiographs in one study and CT in a subset of patients in two others.21–25 Field margins, when described, were 2–3 cm. Treatment planning was by hand calculation in three, and based on CT images in a subset of patients in the other two publications. Imaging of postoperative thyroid carcinomas was performed in two of three studies with CT and magnetic resonance (MR) imaging used in subsets of patients; margins were 2 cm in one study and based on anatomic landmarks in another.26–28 Treatment planning was by hand calculation in all three publications. Tumor imaging has been used more often in studies of nasal tumor irradiation. Based on seven studies, imaging of the nasal cavity included radiographs (3/7), CT in all patients (3/7), CT in a subset of patients (1/7), and MR imaging in a subset of patients (1/7).29–35 The only reference to field margins was anatomic landmarks used to delineate radiation treatment fields (2/7). Treatment planning was by hand calculation in all patients (3/7) or a subset of patients (1/7); and CT-based computer planning was done in all patients (3/7) or a subset of patients (1/7). This historical information illustrates the inconsistency in the manner in which radiation planning is undertaken and described. More attention will have to be paid to this aspect of radiation therapy, particularly when reporting results of external beam radiation therapy. The International Commission on Radiation Units and Measurements (ICRU) reports provide quidelines for various aspects of radiation therapy including prescribing, recording, and reporting photon beam therapy (ICRU report 50).36 This level of standardization of treatment planning and reporting needs to be instituted in veterinary radiation oncology.
One noteworthy aspect of our study is that not all dogs were irradiated nor were they all considered candidates for radiation therapy. The utility of hemoclips needs to be defined based on outcome assessment. There is no agreement among veterinary radiation oncologists on the appropriate margin size based on tumor type, grade, and/or expected local growth pattern. In humans, accurate estimation of locoregional microscopic tumor extension is problematic for some sites.37 There are substantial differences in how radiation treatment fields are defined and whether imaging is used in the treatment planning process.
The placement of hemoclips to define the tumor bed is only one aspect of radiation patient management. Failure to ink surgical margins to assess completeness of resection cannot be compensated for with hemoclip placement or CT planning.38 Additionally, hemoclips alone may result in underestimation of the tumor bed and a combination of clip location and CT-based planning may be needed.39
As noted, only a subset of the dogs (n=13 dogs) were actually irradiated postoperatively. An additional eight dogs were candidates for irradiation. Thus, 21 of 47 dogs (44.7%) either were, or could have been, irradiated. For the remaining 26 dogs, masses were completely excised and/or they had a benign disease and radiation therapy was not indicated. It may be that the extent of the excision, and the placement of hemoclips distant from the incision, reflected the aggressiveness of the resection with intent to provide complete excision. Because the goal of the study was to determine if additional information was provided by placement of hemoclips to delineate the surgical bed, what the relationship is between a surgical incision and location of hemoclips, and how each have utility in describing the surgical field, the results of this study are considered valid despite this weakness.
Although it is known that hemoclips may shift in position between the immediate postoperative period and the start of radiation therapy, this was not addressed in the current study.4 Thus, ideally, the tumor bed should be imaged immediately postoperatively and again prior to radiation therapy. Furthermore, hemoclips may move with the skin and subcutaneous tissues if placed superficially. Hemoclips should be placed deeper into underlying fascia or muscle to provide a more stable location.
Portal imaging may be useful for identification of hemoclip position and for verification of inclusion of all of the tumor bed with a planning target volume. Whether hemoclips will be seen in portal images depends on the portal imaging system, film quality, tumor location, and patient size.
Identification of hemoclips with CT imaging for 3D radiation treatment planning was also not addressed in this study. Localization of hemoclip placement in CT images and their inclusion when contouring the planning target volume is valuable in defining the lateral and deep aspects of the surgical bed. Hemoclips can cause artifacts in CT images but we now use them routinely in the majority of radiation patients to delineate the surgical bed.40 We experience minimal artifact although artifacts can be problematic with smaller body parts and thin small patients, and if the hemoclips are immediately subjacent to the skin. Hemoclips may be beneficial for both photon and electron treatment fields, particularly for trunk tumors that have been resected and are irradiated using electrons (Fig. 2).
Exactly where to place hemoclips in a surgical bed is debatable. They can be placed to outline the gross tumor location. Alternatively they may be placed to outline the extent of the surgical bed. It is important that there be an understanding between the surgeon and radiation oncologist as to the information expected from hemoclip insertion.
Precise target definition in radiation therapy will likely be improved based on information provided by hemoclip insertion. Hemoclip use in this study resulted in an increase in volume of irradiated tissue. Although not addressed in the current study, larger field areas may increase the risk of complications due to irradiation of a larger volume of normal tissue. Future studies based on outcome assessment will be necessary to determine the impact of hemoclips on radiation treatment field size in clinical patients and impact on local radiation side effects.