TIMING OF LEFT HEART BASE DESCENT IN DOGS WITH DILATED CARDIOMYOPATHY AND NORMAL DOGS
Previously presented in abstract form at the British Small Animal Veterinary Association annual congress in Birmingham, UK, April 7–10, 2005.
Abstract
The identification and assessment of myocardial failure in canine idiopathic dilated cardiomyopathy (DCM) is achieved using a variety of two-dimensional and Doppler echocardiographic techniques. More recently, the availability of tissue Doppler imaging (TDI) has raised the potential for development of new ways of more accurately identifying a disease phenotype. Nevertheless, TDI has not been universally adapted to veterinary clinical cardiology primarily because of the lack of information on its utility in diagnosis. We assessed the application of timing of left heart base descent using TDI in the identification of differences between DCM and normal dogs. The times from the onset of the QRS complex on a simultaneously recorded electrocardiograph to the onset (Q–S′), peak (Q–peak S′), and end (Q–end S′) of the systolic velocity peak were measured in the interventricular septum (IVS) and the left ventricular free wall. The duration of S′ was also calculated. The Q–S′ (FW), Q–end S′ (FW), and duration S′ (FW) were correlated with ejection fraction in the diseased group (P<0.05). In addition, Q–S′, Q–peak S′, Q–end S′, and the peak S′ velocity were prolonged in the diseased dogs at both the free wall and in the IVS (P<0.01). The duration of S′ was unaffected by disease status. These findings provide insight into the electromechanical uncoupling that occurs in canine DCM and identifies new TDI parameters that can be added to the range of Doppler and echocardiographic parameters used for detecting myocardial failure in the dog.
Introduction
Ultrasound-derived systolic time intervals (STIs) can be used to assess global left ventricular systolic function.1 They have good agreement with invasively derived STIs and are a sensitive means of detecting a variety of cardiac diseases and pharmacologic cardiac manipulations.1,2 The systolic preejection period (PEP) and left ventricular ejection time (LVET) and their derivatives, total electromechanical systole (QAVC) and the ratio PEP:LVET, are measured by M-mode examination of the aortic valve leaflet excursions or from spectral Doppler traces of the aortic outflow. However, these parameters are subject to alterations with loading and heart rate (HR), and have not been widely adopted for diagnosis or evaluation of canine cardiac disease.1–5 Tissue Doppler imaging (TDI) of the left heart base is a modality that allows measurement of maximal myocardial velocity and the timing of the phases of heart base descent during systole.4 TDI provides an alternative method for assessing the timing of systole and may be of use in the assessment of regional and global left ventricular systolic function, even where there is absence of regional wall motion abnormalities.6,7 TDI assesses Doppler frequency shifts and quantifies myocardial velocities.4,8,9 During data acquisition, Doppler frequency shifts arising from the myocardium are selectively amplified while blood pool Doppler signals are rejected.4 Color two-dimensional TDI (c-TDI) uses autocorrelation techniques to calculate mean instantaneous myocardial velocities along a series of ultrasound scan lines within a two-dimensional sector.8 These velocities are color encoded and superimposed on a gray-scale image in real time.4 Mean myocardial velocities are extracted and transmural myocardial velocity gradients and indices of myocardial deformation such as regional strain and strain rate can be calculated from the stored velocity data during post processing.4,8,10,11
Longitudinal myocardial velocity curves obtained by TDI are characterized by three major peaks in all myocardial segments of the left and right ventricular free wall and the interventricular septum (IVS).4,8,12–14 An initial positive systolic velocity peak (S′) is followed by early (E′) and late (A′) negative diastolic peaks.4,8 High-frequency signals of variable polarity and amplitude are seen during the PEP and isovolumic relaxation period.4,8 Measurement of the amplitudes of the major velocity peaks by TDI is adequately repeatable and reproducible in conscious dogs and cats.12,13,15,16
In humans, S′ represents the peak mean myocardial velocity during systole and it corresponds to the peak aortic outflow velocity, and is closely correlated with ejection fraction (EF) and peak positive dP/dt.8,17–20 In addition, the timing of the onset of S′ is synchronized and the duration of S′ is comparable in all myocardial segments,6 and myocardial disease causes a delay in the time of onset of S′ and a greater dispersion in the time of onset and duration of S′ around the heart base.21 Q–peak S′ is also significantly prolonged and is inversely related to the maximal rate of increase in pressure during isovolumic contraction (dP/dt) and EF in hypertrophic, hypertensive, and dilated cardiomyopathy (DCM) in man.17,18 The duration of S′ is significantly reduced in human patients with nonischemic DCM.22
Most interest in TDI in animals has been focused on measurement of myocardial velocity and transmural myocardial velocity gradients to assess systolic and diastolic function in dogs and cats.16,23–27 c-TDI-derived transmural myocardial velocity gradients and regional peak mean myocardial velocities are more sensitive than standard echocardiographic techniques for detecting myocardial dysfunction in Golden Retriever muscular dystrophy and occult canine DCM.25 TDI has also been used to assess systolic and diastolic function in a dog with mitral valve dysplasia.26 The use, however, of TDI to measure the timing of the phases of mechanical systole has not, to our knowledge, been described in dogs with naturally occurring DCM. The aims of this study were, therefore, to investigate the application of timing and peak excursion of heart base descent using c-TDI to identify differences between dogs with DCM and dogs without evidence of cardiac disease, to investigate whether or not there was a significant relationship between the HR and TDI-derived time intervals, and to determine whether or not the EF and blood pool-derived STIs were related to the TDI-derived time intervals. To help fulfill these aims, the intraoperator reproducibility of measurements and their correlations with EF and blood pool Doppler-derived STIs were also quantified.
Materials and Methods
Normal dogs were recruited from pet dogs owned by the staff. These dogs had no history of cardiorespiratory or current systemic disease, were normotensive (indirect Doppler blood pressure measurement), had normal clinical and six lead electrocardiographic examinations, and had no significant structural or functional heart disease detected on routine echocardiographic examination. The normal dog group consisted of 17 dogs, six females and 11 males, of various breeds (Labrador Retriever [seven], Cross bred [seven], English Springer Spaniel [two], Boxer [one]), with a mean age of 6.2±3.0 years (range, 2–12 years) and a mean body weight of 25.5±9.1 kg (range, 12.4–41 kg).
The diseased group were recruited from clinical patients with canine DCM.28 Inclusion criteria included the presence of a hypokinetic left ventricular wall, fractional shortening below the reported reference range,29 increased left ventricular internal dimension in diastole and systole, altered chamber geometry, and EF <50% (EF calculated as the percentage reduction in left ventricular volume in systole with ventricular volume calculated using the single plane area method, assessed from the right parasternal long-axis view30). Dogs had to predominantly be in sinus rhythm with no evidence of interventricular conduction disturbances on six lead electrocardiogram.31 The diseased group consisted of 10 dogs, six females and four males, of various breeds; there were three Weimeraners included, and one of each of the remaining breeds (Golden Retriever, Cocker Spaniel, Boxer, Cavalier King Charles, English Springer Spaniel, Deerhound, and Collie Cross). The mean age of the dogs was 5.5±1.0 years (range, 1–10 years), with a mean body weight of 27.8±12.7 kg (range, 9.25–49.2 kg). The International Small Animal Health Council Heart Failure Classification (ISACHC) score ranged from 1b (four dogs) to 3a (two dogs). Two of the dogs were not receiving any medication (ISACHC scores 2 and 3a). The remaining dogs were receiving one or more of the following medications: furosemide, pimobendan, benazepril, ramapril, amiodarone, atenolol, mexilitine, and spironolactone. Concurrent treatment for cardiac disease was not an exclusion criterion, but this is a confounding design factor addressed in the discussion.
For sonography, all animals were restrained in lateral recumbency and imaged using standard techniques and a Vivid System Five ultrasound machine * equipped with a variable frequency 2.5–3.5 MHz flat-phased array transducer.32,33 Ultrasound examinations and data analysis were carried out by an operator (B.C.D.) who was aware of whether the images were from a normal dog or a patient. STIs were measured on a spectral Doppler recording of the aortic outflow obtained from the subcostal transducer position, with the Doppler sampling gate positioned below the aortic valve leaflets.32 The LVET was corrected for HR as follows: LVET index (LVETI)=LVET+(0.55 × HR).1
All c-TDI examinations were performed on a left apical four-chambered view and a lead two electrocardiograph was recorded and displayed simultaneously. The B-mode sector angle and the width and length of the c-TDI sector were minimized to optimize recording frame rates.4 Frame rates in excess of 100 frames per second (fps) are needed to resolve all myocardial motion during ejection, and this was achieved in all examinations.4 The Doppler gain was adjusted to achieve optimal myocardial coloring and the Doppler velocity range was set as low as possible to achieve optimal velocity resolution while avoiding aliasing. Velocity profiles were extracted on line during the examination to ensure that representative velocity curves had been obtained and that aliasing had not occurred.4
Two loops of six cardiac cycles were recorded from the basal myocardial segment of the IVS and then from the left ventricular free wall (LVFW) adjacent to the mitral valve annulus. The recorded loops were transferred to a dedicated personal computer and stored on optical disks† for later analysis using dedicated TDI software.‡
Mean myocardial velocity curves were extracted from the septum and the LVFW using a 15 × 15 pixel sampling area positioned over the myocardium immediately adjacent to the mitral annulus (Fig. 1). The manual tracking facility on the Echopac® software§ was used to track this position throughout the cardiac cycle. The resultant velocity curves were measured using the measurement and analysis system of this software. The first five fully resolved velocity curves were measured and the mean of the five measurements for each parameter is used in the final analysis.

Site of tissue Doppler imaging sampling gate placement within the interventricular septum and posterior wall. ▪, represents the location at which the sampling gate was positioned.
The time to onset (Q–S′), the peak S′ (Q–peak S′), and the end of the S′ wave (Q–end S′) were measured using the beginning of the QRS complex on the simultaneously displayed electrocardiograph as a reference point.6,18 The duration of S′ was calculated (Fig. 2) as was the peak mean systolic velocity, S′. The reference points for the timing of these events were taken as the point at which the acceleration and deceleration slopes of the S′ crossed the baseline. These points were extrapolated down to the baseline in traces where the curve did not cross the baseline.4
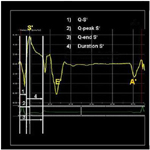
Time intervals measured by tissue Doppler imaging (TDI) (A): A longitudinal myocardial velocity curve recorded from the base of the interventricular septum in a normal dog showing the reference points used for measuring the TDI time intervals. This trace represents a time–velocity curve with velocity (cm/s) on the “y-axis” and time on the x-axis. S′, peak systolic velocity; E′, peak early diastolic velocity; A′, peak late diastolic velocity; Q–S′, beginning of the QRS complex to the onset of the systolic wave; Q–peak S′, beginning of the QRS complex to the peak systolic velocity; Q–end S′, beginning of the QRS complex to the end of the systolic wave; Duration S′, duration of the systolic wave.
For assessing reproducibility, four normal dogs underwent four, nonconsecutive, echocardiographic examinations over a 5-day period (intraobserver/operator, between day variability). The conditions under which these examinations were performed and the order in which the data was acquired were standardized for all examinations. All dogs were restrained in left recumbency by the same handler (K.E.S.) and scanned by the same operator (B.C.D.), as described above. All scans were measured nonconsecutively by the same observer (B.C.D.) and the results for each scan were compared. The examinations were randomized and the operator was unaware whether data were from a normal dog or patient. Analysis of interobserver or within-day variability was not undertaken.
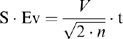
The comparison between the TDI time intervals at the septum and the free wall and the comparison between the spectral Doppler-derived STIs and TDI-derived time intervals were performed using an unpaired Student's t-test. The relationship between the R–R interval and the TDI time intervals was investigated using a simple linear regression model. When this analysis was performed, it was discovered that several of the TDI time intervals were related to the HR. Because these intervals are inherently related to one another, it was decided that all TDI time intervals should be corrected for HR. This was achieved by dividing each parameter by the square root of the R–R interval measured on the simultaneously recorded electrocardiograph.
A Pearson correlation was used to assess the correlation between the Doppler-derived STI and TDI-derived time intervals and between the TDI-derived time intervals and EF. The significance level for these analyses was set at P<0.05.
Results
There was no statistically significant difference between the diseased and nondiseased group with respect to age, body weight, or gender distribution. As expected, the dogs with DCM differed significantly from the unaffected dogs in the majority of their standard echocardiographic parameters (Table 1).
| Parameter | Normal GroupMean±SD | Diseased GroupMean±SD | P-Value |
|---|---|---|---|
| LVDd (mm) | 43.26±5.97 | 68.57±13.92 | <0.001 |
| LVDs (mm) | 29.64±5.29 | 61.38±11.63 | <0.001 |
| PWd (mm) | 8.53±1.26 | 8.13±1.96 | 0.581 |
| PWs (mm) | 12.17±1.83 | 8.70±1.78 | <0.001 |
| IVSd (mm) | 9.31±1.35 | 8.60±1.70 | 0.227 |
| IVSs (mm) | 12.89±1.66 | 10.01±2.15 | 0.002 |
| FS (%) | 31.75±4.26 | 11.19±2.22 | <0.001 |
| EF (%) | 54.13±5.50 | 19.14±14.55 | <0.001 |
| ESVI (ml/m2) | 28.94±7.02 | 134.32±45.43 | <0.001 |
| AO (mm) | 20.94±2.68 | 21.14±5.33 | 0.916 |
| LAD (mm) | 23.82±3.15 | 38.27±12.09 | 0.005 |
| R–R (s) | Med: 0.62, R: 0.50–1.07 | 0.46±0.12 | <0.001 |
| PEP (s) | 0.08±0.01 | 0.12±0.02 | <0.001 |
| LVET (s) | 0.19±0.02 | 0.14±0.01 | <0.001 |
| LVETI (s)* | 0.24±0.01 | 0.22±0.01 | <0.001 |
| QAVC (s) | 0.26±0.02 | 0.26±0.01 | 0.059 |
| Ratio PEP:LVET | Med: 0.39, R: 0.31–0.46 | 0.81±0.16 | <0.001 |
| AV (m/s) | 1.32±0.19 | 0.88±0.15 | <0.001 |
| PV (m/s) | 0.84±0.19 | 0.67±0.19 | 0.039 |
| EPSS (mm) | 0.50±0.14 | 25.92±6.63 | <0.001 |
- Med, median; R, range; LVDd, left ventricular diameter in diastole; LVDs, left ventricular diameter in systole; PWd, posterior wall thickness in diastole; PWs, posterior wall thickness in systole; IVSd, interventricular septal thickness in diastole; IVSs, interventricular septal thickness in systole; FS, fractional shortening; EF, ejection fraction; ESVI, end systolic volume index; AO, aortic internal diameter at end systole; LAD, left atrial internal diameter at end systole; PEP, preejection period; LVET, left ventricular ejection time; LVETI, left ventricular ejection time index; QAVC, total electromechanical systole; AV, aortic outflow velocity; PV, pulmonic outflow velocity; EPSS, E-point-to-septal-separation; SD, standard deviation; HR, heart rate.
- * Calculated as LVET+(0.55 × HR).1
The intraoperator variability for all TDI-derived time intervals was low with CV <10% for all parameters (Table 2). Q–end S′ was the most repeatable parameter at both measuring sites (CV=1.75%±0.58 and 1.34%±0.44 at the septum and the free wall, respectively). The least repeatable parameter was peak S′ velocity measured at the free wall (CV=12.03%).
| Coefficient ofVariation (%) | 95% ConfidenceInterval | |
|---|---|---|
| Q–S′ (IVS) | 8.664 | 2.867 |
| Q–peak S′ (IVS) | 7.890 | 2.611 |
| Q–end S′ (IVS) | 1.752 | 0.580 |
| Duration S′ (IVS) | 3.600 | 1.191 |
| Peak S′ (IVS) | 7.809 | 2.584 |
| Q–S′ (FW) | 6.283 | 2.064 |
| Q–peak S′ (FW) | 5.314 | 1.759 |
| Q–end S′ (FW) | 1.341 | 0.444 |
| Duration S′ (FW) | 3.196 | 1.058 |
| Peak S′ (FW) | 12.027 | 3.980 |
- This table shows the results of the intraoperator reproducibility study, with results reported as coefficients of variation and 95% confidence intervals for each of the measured parameters. TDI, tissue Doppler imaging; Q–S′, beginning of the QRS complex to the onset of the systolic wave; Q–peak S′, beginning of the QRS complex to peak systolic velocity; Q–end S′, beginning of the QRS complex to the end of the systolic wave; duration S′, duration of the systolic wave; peak S′, maximal velocity of the systolic wave; IVS, interventricular septum; FW, left ventricular free wall.
Significant positive relationships were found between the R–R interval and duration S′ (IVS), Q–end S′ (IVS), duration S′ (FW), and Q–end S′ (FW) in the normal group (Table 3). A significant relationship was also noted between R–R interval and duration S′ (IVS), Q–end S′ (IVS), duration S′ (FW), and Q–end S′ (FW) in the diseased group (Table 3). The peak S′ velocity, time from Q–peak S′, and Q–S′ durations were not linearly related to the R–R interval at either site.
| Normal Group | Diseased Group | |||
|---|---|---|---|---|
| R 2 Value | P-Value | R2 Value | P-Value | |
| Duration S′ (IVS) | 0.458 | 0.003 | 0.575 | 0.003 |
| Q–S′ (IVS) | 0.010 | 0.786 | 0.002 | 0.903 |
| Q–peak S′ (IVS) | 0.052 | 0.525 | 0.045 | 0.558 |
| Q–end S′ (IVS) | 0.295 | 0.024 | 0.698 | 0.002 |
| Peak S′ (IVS) | 0.055 | 0.515 | 0.262 | 0.130 |
| Duration S′ (FW) | 0.374 | 0.010 | 0.740 | 0.001 |
| Q–S′ (FW) | 0.020 | 0.699 | 0.062 | 0.488 |
| Q–peak S′ (FW) | 0.06 | 0.485 | 0.038 | 0.592 |
| Q–end S′ (FW) | 0.448 | 0.006 | 0.595 | 0.009 |
| Peak S′ (FW) | 0.001 | 0.970 | 0.156 | 0.771 |
- Q–S′, beginning of the QRS complex to the onset of the systolic wave; Q–peak S′, beginning of the QRS complex to the peak of the systolic wave; Q–end S′, beginning of the QRS complex to the end of the systolic wave; Duration S′, duration of the systolic wave; Peak S′, maximal velocity of the systolic wave; IVS, interventricular septum; FW, left ventricular free wall; TDI, tissue Doppler imaging.
HR-corrected Q–S′ (IVS), Q–S′ (FW), Q–peak S′ (IVS), Q–peak S′ (FW), Q–end S′ (IVS), and Q–end S′ (FW) were all significantly longer in the diseased than in the normal group. There was no significant difference between the two groups for HR-corrected duration S′ (IVS) and HR-corrected duration S′ (FW). Peak S′ (IVS) and peak S′ (FW) were significantly lower in the diseased group (Table 4).
| Normal (s) | Diseased (s) | P-Value | |
|---|---|---|---|
| Duration S′ (IVS) | 0.201±0.018 | 0.162±0.034 | 0.409 |
| Q–S′ (IVS) | 0.085±0.016 | 0.124±0.027 | 0.001 |
| Q–peak S′ (IVS) | 0.128±0.021 | 0.190±0.036 | <0.001 |
| Q–end S′ (IVS) | 0.286±0.030 | 0.321±0.030 | 0.010 |
| Peak S′ (IVS) | 8.113±1.726 (cm/s) | 4.948±1.094 (cm/s) | <0.001 |
| Duration S′ (FW) | 0.199±0.029 | 0.199±0.020 | 0.974 |
| Q–S′ (FW) | 0.085±0.018 | 0.158±0.041 | 0.001 |
| Q–peak S′ (FW) | 0.124±0.028 | 0.211±0.048 | <0.001 |
| Q–end S′ (FW) | 0.283±0.039 | 0.352±0.029 | <0.001 |
| Peak S′ (FW) | 10.363±2.190 | 5.262±2.001 | <0.001 |
- Q–S′, beginning of the QRS complex to the onset of the systolic wave; Q–peak S′, beginning of the QRS complex to the peak of the systolic wave; Q–end S′, beginning of the QRS complex to the end of the systolic wave; Duration S′, duration of the systolic wave; Peak S′, maximal velocity of the systolic wave; IVS, interventricular septum; FW, left ventricular free wall; TDI, tissue Doppler imaging.
There was no significant correlation between the HR-corrected TDI intervals or the peak S′ velocity and the EF in the normal group. The DCM group was characterized by a significant and strong negative correlation between EF and HR-corrected Q–S′ (FW) (P=0.017, r=−0.730) and Q–end S′ (FW) (P=0.044, r=−0.645), and a significant positive correlation between the EF and duration S′ (FW) (P=0.048, r=0.637) (Fig. 3). The remaining parameters did not have a significant correlation with EF.
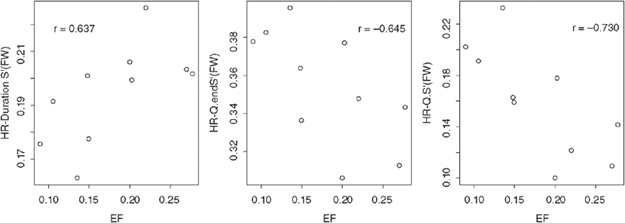
Scatter plots of the ejection fraction vs. heart rate-corrected S′ Duration (FW), Q–end S′ (FW), and Q-S′ (FW). HR, heart rate; HR–Duration S′ (FW)′, heart rate-corrected duration of the systolic wave measured from the left ventricular free wall (LVFW); HR–Q–end S′, HR-corrected beginning of the QRS complex to the end of the systolic wave measured from the LVFW; HR–Q–S′ (FW), HR-corrected beginning of the QRS complex to the onset of the systolic wave measured from the LVFW; EF, ejection fraction.
There was no significant difference between the time intervals recorded at the IVS and the LVFW in the normal group. However, the peak S′ velocity was significantly higher when assessed from the free wall site than when assessed from the IVS (P=0.04) in normal dogs. A significant difference was found between Q–S′ at the two sample points in the diseased group (P=0.01). None of the remaining TDI-derived STIs varied between the two sites and the peak S′ velocity did not vary between the free wall and the IVS in the diseased dogs.
Significant, positive correlations were found between the PEP and Q–S′ (IVS) (P=0.02, r=0.588), PEP and Q–S′ (FW) (P=0.008, r=0.617), LVET and duration S′ (IVS) (P=0.008, r=0.619), and LVET and duration S′ (FW) (P=0.006, r=0.651) in the normal group. A significant and strong positive correlation was also found between PEP and Q–S′ (FW) (P=0.008, r=0.780) and between LVET and duration S′ (FW) (P=0.007, r=0.788) in the diseased dogs. No significant correlation was found between PEP and Q–S′ (IVS) or between LVET and duration S′ (IVS) in the DCM group.
In the normal group, the LVET was significantly longer than the duration S′, P=0.01 and 0.008, respectively, for the septal and free wall measurements. Furthermore, within this group, the total electromechanical systole (QAVC) was significantly longer than Q–end S′ for both the septal and the free wall measurements, P=0.002 and 0.043, respectively. In the DCM group, the PEP was also significantly longer than Q–S′ (IVS) (P<0.001) and QAVC was significantly longer than Q–end S′ (IVS) (P=0.043).
Discussion
The results of this study demonstrate that the velocity and timing of systolic heart base descent can be assessed with a high degree of reproducibility using c-TDI in dogs, that these measurements correlate well with other more commonly used indices of global left ventricular systolic function, and that these parameters are significantly different when dogs with DCM are compared with normal dogs. The lack of a gold standard for measurement of systolic function in the pet-owned dog population and the lack of invasive-derived measurements from our study population are limiting factors, but these do not detract from the conclusion that these TDI measurements are of value in the identification of systolic dysfunction in DCM dogs or that these measurements can be helpful in separating a diseased from a normal population.
We identified a significant linear relationship between HR and both the duration of the S′ wave (S′ duration) and the time to the end of the S′ wave (Q–end S′), in both groups of dogs, at both the IVS and the free wall. Because the TDI intervals being assessed were closely related to one another, the decision was made to correct this group of parameters for the affects of HR. This process involved measuring the R–R interval on all tracings and dividing the intervals by the square root of the R–R interval. This is a cumbersome process and may serve as a potential limitation in a clinical setting.
The dogs with DCM in this study had a significant variation in the Q–S′ at the two different aspects of the mitral annulus, a significant reduction in the peak S′ velocity, and a delayed Q–peak S′ and Q–end S′ in both the IVS and the LVFW. There was no significant difference between the groups for the duration of S′, indicating that the duration of longitudinal left ventricular shortening during systole is dependent on factors other than the contractile function of cardiomyocytes.
The peak velocity of heart base descent was significantly lower in the myocardial failure group. This measurement has shown promise for detecting systolic dysfunction in canine myocardial failure patients. Breed variations have, however, been reported 34 and may confound the widespread use of this parameter until breed-specific values are available. Some investigators have also reported an affect of HR on peak S′,12 although no such affect was identified in the current study. The peak S′ velocity was significantly higher at the free wall compared with the IVS in the normal dogs, although there was no such difference in the dogs with DCM. Previously, it has been reported that in normal humans the peak S′ velocity is higher at the free wall site than at the IVS,6 but this difference does not persist in people with myocardial hypertrophy.21
The time of onset of S′ (Q–S) was in agreement with what has been previously reported for its blood pool Doppler equivalent PEP,1 not significantly effected by R–R interval and was negatively correlated with EF in the free wall and the IVS. The Q–S′ interval was significantly longer at both sites on the heart base in the diseased group than in the normal group, indicating a delay in longitudinal shortening of the left ventricle in these dogs. We postulate that this delay can be attributed to the greater time taken for the diseased myocardium to generate sufficient longitudinal contractile force during early systole and an altered pattern of contraction of the diseased left ventricle.
A significant correlation was found between EF and Q–S′ (FW), Q–end S′ (FW), and the duration S′ (FW) in the DCM group. Q–S′ and Q–end S′ were significantly longer in the diseased than in the normal group at both measuring sites indicating a delay to onset of systole, and delay to the completion of systole (with no significant alteration in the duration of systole). In humans, the time to peak S′ is inversely related to dP/dt and EF at the free wall, and is prolonged significantly in hypertrophic, hypertensive, and DCM.17,18 Unfortunately, those studies did not investigate any other TDI intervals. While we did not identify a significant association between Q–peak S′ (FW) and EF in the dogs with DCM (P=0.059, r=−0.613), our results may be biased by the small sample size.
There was good synchrony of systolic longitudinal shortening between the examined myocardial segments around the heart base in the normal group, which agrees with previous reports in humans and dogs.4,13 However, in the diseased group, the Q–S′ was longer at the free wall site than at the IVS. If this alteration represented asynchrony of longitudinal shortening, one would expect the remaining STIs to be affected, none of which were significantly different between the two sites. Therefore, the synchrony of heart base descent in dogs with DCM may warrant further investigation.
In the present study, a significant correlation was found between PEP and Q–S′ and between LVET and duration S′ at the IVS and at the free wall in the normal group. This is in agreement with studies in man in which a similar close coupling was found between systolic myocardial velocities and ventricular emptying during systole.6,22 This correlation was, however, lost at the septal site in the diseased group. Septal movement is influenced by both right and left ventricular function, whereas the LVFW movement is less influenced by right ventricular function.17 STIs measured by blood pool Doppler assessment of aortic outflow reflect global left ventricular systolic function, and, therefore, one would expect the values for the free wall parameters to be more closely correlated with these values. DCM can affect both the left and right ventricles. To our knowledge, the influence of right ventricular function on the TDI measurements recorded from the IVS has not been assessed in the dog. However, it could be hypothesized that alterations in the right ventricular function in the diseased dogs may have influenced the TDI measurements recorded from this site.
With respect to absolute measurements, PEP was longer than Q–S′ in both myocardial segments in the normal and diseased groups, although this difference only reached significance at the IVS. This is in agreement with what has previously been found in humans, where the onset of S′ has been shown to precede left ventricular ejection by a mean of 10 ms.6 Longitudinal shortening of the left ventricle begins during the isovolumetric phase of systole causing descent of the heart base before the onset of left ventricular ejection, and this accounts for the disparity in the timing of these two events.30
QAVC was significantly longer than Q–end S′ in both myocardial segments in the normal group. QAVC was also longer than Q–end S′ in both myocardial segments in the diseased group, but this difference only reached significance for Q–end S′ (IVS). This finding is in agreement with what has previously been shown in humans, and is due to the inertia of the blood column in the left ventricular outflow tract and ascending aorta.6
We recognize that there are certain limitations that need to be considered in the interpretation of our findings. The lack of invasive data for client-owned dogs is a problem as mentioned earlier, and validation of our findings would also be improved by increased group sizes. In addition, the variation in the TDI measurements over several different days was not analyzed, and, therefore, we could not truly assess the repeatability of the measurements. The determination of normality is a major confounding factor in a study of this nature, but we have used the best possible consensus opinion to identify both normal and diseased dogs.28 We are satisfied that the diagnosis of DCM was correct, although we could only confirm this in one dog at post mortem examination. In an attempt to avoid inadvertent inclusion of dogs with occult disease, the normal dogs were purposely selected from breeds not known to be overly represented in prevalence reports of canine DCM. It could be argued that this enters an additional confounding factor of breed variability,34 but this can only be resolved with breed-specific studies. The echocardiographer was not blinded to the group the presenting dog was in. This may have introduced an additional bias to the study. In addition, we included dogs that were being treated. We cannot state the possible effects of treatment on our results. However, the dogs included in this study were clinical patients, which frequently presented on medication. We did not feel that it was justified to remove these medications to perform this study.
nt on these measurements before they may be employed in a clinical setting.
ACKNOWLEDGMENTS
The authors would like to thank Dr. DJ Shaw for statistical guidance. KES was sponsored by the Petplan Charitable Trust and the Feline Advisory Bureau, and RW was sponsored by Boehringer Ingelheim.




