The effects of concentrations of lysine in media on differentiation of 3T3-L1 preadipocytes
ABSTRACT
Intramuscular fat content is increased by feeding of low lysine diets in pigs. Reduction in dietary lysine intake results in low plasma lysine concentration and low cytosolic lysine concentration in skeletal muscles. From these observations, we hypothesized that low plasma lysine concentration in pigs fed on low lysine diets reduced supply of lysine from blood circulation to preadipocytes, and this limited supply of lysine might promote adipocyte differentiation in porcine muscles. In order to verify the hypothesis, we investigated the effects of low concentrations of lysine in culture medium on differentiation of 3T3-L1 preadipocytes. Low concentration of lysine suppressed lipid accumulation and messenger RNA (mRNA) expression and enzyme activity of fatty acid synthase. mRNA expressions of peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) were lower in cells cultured in low lysine medium. On the other hand, mRNA and protein expressions of C/EBPβ and C/EBPδ were not inhibited by low concentrations of lysine in culture medium. These results indicate that low lysine concentrations in culture medium inhibit differentiation of 3T3-L1 preadipocytes through inhibiting the mRNA expressions of PPARγ and C/EBPα.
INTRODUCTION
Intramuscular fat (IMF) content is an important pork quality trait. Taste, juiciness and tenderness in pork are influenced by IMF content (Hodgson et al. 1991; Castell et al. 1994). However, the detailed mechanism of IMF accumulation in pigs is still unknown. There are several nutritional approaches to increase IMF content in muscle of pigs. Some studies have shown that IMF content is increased by feeding of low lysine diets in pig (Witte et al. 2000; Katsumata et al. 2005).
The major component of IMF, triglyceride, is stored partly within the myofibers and mostly within the intramuscular adipocytes (Essen-Gustavsson et al. 1994). Therefore, increasing both number and size of intramuscular adipocytes may contribute to IMF deposition. Lee and Kauffman (1974) reported that IMF deposition in growing pigs was not only due to increase in size of intramuscular adipocytes but also due to increase in its number. Ashihara et al. (2007) reported that energy and protein restriction during nursing periods resulted in higher number of intramuscular adipocytes when pigs reached the market weight as compared with control pigs. Therefore, higher IMF content of pigs fed a low lysine diet may be due to increase in the number of intramuscular adipocytes. Adipocyte numbers increase through the differentiation from preadipocytes. However, it is not known whether the feeding of low lysine diet directly alters adipocyte differentiation. Reduction in dietary lysine intake results in low plasma lysine concentrations (Katsumata et al. 2003) and low cytosolic lysine concentrations in skeletal muscles (M. Katsumata, unpubl. data, 2008). This indicates that supply of lysine to intramuscular preadipocytes is limited when pigs were fed with a low lysine diet. From these observations, we hypothesized that low plasma lysine concentration in pigs fed with a low lysine diet reduced supply of lysine from blood circulation to preadipocytes, and this limited supply of lysine might promote adipocyte differentiation in porcine muscle.
3T3-L1 preadipocyte is commonly used as an adipocyte differentiation model system for investigation of the molecular mechanisms regulating adipogenesis (Green & Meuyh 1974). Thus, it is one of the most appropriate models for studying adipogenesis. The process of 3T3-L1 preadipocyte differentiation is controlled by transcription factors such as CCAAT/enhancer-binding proteins (C/EBPβ, C/EBPδ and C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) (Gregoire et al. 1998; Farmer 2006; Rosen et al. 2007). The messenger RNA (mRNA) and protein levels of C/EBPβ and C/EBPδ were the highest 2 days after the induction (Yeh et al. 1995). C/EBPβ participates in the regulation of mitotic clonal expansion that is considered as a critical event for adipogenesis. Thereafter, C/EBPβ and C/EBPδ transcriptionally activates both PPARγ and C/EBPα expressions (Gregoire et al. 1998; Lane et al. 1999). PPARγ and C/EBPα coordinately activate the expression of adipocyte-specific genes (Gregoire et al. 1998).
In order to verify our hypothesis, we used 3T3-L1 preadipocytes to investigate the effects of lysine concentrations in culture media on adipocyte differentiation. For this purpose, we measured intracellular triglyceride content and mRNA expression of adipogenic transcription factors as markers of adipocyte differentiation.
MATERIALS AND METHODS
Cell culture
The 3T3-L1 preadipocytes were purchased from the American Type Culture Collection (Manassas, VA, USA). Experiments were performed using the five modified Dulbecco's modified Eagle's medium (DMEM) prepared in our laboratory. The modified DMEM containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA) were prepared by adding 1 volume of FBS to 9 volumes of each modified DMAM. The concentrations of lysine in each modified DMEM containing 10% FBS were as follows: 1 × (0.92 mmol/L); 1/2 × (0.46 mmol/L); 1/8 × (0.12 mmol/L); 1/16 × (0.06 mmol/L); and 1/40 × (0.02 mmol/L). The 1 × medium contained the same concentration of lysine with standard DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA). Thus, we called 1 × medium ‘control’, whereas the lowest lysine concentration medium, we called 1/40 × ‘low lysine’ in this study. Other amino acid concentrations were the same as the standard DMEM. The lysine concentration of 1/8 × (0.12 mmol/L) was similar to the plasma lysine concentrations in pigs fed a control diet, and the lysine concentration of 1/16 × (0.06 mmol/L) is similar to the plasma lysine concentrations in pigs fed a low lysine diet (Katsumata et al. 2003). 3T3-L1 cells were plated at a density of 0.5 × 104 cells/cm2 and cultured in standard DMEM containing 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomysin (Nacalai Tesque, Kyoto, Japan) for 5 days. At confluence, medium was replaced with modified DMEM containing 10% FBS, 0.5 mmol/L of 1-methyl-3-isobutylxanthine (IBMX) (Wako Pure Chemical Industries, Osaka, Japan), 0.25 µmol/L dexamethasone (Wako Pure Chemical Industries) (from day 0 to day 2). Forty-eight hours later, this medium was replaced every other day with modified DMEM containing 10% FBS, 5 µg/mL insulin (Sigma-Aldrich) for the remaining 6 days (from day 2 to day 8). At the time points indicated in figures, cells were either harvested for Oil Red O staining or triglyceride assay or real-time polymerase chain reaction (PCR) or Western blot analysis.
Oil Red O staining
Cells were washed twice with phosphate buffered-saline (PBS) on day 8 of the differentiation period, then fixed with 10% formalin for 10 min at room temperature (RT). After rinsing in 60% isopropanol for 1 min, cells were stained with Oil Red O solution (Wako Pure Chemical Industries) for 20 min at RT. Cells were counterstained with Mayer's hematoxylin solution (Wako Pure Chemical Industries) for 5 min at RT and then rinsed with PBS.
Triglyceride assay
Cultured cells on 12-well plates were washed twice with PBS, scraped off into 0.4 mL of 25 mmol/L Tris-HCl (pH 7.5) containing 1 mmol/L ethylenediaminetetraacetic acid (EDTA), and then homogenized (Polytron, PT-MR2100). Triglyceride (TG) in cell lysate was extracted with the same volume of chloroform-methanol (2:1, v/v) and quantified enzymatically using a Triglyceride G-test Kit (Wako Pure Chemical Industries).
RNA isolation and real-time PCR
Total RNA was extracted from 3T3-L1 cells on 6-well plates by the guanidine thiocyanate/phenol-chloroform extraction method using TRIzol Reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. RNA quality was assessed by agarose gel electophoresis. Complementary DNA (cDNA) was synthesized with random hexamer (TaKaRa, Osaka, Japan) and ReverTra Ace (TOYOBO CO., Osaka, Japan). Real-time PCR primers were purchased from Qiagen (Tokyo, Japan) (QuantiTect Primer Assays) for mouse PPARγ (QT00100296), mouse C/EBPα (QT00311731), mouse C/EBPβ (QT00320313), mouse C/EBPδ (QT00312809) and mouse 18SrRNA (QT01036875). Fatty acid synthase (FAS) forward- and reverse-primer sequences were 5′-AGATCCTGGAACGAGAACACGAT-3′, 5′-GAGACGTGTCACTCCTGGACTTG-3′, respectively (Schmid et al. 2005). Gene expression level was measured by real-time PCR using a LightCycler (Roche Diagnostics, Mannheim, Germany) instrument with the QuantiTect SYBR Green PCR system (Qiagen). We used 18SrRNA expression as an internal standard.
Western blot analysis
The 3T3-L1 cells on 10 cm diameter dishes were harvested and lysed in RIPA Lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA), according to the manufacturer's instructions. Protein concentrations of cell lysate were determined by BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Equal amounts of protein were boiled for 3 min and analyzed by electrophoresis in a 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel (ATTO Corporation, Tokyo, Japan), and transferred to a nitrocellulose membrane (Tokyo Roshi Kaisha, Tokyo, Japan). Subsequently, the membranes were treated with blocking buffer (5% nonfat dry milk in PBS) for 1 h. The blocked membranes were probed with primary antibodies overnight at 4°C and further incubated with a secondary antibody conjugated with horseradish peroxidase for 1 h at RT. Bound IgG was detected using an enhanced chemiluminescence system (GE Healthcare UK, Little Chalfont, UK). The primary antibodies used in this study were anti-C/EBPβ (rabbit polyclonal IgG, Cell Signaling Technology, Danvers, MA, USA, #3087) 1:1000; anti-C/EBPδ (rabbit polyclonal IgG, Cell Signaling Technology, #2318) 1 : 1000.
Statistical analysis
Statistical significance of effects of concentrations of lysine in the culture media on 3T3-L1 differentiation were assessed by one-way analysis of variance (ANOVA) procedure by the GLM procedure of Statistical Analysis Systems version 9.1.3 (SAS Institute Inc., Cary, NC, USA). Results are presented as least square means ± pooled standard errors. Differences with probabilities of < 0.05 were considered significant.
RESULTS
Intracellular TG content and lipid droplets
We investigated the effects of five levels of lysine concentrations on TG accumulation and lipid droplet formation of 3T3-L1 cells on day 8 after the induction of differentiation. 3T3-L1 preadipocytes were induced to adipocytes by media containing various concentrations of lysine, and TG content on day 8 is shown in Figure 1A. TG content in 3T3-L1 cells induced by the higher lysine concentrations reached as high as 3 µg/104 cells on day 8 whereas that of the cells cultured in the 1/40 × lysine (low lysine) medium was only 0.74 µg/104 cells (Fig. 1A). At 8 days, 3T3-L1 preadipocytes after induction in 1 × lysine (control) and 1/40 × lysine (low lysine) medium, were stained with Oil Red O (Fig. 1B). The number of lipid droplets in the control cells was larger than that of the cells cultured in the low lysine medium. The effect of low lysine medium on accumulation of TG throughout the differentiation period is shown in Figure 2. TG content of cells cultured in the low lysine medium on 6 and 8 days after the induction was lower than those of the control cells (P < 0.05).
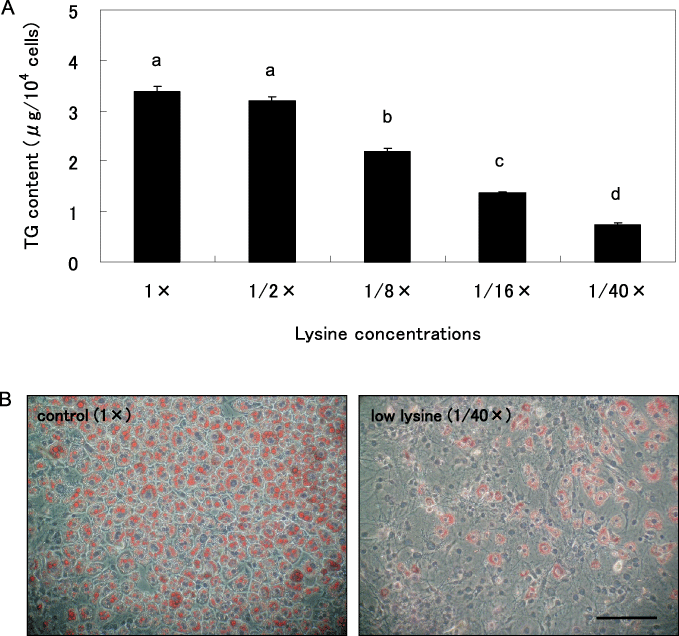
Effect of lysine concentrations in differentiating medium on 3T3-L1 preadipocyte differentiation. 3T3-L1 preadipocytes were differentiated in five levels of lysine concentrations for 8 days. Triglyceride (TG) µg per 104 cells were determined (A). Intracellar lipid was stained with Oil Red O (B). Values are mean ± SE n = 6. Means with different letters are significantly different (P < 0.05). Scale bar: 200 µm.
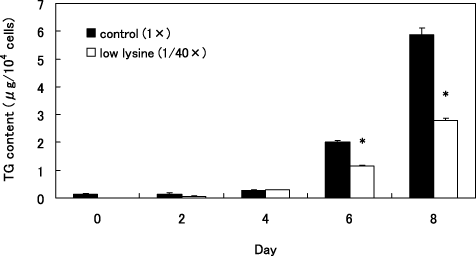
Effect of five levels of lysine concentrations on triglyceride (TG) accumulation of 3T3-L1. 3T3-L1 preadipocytes were differentiated in control (1×) medium and low lysine (1/40×) medium for 8 days (from day 0 to 8). Value are mean ± SE n = 6. *P < 0.05 versus control (1×).
FAS mRNA and enzyme activity
There were no differences in mRNA expressions of 18SrRNA that we used as an internal standard between the 1 × (control) cells and the 1/40 × lysine (low lysine) cells throughout the differentiation period. The mRNA expressions of FAS in the control cells on 4 and 6 days after the induction were twice as high as those of the pre-induction state (Fig. 3A). Further, the enzyme activities of FAS in the control cells on 4, 6 and 8 days after the induction were approximately 5–6 times higher than those of the pre-induction state (Fig. 3B). On the fourth day, both mRNA expression and enzyme activity in cells cultured in the low lysine medium were lower than those of the control cells (P < 0.05) (Fig. 3A,B). The effect of the low lysine treatment reducing the expression of mRNA and the activity of the enzyme was maintained until the eighth day.
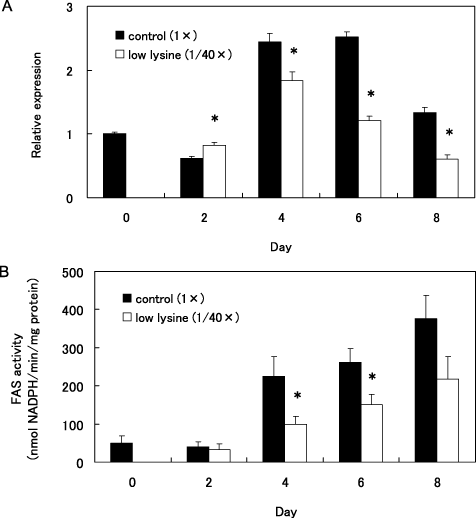
Effect of low lysine treatment on messenger RNA (mRNA) expression and enzymatic activity of fatty acid synthase (FAS). 3T3-L1 preadipocytes were differentiated in control (1×) medium and low lysine (1/40×) medium for 8 days (from day 0 to 8). The expression level of FAS mRNA was measured by real-time polymerase chain reaction and normalized with the 18SrRNA expression level (A). FAS activity per mg protein was determined (B). Values are mean ± SE n = 6. *P < 0.05 versus control (1×).
Expression of C/EBPs and PPARγ mRNA
Expression levels of C/EBPβ and C/EBPδ mRNA in the control cells on day 2 after the induction was approximately five times higher than those of the pre-induction state. Thereafter, expression level of these genes in the control cells gradually declined to the pre-induction level on day 8. The low lysine treatment did not suppress the expressions of mRNAs of these genes (Fig. 4A,B). Protein expression of C/EBPβ and C/EBPδ was not suppressed by the low lysine treatment (data not shown). mRNA expression level of PPARγ and C/EBPα gradually increased from day 2 after the induction of differentiation in the control cells. Expression level of both genes on day 8 in cells cultured in the low lysine medium was lower than that of the control cells (P < 0.05) (Fig. 4C,D).
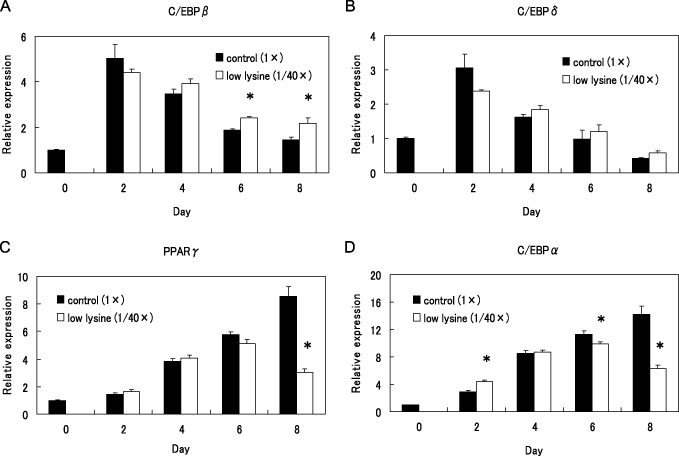
Effect of low lysine treatment on messenger RNA (mRNA) expression of CCAAT/enhancer binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARγ). 3T3-L1 preadipocytes were differentiated in control medium and low lysine medium for 8 days (from days 0 to 8). The expression levels of C/EBPβ (A), C/EBPδ (B), PPARγ (C) and C/EBPα (D) mRNA were measured by real-time polymerase chain reaction and normalized with the 18SrRNA expression level. Values are mean ± SE n = 6. *P < 0.05 versus control (1×).
DISCUSSION
Our hypothesis was that low plasma lysine concentration in pigs fed on low lysine diet reduced supply of lysine from blood circulation to preadipocytes, and this limited supply of lysine might promote adipocyte differentiation in porcine muscle. In order to verify this hypothesis, we investigated whether or not low concentration of lysine in culture medium would promote differentiation of 3T3-L1 preadipocytes. Adipocyte differentiation is characterized by accumulation of TG in cells. The accumulation level of TG in 3T3-L1 cells cultured in the low lysine media (1/8×, 1/16× and 1/40×) was lower than those of the control cells. Therefore, it seems that differentiation of 3T3-L1 preadipocytes was inhibited by the low concentrations of lysine in media. Furthermore, the levels of mRNA expression and the activity of FAS were also down-regulated by the low concentration of lysine (1/40×) in the media. These results suggest that the accumulation of TG in the cells was inhibited by the low concentrations of lysine in the medium via suppressing fatty acid synthesis both at the gene expression and the enzyme activity levels. The results are inconsistent with our hypothesis.
In recent years, mechanisms by which nutrients exert direct effects on cell growth and function have been well elucidated. The well-characterized signaling pathway regulated by amino acids is the pathway of mammalian target of rapamycin (mTOR) (Kimball & Jefferson 2006). mTOR is a nutrient-sensing kinase that integrates the inputs from amino acids, growth factors, and the energy status of the cell (Kimball & Jefferson 2004). Some studies showed that amino acid restriction suppressed the activity of mTOR in HEK293 cells and CHO-IR cells or human T-lymphoblastoid Jurkat cells (Hara et al. 1998; Iiboshi et al. 1999). Furthermore, it is suggested that mTOR plays an important role in adipocyte differentiation. Some studies demonstrated that inhibition of mTOR with rapamycin had negative effects on adipocyte differentiation in the 3T3-L1 preadipocyets (Cho et al. 2004; Kim & Chen 2004). In the present study, the low lysine concentration (1/40×) in the medium inhibited mRNA expression of PPARγ without inhibiting mRNA and protein expressions of C/EBPβ and C/EBPδ. These results are consistent with the data from Kim & Chen (2004), who reported that inhibition of mTOR with rapamycin suppressed mRNA and protein expressions of PPARγ without affecting those of C/EBPβ and C/EBPδ. Hence, we infer that the low lysine concentrations in the media might inhibit adipocyte differentiation through suppressing mTOR pathway.
The differentiation of adipocytes is induced by various serum adipogenic factors such as insulin, growth hormone and glucocorticoid hormones (Green & Kehinde 1975; Morikawa et al. 1982; Schiwek & Loffler 1987). In rats, serum corticosterone (one of the glicocorticoid hormones) concentration was elevated by feeding of lysine-deficient diets (Cree & Schalch 1985). Therefore, the elevated glucocorticod hormones by feeding of low lysine diets may promote adipocyte differentiation. However, there are no reports showing that low lysine diets elevate the concentrations of serum glucocorticoid hormones in pigs. Further study is necessary for clarifying the effect of feeding of low lysine diets on the concentration of serum adipogenic factors in pigs.
In this study, the low lysine concentrations in culture media did not promote 3T3-L1 adipocyte differentiation. This result was inconsistent with our hypothesis. Most in vitro studies on adipocyte differentiation and metabolism have been performed using the murine preadipocyte cell lines, especially 3T3-L1 preadipocyte. Thus, we used the 3T3-L1 preadipocyte in this study. However, some studies show that the responsiveness to inducers of adipocyte differentiation may often vary according to the origin of the cells, such as the species and age of the donor (Ailhaud et al. 1992; Boone et al. 1999; Nakajima et al. 2003). Furthermore, Gardan et al. (2006) reported that lipid metabolism and secretory function of intramuscular adipocytes differ from that of nonmuscular adipocytes. Therefore, the responsiveness of adipocyte differentiation to lysine or amino acid concentrations may vary among animal species or regional differences. Indeed, lysine concentrations in culture media affected differentiation of 3T3-L1 preadipocyte, a commonly used cell line as an adipocyte differentiation system, in the present study. However, it will be necessary to conduct further studies with porcine adipocytes, ideally with porcine intramuscular adipocytes, in order to elucidate the role of lysine in enhancing IMF accumulation in pig muscle.
In summary, our results presented here showed that low concentration of lysine in culture medium inhibited differentiation of 3T3-L1 preadipocytes and this inhibitory effect was mediated by the reduction of PPARγ and C/EBPα mRNA expression.




