Reactions of N-N and N-O compounds with horseradish peroxidase and peroxidases from Mycobacterium tuberculosis
Abstract
Several N-N-and N-O-containing compounds were analysed for their ability to act as substrates for horseradish peroxidase and peroxidases in Mycobacterium tuberculosis extracts. Aminoguanidine, diaminoguanidine, isoniazid, hydroxylamine and hydrazine were found to be weak substrates for horseradish peroxidase in reaction I and to inhibit the reaction of horseradish peroxidase with hydrogen peroxide. The same compounds inhibited the reaction of Mycobacterium tuberculosis peroxidase-catalase with hydrogen peroxide, and hydroxylamine was found to be a weak substrate for this enzyme. In growth inhibition experiments, diaminoguanidine inhibited the growth of M. tuberculosis H37Rv at 50 μg/mL, but not the growth of two isoniazid-resistant strains. Isonicotinic acid hydroxamate inhibited the reaction of the peroxidases with hydrogen peroxide, but was not itself a substrate and had no growth-inhibitory effects. On the basis of these results we suggest that the effect of isoniazid on growth of M. tuberculosis results from increased oxidative stress due to inhibition of catalase-peroxidase as well as from generation of toxic radicals with the structure 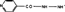 .
.




