CTLA4-Ig Restores Rejection of MHC Class-II Mismatched Allografts by Disabling IL-2-Expanded Regulatory T Cells
Authors' contributions: †L.M.C and B.V. are first co-authors; L.M.C. and B.V. designed and performed research, analyzed data, made the figures and wrote the paper; P.L. performed research; K.F. wrote the paper; O.L. supervised research and wrote the paper; A.L.M. designed and supervised research and wrote the paper.
Abstract
Allograft acceptance and tolerance can be achieved by different approaches including inhibition of effector T cell responses through CD28-dependent costimulatory blockade and induction of peripheral regulatory T cells (Tregs). The observation that Tregs rely upon CD28-dependent signals for development and peripheral expansion, raises the intriguing possibility of a counterproductive consequence of CTLA4-Ig administration on tolerance induction. We have investigated the possible negative effect of CTLA4-Ig on Treg-mediated tolerance induction using a mouse model of single MHC class II-mismatched skin grafts in which long-term acceptance was achieved by short-term administration of IL-2/anti-IL-2 complex. CTLA4-Ig treatment was found to abolish Treg-dependent acceptance in this model, restoring skin allograft rejection and Th1 alloreactivity. CTLA4-Ig inhibited IL-2-driven Treg expansion, and prevented in particular the occurrence of ICOS+ Tregs endowed with potent suppressive capacities. Restoring CD28 signaling was sufficient to counteract the deleterious effect of CTLA4-Ig on Treg expansion and functionality, in keeping with the hypothesis that costimulatory blockade inhibits Treg expansion and function by limiting the delivery of essential CD28-dependent signals. Inhibition of regulatory T cell function should therefore be taken into account when designing tolerance protocols based on costimulatory blockade.
Abbreviations:
-
- CFSE
-
- carboxyfluorescein diacetate succinimidyl ester
-
- Cplx
-
- complex
-
- CTLA4-Ig
-
- cytotoxic T lymphocyte antigen 4
-
- Ctrl
-
- control
-
- dLNs
-
- draining lymph nodes
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- Foxp3
-
- fork-head box P3
-
- GFP
-
- green fluorescent protein
-
- GILs
-
- graft infiltrating lymphocytes
-
- HCV
-
- hepatitis virus C
-
- ICOS
-
- inducible costimulatory molecule
-
- IFN-γ
-
- interferon gamma
-
- mAb
-
- monoclonal antibody
-
- MFI
-
- mean fluorescence intensity
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PBS
-
- phosphate-buffered saline
-
- SEM
-
- standard error of the mean
-
- Teff
-
- effector T cell
-
- Th
-
- T helper cell
-
- Treg(s)
-
- regulatory T cell(s)
Introduction
Treg expansion represents a promising immunotherapy to induce functional tolerance in clinical settings (1–5). Previous work has demonstrated that in vivo Treg expansion in mice can be achieved by treatment with interleukin (IL)-2 in complex with a particular anti-IL-2 monoclonal antibody (6). In line with this, two recent clinical trials reported the efficacy of low dose IL-2 treatment to expand Tregs in refractory graft versus host disease (7) and in HCV-induced vasculitis (8), paving the way for novel treatments of several human immunological disorders such as autoimmune diseases and acute allograft rejection. Immune interventions aiming at blocking effector T cell responses through costimulatory blockade also represent efficient strategies to inhibit unwanted immune responses in vivo (9–12). This was recently suggested by the capacity of CTLA4-Ig compared to cyclosporine in improving renal function in kidney-transplanted patients (13–15). Transplantation tolerance most probably relies on a delicate equilibrium between regulatory and effector mechanisms. This raises the possibility that the combination of treatments aiming at inhibiting effector function while expanding Tregs numbers or function may represent valuable strategies to achieve immune tolerance. To test this hypothesis, we studied the effect of a therapeutic combined strategy using CTLA4-Ig and an IL-2-induced Treg expansion on allograft survival. In this setting, we demonstrate that CTLA4-Ig prevents graft acceptance induced by exogenous IL-2 therapy through inhibition of Treg homeostasis and suppressive capacities.
Materials and Methods
Mice
C57Bl/6.C-H-2bm12 (bm12) and CD90.1 C57Bl/6 (B6) mice were obtained from The Jackson Laboratory. Wild type B6 mice were purchased from Harlan, Netherlands. B6 Foxp3gfp knockin mice that expressed Foxp3 as a Foxp3-GFP fusion protein were obtained from Pr Marisa Alegre, University of Chicago, Illinois (16). Animals were bred in our specific pathogen-free animal facility and used at eight to twelve weeks of age. All animals received human care in compliance with the Principles of Laboratory Animal Care formulated by the National Institute of Health (NIH publication No. 86–23, revised 1985) and protocols were approved by the local committee for animal welfare.
Skin grafting
Mice were anesthetized with a mixture of 5% xylazine and 10% ketamine in phosphate-buffered saline (PBS) according to body weight. Skin grafting was performed according to an adaptation of the method of Billingham and Medawar (17). Grafts were monitored daily after the removal of the bandage on day 8 and considered rejected when more than 75% of epithelial breakdown had occurred.
Treatment with IL-2/anti-IL-2 complex, CTLA4-Ig and antibodies
IL-2/anti-IL-2 complex was formed by incubating 1 μg recombinant mouse IL-2 and 9 μg of Functional Grade Purified anti-mouse IL-2 (Clone: JES6–1A12, eBioscience) for 30 min at 37°C. The complex was administered at days 0, 1 and 2 posttransplantation of each experiment. Concomitant treatment with CTLA4-Ig consists of an intraperitoneal injection of 1 mg abatacept (Orencia®, Bristol-Myers Squibb Eeig, braine l'alleud, Belgium) on days 0, 1 and 2, except for the experiment shown in Figures 4(A) and (B) where CTLA4-Ig was administered on days 6, 7 and 8. For control antibody, CTLA4-Ig was compared to purified human total IgG (>96% purity, Sandoglobuline®). Anti-CD28 mAb (Clone: 37.51, bioXcell [West Lebanon, NH, USA]; 500 μg) was administrated intraperitoneally on days 0, 1 and 2. Anti-ICOS mAb (Clone: 7E.17G9, bioXcell; 250 μg) was administrated intraperitoneally on days –1 and 1. Purified Gamma globulin from Syrian hamster (Jackson Immunoresearch Laboratories) and purified rat IgG mAb (LODNP, LO-IMEX, Université Catholique de Louvain, Brussels, Belgium) were used as control antibody for anti-CD28 and anti-ICOS mAbs, respectively.

Long-lasting Treg-mediated suppression of allograft rejection requires persistent B7 costimulation. (A, B) Two groups of B6 recipients were grafted with bm12 skins and treated with the IL-2/anti-IL-2 complex at days 0, 1 and 2. Mice were then treated or not with 1 mg of CTLA4-Ig at days 6, 7 and 8 (n = 5/group). (A) Graft survival was compared. (B) At day 40, all recipients described in (A) were euthanized to determine the amounts of CD4+ Foxp3+ Tregs in the spleen and dLNs. (C, D) Two groups of B6 recipients were grafted with F1(bm12 × B6) skins and treated (n = 6) or not (n = 9) with 1 mg of CTLA4-Ig at days 0, 1 and 2. (C) Graft survival was compared. (D) At day 28, recipients were euthanized to determine the amounts of CD4+ Foxp3+ Tregs in the spleen and dLNs. Results are expressed as mean ± SEM in all panels (*p < 0.05). In all panels, data are representative of two independent experiments.
Histologic examination
Skin graft histology was performed at day 15 posttransplantation on paraffin embedded tissue sections (5 μm) stained with hematoxylin/eosin or toluidine blue. A single section of skin graft was considered for each experimental animal. Mast cells stained in purple with the toluidine blue were counted blindly by two independent operators. Three nonoverlapping fields were evaluated for each skin graft.
RNA extraction and real-time (RT)-PCR
Total RNA was extracted from skin grafts using the MagnaPure LC RNA Isolation Kit III for tissue (Roche Diagnostics). Reverse transcription and RT PCR were performed using LightCycler-RNA Master Hybridization Probes (one-step procedure) on a Lightcycler apparatus (Roche Diagnostics). The number of mRNA copies was evaluated by using standard curve for each gene of interest and was normalized to β-actin as a housekeeping gene. Primer and probe sequences were as follows: for β-actin: forward-CCGAAGCGGACTACTATGCTA, reverse-TTTCTCATAGATGGCGTTGTTG, probe-ATCGGTGGCTCCATCCTGGC; for IFN-γ: forward-GGATGCATTCATGAGTATTGC, reverse-GCTTCCTGAGGCTGGATTC, probe-TTTGAGGTCAACA-ACCCACAGGTCCA; for CXCL9: forward-GAACCCTAGTGATAAGGAATGCA, reverse-CTGTTTGAGGTCTTTGAGGGATT, probe-ATCAGCACCAGCCGAG-GCACG; for CXCL11: forward-GATGAACAGGAAGGTCACAGC, reverse-GCTTTCTCGATCTCTGCCATT, probe-CCGATGCAAAGACAGCGCCC.
Flow cytometry
Antibodies against mouse CD4, CD25, ICOS, CD90.1, CD90.2, CTLA4, Ki67, CD16/CD32 (BD Biosciences), Foxp3, CD39 (eBioscience), LAP (membrane bound TGF-β) (Biolegend) and Bim (Cell Signaling Technology) were used. Flow cytometry analyses were performed on a CyAn-LX cytometer using Summit 4.1 software. Cell suspensions were incubated for 10 min with Fc block then stained for surface markers for 20 min. Foxp3, Bim and Ki67 staining was performed by using eBioscience Fixation/Permeabilization according to the manufacturer's instructions. Graft infiltrating lymphocytes were isolated as previously described (17). BD FACS Lysing Solution was used for lysing red blood cells for peripheral blood mononuclear cells (PBMCs) samples.
Mixed lymphocyte culture, in vitro suppression assay and cytokine detection
For mixed lymphocyte cultures, responder cells (2.5 × 106 cells/mL) isolated from draining lymph nodes (inguinal and axillary) were stimulated with irradiated splenocytes (2000 Rad; 2.5 × 106 cells/mL) from either B6 (syngeneic), bm12 (allogeneic) mice in 48-well, flat-bottom plates. Cultures were incubated in 5% fetal calf serum-supplemented complete RPMI 1640 medium. For in vitro suppression assays, CD90.1 CD4+ T cells were isolated using a CD4 negative isolation kit (Miltenyi), then labeled with 1 μM CFSE for 30 min at 37°C and used as responder cells. CD90.2 Foxp3-GFP+ CD4+ cells were isolated with a MOFLO cell sorter and used as suppressor cells. Responders were used at a fixed concentration of 105 cells per well and stimulated with 2 μg/mL of soluble anti-CD3 antibody in the presence of 4 × 105 RAG−/− spleen cells used as feeder cells. Cells were stimulated in 96-well, flat-bottom plates in triplicate. IFN-γ in culture supernatants was detected by ELISA at 48 h for mixed lymphocyte reaction (Figure 1D) and 72 h for the suppression assays, according to the manufacturer's instructions (R&D, DUOSET IFN-γ detection kit). The detection threshold was <30 pg/mL.

IL-2/anti-IL-2-induced acceptance of an MHC class II mismatched skin graft is prevented by CTLA4-Ig. (A) bm12 skins were transplanted on B6 recipients that were divided in 3 groups: IL-2/anti-IL-2 complex-treated recipients (Cplx) (n = 14), recipients coinjected with IL-2/anti-IL-2 complex+CTLA4-Ig (Cplx+CTLA4-Ig) (n = 12), and untreated recipients (Ctrl) (n = 6). Graft survivals were compared. Cumulative data of three independent experiments are shown. (B) Graft infiltrating lymphocytes (GILs) and draining lymph nodes (dLNs) cells were isolated on day 12 posttransplantation from mice treated as described in (A). Foxp3 expression by CD4+ T cells is shown. Representative histograms of GILs are shown and a pool of individual mice is represented (n = 3–5/group). (C) Intragraft mRNA expression of Th1 related gene was measured at day 15 posttransplantation in each group and compared to basal mRNA expression in native skins (n = 5–9/group). (D) At day 20 posttransplantation, dLNs cells (2 × 106/mL) from recipients in each group, or ungrafted mice (Naïve), were stimulated with both B6 (Syngeneic) and bm12 (Allogeneic) irradiated spleen cells (2.5 × 106/mL). IFN-γ production was quantified (n = 4–9/group). (E) Pictures 1, 2, 3: Hematoxylin/eosin staining of grafts harvested from recipients described in (A). Pictures 4, 5, 6: Graft infiltrating mast cells (black arrows) were observed after toluidine blue staining. A representative picture of each group is shown (original magnification, ×200). Pictures 7, 8, 9: macroscopic aspect of grafts in each group at the indicated time posttransplantation. (F) Graft infiltrating mast cells were counted in each group. Each point represents the average of three fields per graft for an individual mouse. In all panels, the mean is represented and error bars are SEM (*p < 0.05, ***p < 0.001) and data are representative of two independent experiments unless otherwise specified.
Statistical analyses
Statistical analyses of differences between groups were performed using the two-tailed Mann–Whitney test. Graft survival curves were compared by the log-rank test. A p < 0.05 was considered statistically significant.
Results
IL-2/anti-IL-2-induced long-term acceptance of an MHC class II mismatched skin graft is prevented by CTLA4-Ig
To examine the possible synergistic effect of costimulatory blockade and Treg expansion on transplantation tolerance, CTLA4-Ig treatment was administered concomitantly with IL-2 coupled to a specific anti-IL-2 monoclonal antibody (IL-2/anti-IL-2 complex) in a model of MHC class II mismatched allograft rejection. Skin from bm12 donors were grafted on C57Bl/6 (B6) recipients injected, or not, with IL-2/anti-IL-2 complex (6,18) at days 0, 1 and 2 posttransplantation. Approximately 75% of IL-2/anti-IL-2 complex-treated mice retained their graft in excess of 50 days, whereas all control mice rejected their graft within 2 weeks (Figure 1A). This maintenance of long-term graft survival was dependent on Tregs because a subsequent Treg depletion with an anti-CD25 antibody restored 100% of rejection in IL-2-treated recipients (Figure S1A). Much to our surprise, coadministration of CTLA4-Ig antagonized the tolerogenic effect of IL-2/anti-IL-2 complex, as shown by the complete rejection of class II mismatched skins from mice undergoing combined immunotherapy (Figure 1A). Of note, CTLA4-Ig alone did not delay the spontaneous rejection of bm12 skin grafts in B6 recipients. In this setting, lower amounts of splenic Tregs were detected after CTLA4-Ig administration (Figures S1B and S1C). Because IL-2/anti-IL-2 complex is known to promote graft acceptance in a Treg-dependent fashion, we evaluated the effect of CTLA4-Ig on both effector and regulatory T cell functions and numbers. CTLA4-Ig coinjection abolished the IL-2-driven accumulation of Tregs both within the grafted skin and the corresponding draining lymph nodes (dLNs; Figure 1B). Costimulatory blockade restored the typical Th1-like response seen in untreated mice, as witnessed by the increased level of intragraft mRNA coding for classical Th1-related cytokine (IFN-γ) and chemokines (CXCL9, CXCL11; Figure 1C). In line with this, the antidonor immune response was exacerbated in mice undergoing combined IL-2 / CTLA4-Ig therapy when compared to IL-2-only treated recipients. This was attested by the increase of IFN-γ production by draining lymph node cells upon allogeneic stimulation in case of IL-2/CTLA4-Ig cotreatment (Figure 1D). Histological analyses confirmed skin graft destruction in both untreated and IL-2/CTLA4-Ig treated mice, while healthy skin grafts were observed in IL-2-only treated recipients (Figure 1E). Notably, CTLA4-Ig treatment affected the recruitment of mast cells (Figures 1E and F), known to be involved in Treg-mediated allograft tolerance (19). Taken together, these observations indicate that expansion of Tregs by exogenous IL-2 therapy is sufficient to prevent a CD4-mediated allograft rejection. However, CTLA4-Ig restores alloreactivity suggesting that CD28 signaling is required for IL-2-expanded Treg capacities.
CD28 signaling on Treg is critical for their IL-2-induced in vivo expansion and immunosuppressive capacity
We next determined whether CTLA4-Ig could inhibit exogenous IL-2-induced Treg expansion and/or suppressive capacities. Treg expansion was evaluated using Foxp3-GFP reporter ungrafted mice. Coadministration of CTLA4-Ig led to a significant reduction of peripheral Tregs in both peripheral blood (in particular at days 6 and 9 postIL-2/anti-IL-2 administration), (Figure 2A) and spleen at day 5 (Figure 2B). Of note, this negative effect on Tregs was not observed when purified human IgG, used as control antibody, were coinjected with the IL-2 complexes. However, we cannot exclude that both proliferation and survival of Tregs might be altered under costimulatory blockade. As shown in Figure (S2), an enhancement of Treg-intranuclear Ki-67 expression reflecting a strong mitotic activity was observed in complex-treated mice in comparison to untreated mice. In contrast, Ki-67 expression on Tregs was severely decreased upon CTLA4-Ig and IL-2/anti-IL-2 coinjection. In parallels, the study of Bim expression among expanded-Tregs was performed with regard to the effect of CTLA4-Ig on their survival. Indeed, Bim has been described as a typical Treg-associated proapoptotic molecule (20). We detected a smaller percentage of Bim+ Tregs in the IL-2/anti-IL-2-treated animals while the CTLA4-Ig cotreatment prevented this downregulation (Figure S2). The previous observations are compatible with published reports highlighting the requirement of CD28 signaling for Treg homeostasis (21–23). Accordingly, in vivo treatment with an agonist anti-CD28 mAb counteracted the inhibitory effect of CTLA4-Ig on IL-2-driven Treg expansion (Figures 2A and B). Of note, the injection of this anti-CD28 mAb alone (without exogenous IL-2) was not sufficient to expand Tregs (data not shown). A phenotypical characterization of Tregs was performed in IL-2/anti-IL-2-treated mice coinjected or not with CTLA4-Ig and anti-CD28 mAb, using Tregs from untreated mice as a control. IL-2/anti-IL-2-expanded Tregs showed an increased expression of Foxp3, inducible costimulator molecule (ICOS), membrane bound TGF-β (mbTGF-β), CD25, CTLA4 and CD39 in comparison to control Tregs (Figures 2C and S3A). The upregulation of these markers was significantly reduced by the CTLA4-Ig administration, and restored by anti-CD28 coinjection. Similarly, ex vivo suppression assays revealed the increased regulatory function of IL-2/anti-IL-2-expanded Tregs that was inhibited back to control levels by CTLA4-Ig cotreatment. Again, coadministration of agonist anti-CD28 mAbs restored the high suppressive capacity of IL-2-treated Tregs in this setting (Figure 2D). Because ungrafted animals may not be representative of recipients under conditions of ongoing alloresponses, all of these studies over Treg expansion, phenotype and functions were repeated in bm12-grafted recipients and showed similar results (data not shown).
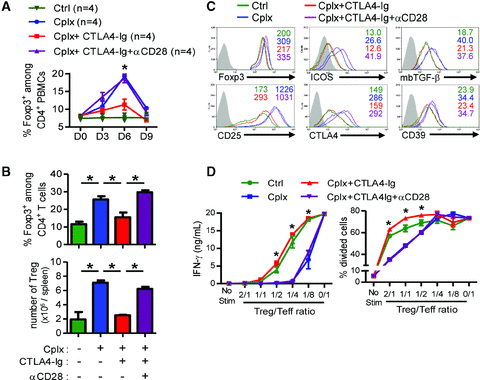
CTLA4-Ig inhibits IL-2/anti-IL-2-induced Treg expansion and immunosuppressive capacity by blocking CD28 signaling. Four groups of B6 Foxp3-GFP ungrafted mice were constituted: no treatment (Ctrl), IL-2/anti-IL-2 complex (Cplx), complex with CTLA4-Ig cotreatment (Cplx+CTLA4-Ig), and complex with CTLA4-Ig and anti-CD28 agonist mAb cotreatment (Cplx+CTLA4-Ig+aCD28). (A) Expression of Foxp3 among CD4+ PBMCs is shown at days 0, 3, 6 and 9 (n = 4/group). (B) On day 5 after injection, spleen cells were isolated and Foxp3-GFP expression on CD4+ T cells was measured (n = 3/group). Percentages and absolute counts of Tregs are represented. (C) The phenotype of splenic CD4+ Foxp3+ Tregs was analyzed at day 5 with respect to the expression of Foxp3, ICOS, membrane-bound TGF-β, CD25, CTLA4 and CD39. Gray represents isotype control or CD4+ effector cells for Foxp3-GFP. Values represent the mean flurorescence intensity (D) In vitro suppression of CFSE labeled CD4+CD90.1+ T cell proliferation (Teff) by various number of CD4+ Foxp3-GFP+-sorted cells (Treg) isolated from mice in each group. Proliferation of effector cells (Teff) was assessed by CFSE dilution and IFN-γ was quantified in culture supernatants. Control antibody from Syrian hamster and purified human total IgG were used in control experiments for anti-CD28 mAb and CTLA4-Ig, respectively. No observable effect of these control antibodies was noticed (data not shown). In all panels, one representative experiment of three is shown and the results are expressed as mean ± SEM (*p < 0.05).
Potent ICOS+ Treg induction by IL-2 is abolished by CTLA4-Ig coadministration
The notable upregulation of ICOS, a molecule whose Treg-associated expression is correlated with higher suppressor activity (24–27), led us to examine in more details the effect of CTLA4-Ig treatment on the subset of ICOS-expressing Tregs expanded by exogenous IL-2. Administration of IL-2/anti-IL-2 complex led to a significant increase in ICOS+ Tregs, from less than 10% in untreated control mice, up to 40% in IL-2/anti-IL-2-treated animals, a response that was almost completely antagonized by coadministration of CTLA4-Ig (Figure 3A). Notably, while IL-2/anti-IL-2 promoted both ICOS+ and ICOS− Treg expansion, CTLA4-Ig coadministration only abrogated the ICOS-expressing subset of Foxp3 positive cells both in spleen (Figure 3B) and in lymph nodes (data not shown). ICOS+ expanded Tregs expressed higher levels of mbTGF-β, CTLA4 and lower levels of Bim when compared to their ICOS− counterpart expanded Tregs (Figure 3C and S3B), and displayed enhanced suppressive capacities in vitro (Figure 3D). To directly assess the putative role of ICOS on Treg-mediated suppression, bm12 skins were grafted on IL-2/anti-IL-2-treated B6 recipients coinjected, or not, with anti-ICOS antibody on days –1 and 1. At day 25 posttransplantation, 80% of recipients cotreated with anti-ICOS antibody rejected their graft, whereas no rejection was observed in the IL-2/anti-IL-2-treated group (Figure 3E), a biological response that correlated with the reduced expansion of Tregs in anti-ICOS treated mice (Figure 3F).
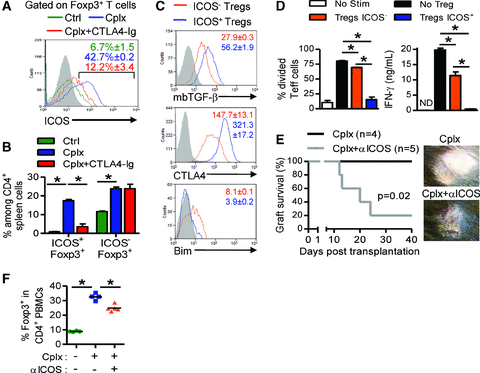
Potent ICOS+ Treg induction by IL-2/anti-IL-2 is abolished by CTLA4-Ig co-administration. (A–C) B6 Foxp3-GFP mice were treated with the IL-2/anti-IL-2 complex alone (Cplx) (n = 3) or in combination with CTLA4-Ig (Cplx+CTLA4-Ig) (n = 3). Untreated mice served as control (Ctrl) (n = 4). CD4+Foxp3+ Tregs from each group were studied by flow cytometry at day 5 posttreatment with respect to their ICOS expression. (A) ICOS expression on spleen Tregs is shown. Gray represents isotype control staining. (B) Percentages of ICOS− and ICOS+ Tregs among total CD4+ splenocytes in each group. (C) Differential expression of mbTGF-β, CTLA4 and Bim in both ICOS− Tregs and ICOS+ Tregs in IL-2/anti-IL-2 complex treated mice. Gray represents isotype control staining. Values represent mean fluorescence intensity and are expressed as mean ± SEM. (D) IL-2/anti-IL-2-expanded Tregs from Foxp3-GFP mice were isolated and sorted on the basis of their ICOS expression. Then, suppressive capacities of ICOS− and ICOS+-expanded Tregs were compared in vitro. CFSE-labelled CD90.1 CD4+ cells were cultured with ICOS− or ICOS+ Tregs in a 1/2 Treg/Teff ratio for 72 h. Proliferation of CD90.1 cells (Teff) was assessed by CFSE dilution in divided cells (left) and IFN-γ production was quantified in culture supernatants (right). Results are expressed as mean ± SEM (*p < 0.05). (E) bm12 skin was transplanted on IL-2/anti-IL-2 treated B6 recipients injected with anti-ICOS mAbs (Cplx+αICOS) (n = 5) or with purified rat IgG control antibody (Cplx) (n = 4). Grafts survival was compared. (F) Percentages of Tregs among CD4+ PBMCs at day 5 posttransplantation from recipients described in (e). Untouched mice served as control (Ctrl) (n = 4). The mean is represented (*p < 0.05). In all panels, data are representative of two to three independent experiments.
Long-lasting Treg-mediated suppression of allograft rejection requires persistent B7 costimulation
To evaluate whether CD28-mediated signaling was only required during the early expansion phase in response to exogenous IL-2, CTLA4-Ig administration was delayed until day 6 postgraft, after the peak of Treg expansion. Despite a delayed blockade protocol, mice injected with CTLA4-Ig rejected MHC class II mismatched skin grafts (Figure 4A) and displayed reduced numbers of peripheral Tregs (Figure 4B), indicating that these cells required continuous CD28 engagement for adequate regulatory functions. This conclusion was further strengthened using a distinct animal model of regulatory T cell-dependent, spontaneous acceptance of semiallogeneic skin grafts (28). B6 mice were grafted with semiallogeneic (bm12 × B6) skins and treated with CTLA4-Ig as previously described. Costimulatory blockade led to a significant increase in rejection episodes (Figure 4C), concomitant with reduced numbers of circulating Tregs (Figure 4D). These observations confirm the capacity of CTLA4-Ig to antagonize CD28-mediated signaling important for optimal peripheral Treg expansion and function.
Discussion
Our results unravel detrimental effects of CTLA4-Ig on a Treg-based protocol for suppression of allograft rejection. Although CTLA4-Ig fusion protein has been mainly described as an efficient antirejection therapy by blocking the CD28 costimulation on naïve T cells (14,29), our features clearly raise concerns about its use in tolerance induction protocols through its impact on Treg homeostasis and suppressive functions. The importance of these CD28/B7 interactions in regulatory immune function has been previously documented by a worsening of autoimmune diabetes in CD28−/− or B7.1/B7.2−/– NOD mice compared with wild type NOD mice (21–23). Similarly, the administration of anti-B7.1 and anti-B7.2 blocking antibodies induced rejection of spontaneously accepted MHC class II disparate heart allografts (30). Very recently, a study investigating the impact of B7/CD28 blockade by hCTLA4Ig in various allogeneic combinations of heart allografts reported an accelerated rejection of bm12 cardiac allografts by B6 recipients (31). In this combination like in the present F1 (bm12 × B6) skin graft model, spontaneous long-term allograft survival depends on Tregs and the deleterious effect of hCTLA4-Ig was accompanied with an unfavorable effector/regulatory T cell ratio (31). This is concordant with our own data showing that IL-2-mediated Treg expansion is prevented by CTLA4-Ig because of the starvation of available B7 molecules. Although a specific deficiency of CTLA4 in Foxp3+ CD4+ T cells dramatically enhances the number of peripheral Tregs (32), CTLA4-Ig administration (by blocking B7 interactions with both CTLA4 and CD28) had the opposite effect in our experiments. This remains in agreement with the established requirement of CD28 signaling for Treg maintenance (23). In this sense, we demonstrated that restoring a specific CD28 signaling was sufficient to counteract the deleterious effect of CTLA4-Ig on expanded Tregs. Noteworthy, CTLA4 signaling has been reported to be necessary for tolerance induction of full mismatched islet and skin allografts after donor specific transfusion and anti-CD40L blockade (33,34). The blockade of the B7/CTLA4 negative costimulatory signal favors potent activation of T effector cells (Teff) resulting in allograft rejection. It is not excluded that a same phenomenon could partially explain the rejection observed in the IL-2 and CTLA4-Ig cotreated group through a B7 starvation capped by CTLA4-Ig. Therefore, a specific CD28 blockade without affecting CTLA4 signaling on T cell surfaces seems to be an effective strategy for dampening immune response without affecting Tregs as demonstrated by Poirier et al. in nonhuman primate models of heart and kidney transplantation (35).
In our experiments like in others (30), allograft acceptance results from a subtle balance between Tregs and alloreactive CD4+ Teff. In consequence, we conclude that CTLA4-Ig-mediated costimulatory blockade has a predominant effect on Tregs counterbalancing the Teff/Treg ratio leading to allograft rejection. This is the case after IL-2/anti-IL-2 treatment as well as in the models of spontaneous acceptation of F1 (bm12 × B6) skin graft or cardiac allografts (31). Furthermore, we observed that CTLA4-Ig alone did not delay bm12 allograft rejection by B6 recipients demonstrating that B7/CD28 is not required for rejection. Besides, by using bm12-grafted B6 foxp3-GFP recipients treated or not with CTLA4-Ig, the stimulation of CD4posGFPneg Teff cells isolated from draining lymph nodes with donor-typed stimulators did not show any decrease in IFN-γ production (data not shown). Overall, these results indicate that CTLA4-Ig alone does not adequately control CD4+ mediated anti-bm12 alloreactivity.
Our results seem to be in contradiction with previous reports demonstrating a beneficial effect of CTLA4-Ig in experimental and clinical studies (9,14,15,36). First, the absence of CD8 alloreactivity clearly weakens CTLA4-Ig beneficial effects. Indeed, a previous study reported prolonged cardiac allograft survival by CTLA4-Ig in a full MHC class I plus II BALB/c to B6 combination involving CD8 T cell alloreactivity (31). In our experiments, the single MHC class II disparity rules out CD8+ T cell alloresponses. Moreover, the restricted disparity to three amino acids in one of the I-Ab chains is likely to render this model prone to regulation and more sensitive to a deleterious effect of CTLA4-Ig on Treg-mediated suppression of allograft rejection. Second, a major difference regarding clinical reports relies on the fact that patients always receive a combination of immunosuppressive drugs, most probably minimizing the effect of CTLA4-Ig on Tregs in terms of allograft outcome. Nevertheless, if tolerance induction protocols are considered, this aspect becomes critical because patients are weaned off immunosuppression. It is not excluded that the higher frequency of acute rejection episodes observed in the CTLA4-Ig arm of the Benefit study (14) somehow reflects Treg dysfunction. This hypothesis is supported by the reduced levels of Foxp3 mRNA detected in renal biopsies of these patients 12 months after kidney transplantation (13). A potential limitation to translate our findings in clinical transplantation lies in the formulation of the CTLA4-Ig. Indeed, we used the Abatacept form of CTLA4-Ig whereas studies in kidney-transplanted patients used Belatacept, a CTLA4-Ig with higher affinity for B7 molecules.
In the absence of CTLA4-Ig, IL-2/anti-IL-2 treatment enhanced the expression of ICOS on Tregs allowing us to distinguish two subsets of expanded Tregs: ICOS− and ICOS+ cells. In our hands like in other studies, ICOS+ Tregs exhibited higher suppressive capacities than ICOS− Tregs (24–26,37). Whether IL-2/anti-IL-2 complex induces ICOS expression on preexistent Tregs or expand preferentially preexistent ICOS+ Tregs remains to be determined. Importantly, the percentage of ICOS+ Tregs (not ICOS−) dropped after CTLA4-Ig perhaps suggesting that CTLA4-Ig prevents allograft acceptance by inhibiting only the induction of potent ICOS+ Tregs. In our model of Treg-mediated allograft acceptance, ICOS blockade restored rejection. This suggests that ICOS signaling plays an important role in Treg suppressive function during alloreactivity.
We conclude that IL-2 and CD28 synergize for Treg expansion, ICOS expression and the strengthening of suppressive capacities. Alloreactivity and skin graft rejection reappeared by starving Tregs of B7-CD28 interactions through the CTLA4-Ig administration. Importantly, the effect of CTLA4-Ig on both Treg expansion and suppressive function was restored by CD28 costimulation. This also raises potential issues about molecules that specifically target CD28 even if they leave CTLA4 intact (33,35). Not only did we find that CTLA4-Ig counteracted Treg expansion, it did not control CD4+ effector T cells in this system. Although CTLA4-Ig is unequivocally considered an efficient and nontoxic immunosuppressive agent for naive T cells, our results raise concerns about its use in protocols of tolerance induction based on Treg immunotherapy.
Acknowledgments
We thank Dr. Philippe Horlait, Laurent Depret, Gregory Waterlot, Christophe Notte and Samuel Vander Bist for the animal care; Dr. Fréderic Lhommé, Frédéric Paulart and Nicolas Passon for technical assistance; Pr. Gilles Blancho for providing us CTLA4-Ig; Pr. Michel Goldman, Pr. Maria Luisa Alegre and Dr. Gabriel Courties for review of the manuscript.
Funding Source : The Institute for Medical Immunology is funded by research grants of the Walloon Region, the FNRS-Belgium and GlaxoSmithKline Biologicals.
Disclosure
B.V. and L.M.C. are Research Fellows of the Société Francophone de Transplantation and the Fonds National de la Recherche Scientifique (FNRS-Belgium), P.L. is a Research Fellow of the Fonds Erasme (Erasme Hospital, Belgium). K.F. is an Associate Professor, Bucknell University. O.L. and A.L.M. are Research Associate of the FNRS-Belgium.
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




