Enhanced De Novo Alloantibody and Antibody-Mediated Injury in Rhesus Macaques
Abstract
Chronic allograft rejection is a major impediment to long-term transplant success. Humoral immune responses to alloantigens are a growing clinical problem in transplantation, with mounting evidence associating alloantibodies with the development of chronic rejection. Nearly a third of transplant recipients develop de novo antibodies, for which no established therapies are effective at preventing or eliminating, highlighting the need for a nonhuman primate model of antibody-mediated rejection. In this report, we demonstrate that depletion using anti-CD3 immunotoxin (IT) combined with maintenance immunosuppression that included tacrolimus with or without alefacept reliably prolonged renal allograft survival in rhesus monkeys. In these animals, a preferential skewing toward CD4 repopulation and proliferation was observed, particularly with the addition of alefacept. Furthermore, alefacept-treated animals demonstrated increased alloantibody production (100%) and morphologic features of antibody-mediated injury. In vitro, alefacept was found to enhance CD4 effector memory T cell proliferation. In conclusion, alefacept administration after depletion and with tacrolimus promotes a CD4+memory T cell and alloantibody response, with morphologic changes reflecting antibody-mediated allograft injury. Early and consistent de novo alloantibody production with associated histological changes makes this nonhuman primate model an attractive candidate for evaluating targeted therapeutics.
Abbreviations:
-
- AMR
-
- antibody-mediated rejection
-
- APC
-
- allophycocyanin
-
- CFSE
-
- carboxyfluorescein succinimidyl ester
-
- CNI
-
- calcineurin inhibitor
-
- DSA
-
- donor-specific antibody
-
- FITC
-
- fluorescein isothiocyanate
-
- IT
-
- immunotoxin
-
- LFA3
-
- lymphocyte function-associated antigen 3
-
- MLR
-
- mixed lymphocyte reaction
-
- MP
-
- methylprednisolone
-
- MST
-
- mean survival time
-
- mTOR
-
- mammalian target of rapamycin
-
- NHP
-
- nonhuman primate
-
- PBMC
-
- peripheral blood mononuclear cell
-
- PE
-
- phycoerythrin
-
- PerCP
-
- peridinin chlorophyll protein
-
- PTC
-
- peritubular capillaritis
-
- rATG
-
- rabbit antithymocyte globulin
-
- Tac
-
- tacrolimus
Background
Despite advancements in immunosuppressive agents and our understanding of rejection, long-term renal allograft survival remains as low as 50% (1–3). Several factors contribute to chronic allograft injury, including nonimmunologic causes such as chronic hypertension, calcineurin inhibitor use, infection and obstruction (1,3,4). Alloimmune causes that lead to chronic rejection are complex and multifactorial, with a significant portion being associated with antidonor HLA antibodies (5–10). The presence of circulating donor-specific antibodies over time causes glomerular injury, interstitial sclerosis and tubular atrophy, leading to chronic and progressive allograft dysfunction (11). The association of alloantibodies with poor outcome has been well described (12–19), and as many as one-third of renal transplant recipients demonstrate chronic antibody-mediated rejection on >12-month biopsy (10,20,21). Follow-up of 4144 patients showed a 200% increased rate of graft loss at 3 years in alloantibody positive patients (22). Still, a definitive therapy for preventing alloantibody production and its ensuing sequelae remains elusive.
Chronic and antibody-mediated rejection (AMR) has been increasingly studied in nonhuman primate (NHP) transplant models. Smith et al. reviewed their NHP experience in renal transplantation with mixed chimerism induction, and elegantly described morphologic changes associated with chronic antibody-mediated rejection. They noted a temporal progression of injury, beginning with donor-specific antibody (DSA) production (48%), C4d complement deposition (29%) and transplant glomerulopathy (22%) followed by graft failure (23,24). Several others have also reported detection of alloantibodies, often induced in the setting of suboptimal therapy in control groups (25–30). While the various regimens yielded DSA in islet, cardiac and renal transplantation models, none reliably produced DSA in 100% animals with histologic support of AMR.
The purpose of a NHP model of AMR is not only to better characterize humoral alloimmunity but also to test the safety and efficacy of available B cell therapeutics in the transplant setting. In order to develop a pragmatic model of AMR for this purpose, the following criteria should be met: (1) 100% DSA production within the first few months after transplantation, (2) histologic evidence of antibody-mediated tissue injury, (3) sustainable graft survival (i.e. avoidance of subtherapeutic regimens that may lead to premature cellular rejection) and (4) pathophysiology that mimics human clinical observations. In the last decade, immunosuppression use for induction has steadily increased, with over 80% of kidney recipients receiving induction agents in 2008. The majority have been depleting agents: 44.8% of all kidney recipients were treated with rATG (Thymoglobulin), 10.7% alemtuzumab (Campath 1-H), 1.5% lymphocyte immune globulin and 1% OKT3 (31). Alemtuzumab with or without steroids conferred the lowest risk of graft failure, followed by rATG with steroids, then without (32). A recent prospective study of 474 patients found significantly reduced acute rejection at 1 year with alemtuzumab (5%) compared to basiliximab or rATG (17%), as well as continued benefit at 3 years (33). Despite its success as an induction agent, however, early clinical trials using alemtuzumab induction uncovered an increased incidence of alloantibody production (40–50%) and antibody-mediated rejection, even when depletion was combined with conventional immunosuppression with calcineurin and mTOR inhibitors (34–39).
To mimic these clinical findings, we employed T cell depletion in a rhesus macaque renal transplantation model. In this study, we share our experience using a recombinant anti-CD3 immunotoxin fusion protein (A-dmDT390-scfbDb(C207)) as induction therapy combined with maintenance immunosuppression to produce a model of early and consistent alloantibody production with histologic evidence of antibody-mediated injury.
Materials and Methods
Animal selection and transplantation
Male rhesus macaques were obtained from Alpha Genesis (Yemassee, SC, USA) and Yerkes National Primate Research Center Field Station (Lawrenceville, GA, USA). All animals weighed 4–7 kg, were tested specific pathogen free, and expressed the FN18 epitope, the immunotoxin binding site. Donor–recipient pairs were selected based on avoidance of MHC class I matches (6 alleles tested), and class II maximal mismatch among available animals. Donor reactivity was confirmed in vitro with carboxyfluorescein succinimidyl ester-labeled (CFSE, Molecular Probes, Eugene, OR, USA) mixed lymphocyte reaction (MLR). Each animal underwent a donor nephrectomy followed by recipient transplantation, with at least 3 weeks between the two operations. This domino approach was used for all animals in the study. All medications and procedures were approved by the Emory University Institutional Animal Care and Use Committee, and were conducted in accordance with Yerkes National Primate Research Center and the National Institutes of Health guidelines.
Immunosuppression agents
Six animals were designated as untreated controls. All animals given immunosuppression received anti-CD3 immunotoxin (A-dmDT390-scfbDb (C207), Massachusetts General Hospital, Dana Farber-Harvard Cancer Center Recombinant Protein Expression and Purification Core Facility, Boston, MA, USA). Four animals were given immunotoxin (IT) plus tacrolimus (Astellas Pharma US, Inc., Deerfield, IL, USA), and another four received IT, tacrolimus, and alefacept (LFA3-Ig, Astellas Pharma US, Inc). IT was administered at 0.025 mg/kg intravenously twice daily from postoperative day (POD) 0–3. To alleviate potential symptoms of cytokine storm, methylprednisolone 125 mg was injected intravenously on days the animals received IT. They also received methylprednisolone on days 4–6 when they were administered bromodeoxyuridine. Tacrolimus (Tac) was started at 0.05 mg/kg intramuscular injection twice daily on the day of transplantation, and titrated throughout the life of the allograft to maintain a trough of 8–12 ng/mL. Alefacept was administered at 0.3 mg/kg once weekly for 8 weeks, starting on POD 3, 0, then 7, 14 etc. Rescue immunosuppression with methylprednisolone 125 mg IV × 3 days was used when the animal exhibited signs of acute rejection.
Allograft and immune monitoring
Peripheral blood was obtained weekly by femoral venipuncture for allograft and immune monitoring. Three milliliter were designated for serum chemistries and preserving serum, 0.5 mL for complete blood count, 1 mL for immune surveillance by polychromatic flow cytometry and 0.5 mL for monitoring tacrolimus trough levels. For flow cytometric analysis, peripheral blood mononuclear cells (PBMCs) were isolated by the Ficoll method using 5 mL of lymphocyte separation medium (Mediatech, Inc, Manassas, VA, USA) per sample. Washed PBMCs were surfaced stained with the following antibodies: Alexa-Fluor 700 conjugated anti-CD3, PerCP-Cy5.5 conjugated anti-CD4, APC-Cy7 conjugated anti-CD8 (BD Pharmingen, San Diego, CA, USA), PE-Cy7 conjugated anti-CD28, eFluor450 conjugated anti-CD95 (Ebioscience, San Diego, CA, USA) and PE conjugated anti-CD25 (Myltenyi Biotec, Auburn, CA, USA). Cells were then processed to detect FoxP3 and Ki-67 using intracellular staining with a Fix/Perm solution (Ebioscience), Pacific Blue conjugated anti-FoxP3 (BioLegend, San Diego, CA, USA) and PE conjugated anti-Ki-67 (BD Pharmingen).
Upon necropsy, rejected renal allograft tissues were incubated for 30 min at 37°C in Clostridium histolyticum collagenase (C8051 Blend Type H, Sigma-Aldrich, St. Louis, MO, USA). Graft and lymph node tissues were crushed through a 100 μm strainer, washed and stained with the above antibodies.
Samples were collected with an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software 9.2. (Tree Star, Ashland, OR, USA)
Detection of donor-specific antibodies
Alloantibody production was retrospectively assessed by flow cytometric crossmatch of donor PBMCs with serially collected recipient serum samples. Donor PBMCs were coated with ChromPure goat IgG (Jackson ImmunoResearch, West Grove, PA, USA) and incubated with recipient serum. Cells were stained with FITC-labeled antimonkey IgG (KPL, Inc. Gaithersburg, MD, USA), PE CD20 (BD Pharmingen) and PerCP CD3 (BD Pharmingen, San Diego, CA). The threshold for alloantibody positivity varies widely across the literature, ranging from a 10 to a 100 shift in mean fluorescence intensity for both CD3+ and CD20+ cells (15,40–43); thus, in this study, a twofold increase in mean fluorescence intensity from pretransplant values was used to determine alloantibody positivity, with all positive shifts being greater than 100 shift in mean fluorescence intensity.
Immunohistochemistry and electron microscopy
Renal allograft tissues were obtained at time of rejection and fixed in 10% neutral-buffered formalin and embedded in paraffin. Embedded tissue blocks were sliced into 5 μm-thick sections, deparaffinized in xylene, and rehydrated through graded ethanol to water for H&E and PAS stains as well as immunohistochemical analysis. For immunohistochemical assessment, the slides were subjected to heat-induced epitope recover in a 10 mM citrate buffer and then rinsed with Tris-buffered saline. The nonspecific sites were blocked by sequential treatment with 3% hydrogen peroxide, avidin-biotin blocking and protein blocking solutions. Tissue sections were labeled with antigen-specific primary antibodies CD3 (DAKO; dilution, 1:200) and C4d (ARP; dilution, 1:40) for T cell and C4d recognition and subsequently visualized using the Dako LSAB + labeled Streptavidin-Biotin kit (Dako). The positive staining were detected by 3,3-diaminobenzidine peroxidation and counterstained with hematoxylin. The slides stained with hematoxylin and eosin, PAS and antihuman CD3 and C4d antibodies were prepared by the Emory Transplant Center immunohistochemist. Formalin-preserved graft tissues were submitted to the Apkarian Integrated Electron Microscopy Core (Emory University, Atlanta, GA, USA). Renal pathologists reviewed in a blinded fashion all histology, immunohistochemistry and electron microscopy images.
In vitro assays
PBMCs from nine MHC-disparate macaque donor–recipient pairs were isolated and prepared into CFSE MLR. Each MLR was cocultured with three dosings of alefacept: 0 μg/mL, 100 μg/mL and 500 μg/mL; recipient lymphocytes without irradiated cell stimulation were used as controls. Upon incubation at 37°C, 5% CO2 for 5 days, the cells were recovered, washed and stained with the following antibodies: Alexa-Fluor 700 conjugated anti-CD3, PerCP-Cy5.5 conjugated anti-CD4, APC-Cy7 conjugated anti-CD8 (BD Pharmingen), PE-Cy7 conjugated anti-CD28, eFluor450 conjugated anti-CD95, PE conjugated anti-CD2 (Ebioscience, San Diego, CA, USA) and APC-Cy7 conjugated anti-CD20 (BioLegend).
Statistics
All statistical analyses were conducted using Statistical Package for the Social Sciences 19.0 (IBM SPSS, Chicago, IL, USA). Continuous variables were ranked and analyzed with the Mann–Whitney U or Kruskall–Wallis tests. Survival graphs were obtained with the Kaplan–Meier analysis. A p-value of less than 0.05 was considered significant.
Results
IT induces profound peripheral depletion with minimal improvement in graft survival
Three male rhesus macaques treated with IT alone experienced renal allograft survival of 8, 10 and 13 days, which was marginally increased (p = 0.048) compared to controls without immunosuppression (Figure 1A). All three animals experienced maximal peripheral T cell depletion (99%+) by day 3 after transplantation (Figure 1B). Despite profound peripheral depletion, IT did not induce T cell depletion uniformly across lymphoid compartments, particularly in lymph nodes (Figure 1C). Consistent with that, in peripheral blood, a dramatic increase in central memory T cells (CD28+CD95+) was observed by day 7 in IT-treated animals (Figure 1D). Histological analysis of rejected grafts revealed acute cellular rejection.

CD3 immunotoxin induces profound peripheral depletion but marginal graft survival benefit. (A) IT-treated animals have a marginally improved graft survival (MST 10 days) compared to untreated controls (MST 7 days, p = 0.048). (B) Animals are administered IT on days 0, 1, 2 and 3; by day 3, they experience >95% peripheral CD3+ cell depletion. By day 7, maximal T cell depletion is observed in all IT-treated animals. (C) Profound depletion is observed in peripheral blood, spleen and bone marrow, but not lymph nodes. The right image is a lymph node procured and stained for CD3+ on posttransplant day 5 (100× magnification). (D) Rapid repopulation of CD4 and CD8 memory cells is observed by day 7.
The addition of tacrolimus and alefacept prolongs graft survival
In 2005, Pearl et al. described a relative sparing of CD4+ effector memory T cells in human kidney recipients receiving T cell depletion with alemtuzumab or thymoglobulin. Calcineurin inhibitors, especially tacrolimus, best inhibited the function of these CD4+ memory cells in vitro (44). We had also observed a significant decrease in acute rejection when adding tacrolimus to human kidney transplant recipients undergoing alemtuzumab-based depletion with sirolimus maintenance therapy. To target the rapidly repopulating CD4+ memory population in our immunotoxin-treated animals, we added tacrolimus as maintenance immunosuppression and observed a prolongation in graft survival (Figure 2A, p = 0.034). Three of four animals rejected with acute cellular rejection and without donor-specific antibodies by an average of 31 days (Figure 1B). The fourth animal survived to 106 days, with C4d deposition observed in his rejected graft. In contrast to the IT-treated animals, the IT + tacrolimus treated animals showed delayed repopulation of memory T cells, with the most pronounced difference in CD4+ central memory T cells (Figure 2C). All IT + tacrolimus treated animals experienced a significant decrease in CD8 effector memory cells one week after induction T cell depletion (mean 12% of CD8+ cells, p = 0.02); however, by the time of graft rejection, this population increased to 58% of CD8+ cells (p = 0.043).

Tacrolimus maintenance added to IT induction prolongs graft survival. (A) In addition to IT, animals were administered tacrolimus with twice daily intramuscular injection, with a trough level maintained between 8–12 ng/mL. Graft survival was prolonged in these animals (MST 50, p = 0.034). (B) Despite prolonged graft survival, three of four animals experienced acute cellular rejection by 1 month (top images are hematoxylin & eosin stained, 100× left, 200× right; bottom images are CD3, 100× magnification). (C) Phenotypic switching from naive to memory T cells was delayed in IT + Tac treated animals. The left flow cytometric diagrams represent peripheral CD3+ populations. The greatest difference was observed in CD4+ central memory T populations (CD28+CD95+, p = 0.034 at 7 days. Note: only one animal survived to day 13 in the IT-alone treated group).
Improved inhibition of effector memory T cells was necessary to prevent early acute rejection in our model. As alloreactive T cells express higher amounts of CD2, they have been shown to be susceptible to and preferentially inhibited by the CD2-specific fusion protein alefacept/LFA3-Ig (45,46). Four transplanted animals received IT, tacrolimus and alefacept (Figure 3A), and experienced graft survival of 38, 63, 73 and 87 days. Although no statistical difference was found in comparison with the IT + tacrolimus group (p = 0.248), Figure 3B illustrates an incremental increase in graft survival with the addition of each immunosuppressive agent. Alefacept-treated animals still experienced a rapid repopulation of CD4+ central memory T cells, with a 285% increase from POD 7 (time of maximal depletion) to POD 21 (p = 0.02). Clinically, these animals demonstrated evidence of rejection at an average of 43 postoperative days, with increasing BUN and creatinine, and decrease in appetite and activity. One week prior to clinical manifestations of rejection, a significant increase from baseline (p = 0.02) in CD8 effector memory T cells could be detected by flow cytometry. Serum creatinine trends are displayed in Figure 3C.
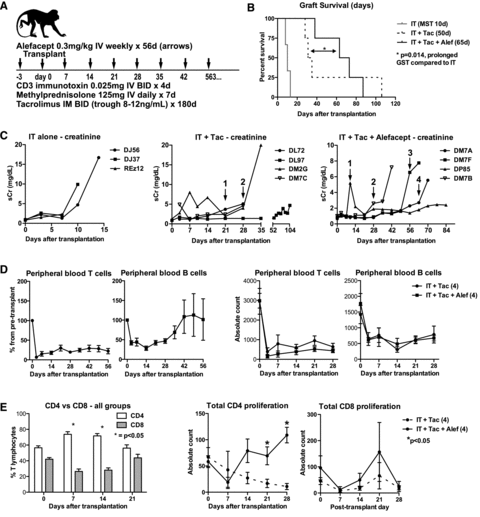
Adding alefacept to IT + tacrolimus decreased early acute rejection but allowed increased CD4+ T cell proliferation. (A) Alefacept (LFA3-Ig) was added to the IT + Tac regimen, administered in intravenous injections on days 3, 0 and then weekly to day 56. (B) Mean survival time for this treatment group was 65 days. Although not statistically prolonged compared to IT + Tac, the two groups had a significant increase in survival time compared to IT alone (p = 0.014). (C) Serum creatinine trends are shown here. A 3-day course of MP was administered for rescue immunosuppression when a sudden increase in creatinine and subtherapeutic tacrolimus levels were detected (arrows with numbers; IT+Tac arrow 1 = DL72, 2 = DM7C; IT+Tac+Alef arrow 1 = DP85, 2 = DM7B, 3 = DM7F, 4 = DM7A). (D) In all 11 IT-treated animals, peripheral T cell depletion was sustained throughout the life of the graft. B cell count initially decreased after transplantation, likely due to MP, but rebounded to at least baseline levels at 6 weeks. On the right are comparisons of peripheral T and B cells between IT+Tac and IT+Tac+Alef groups (p > 0.05). (E) IT+Tac and IT+Tac+Alef treated animals (n = 8) showed a relative sparing of CD4 cells in the first 2 weeks after depletion/transplantation. On the right, alefacept-treated animals (solid line) demonstrated higher CD4+ proliferation than IT + Tac animals. No difference was seen in CD8+ proliferation.
All IT-treated animals sustained low levels of peripheral blood T cells (average 19.8% at 4 weeks, 22% at 8 weeks) throughout the life of their grafts, as depicted in Figure 3D. B cell counts experienced an initial decrease, likely due to methylprednisolone (47,48), but were disproportionately increased in animals surviving past 4 weeks. No difference in T- and B cell absolute counts was observed between IT + tacrolimus and IT + tacrolimus + alefacept treated animals within the 4 weeks (Figure 3D, right). Despite low numbers of T cells, differential proportions of CD4+ and CD8+ were observed, with a relative sparing of CD4+ lymphocytes seen after IT induction: this difference was greatest by 1–2 weeks (p = 0.001) (Figure 3E). While all animals had a skewing toward a CD4+ dominant population, animals treated with alefacept experienced greater CD4+ proliferation (measured by intracellular Ki67+ staining) by weeks 3–4 compared to those without; no difference was observed in CD8+ proliferation. No differences in peripheral blood regulatory T cells (CD4+CD25+FoxP3+) were observed across the treatment groups (data not shown).
Alefacept-treated animals have increased alloantibody production and morphologic evidence of antibody-mediated injury
Alloantibody was detected in all animals treated with alefacept (Figure 4A). Flow crossmatch showed at least a —two- to sixfold mean channel shift by 2–4 weeks for both T and B cells in 100% of alefacept-treated animals. The kinetics of alloantibody production for each of the alefacept-treated animals is shown in Figure 4B.

IT + Tac + alefacept treated animals have increased alloantibody production and antibody-mediated tissue injury. (A) IT + Tac treated animals failed to demonstrate alloantibody production. Four of four IT + Tac + alefacept treated animals had donor-specific antibodies detected by 2–4 weeks after transplantation. (B) Alloantibody kinetics for all animals treated with IT + Tac + alefacept are shown. (C) Transplant glomerulopathy was prevalent in this treatment group (left image, periodic acid Schiff stain, 200×). The space between the two black arrows indicates subendothelial ground substance, representing antibody-induced inflammatory changes (middle image, H&E stain, 200×). C4d deposition in peritubular capillaries was observed in some of the rejected grafts (right image, C4d stain, 200×). (D) The left image is an electron microscopy image of a naive kidney, with the arrows indicating glomerular basement membrane. The middle image shows widening and lamination of glomerular basement membranes. The right image shows duplication of basement membranes, indicative of antibody-mediated injury.
Graft histology was evaluated in a blinded fashion and graded using Banff criteria (Table 1). Among the diagnoses listed in Table 1, glomerulitis, allograft glomerulopathy, mesangial matrix increase, peritubular capillaritis (PTC) and C4d deposition are commonly seen with antibody-mediated injury (4,24,49). These scores were combined to represent an antibody-mediated injury score: the IT alone group had an average combined score of 3.33, IT + tacrolimus 6.25 and IT + tacrolimus + alefacept 6.75. These data suggest that depletion by IT results in antibody-mediated injury that is detectable if the animal and graft survive to at least 4 weeks. The finding that all alefacept-treated animals developed de novo alloantibody makes it the preferred regimen for an AMR model. It should be noted that all animals exhibited varying degrees of PTC; although PTC is indicative of antibody-mediated rejection, it is also related to inflammation due to acute tubular necrosis, pyelonephritis, necrosis and infarction (50), which could confound the scoring system.
| Group | Animal code | Tubilitis | Intimal arteritis | Mononuclear cell interstitial inflammation | Interstitial fibrosis | Tubular atrophy | Vascular fibrous intimal thickening | Glomerulitis | Allograft glomerulopathy | Mesangial matrix increase | Peritubular capillaritis | C4d | Combined score for antibody-mediated injury | Average combined score per group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IT | DJ56 | 2 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 3 | 3.33 |
| DJ37 | 1 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | ||
| REZ12* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 5 | ||
| IT + | DM7C | 1 | 3 | 2 | 0 | 0 | 0 | 2 | 0 | 3 | 3 | 1 | 9 | 6.25 |
| Tac | DM2G | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 4 | |
| DL97 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 0 | 3 | 1 | 1 | 6 | ||
| DL72 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 0 | 2 | 3 | 0 | 6 | ||
| IT + | DM7B | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 4 | 6.75 |
| Tac + | DP85 | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 1 | 3 | 1 | 0 | 8 | |
| Alef | DM7F | 1 | 3 | 1 | 0 | 0 | 3 | 1 | 1 | 3 | 2 | 1 | 8 | |
| DM7A | 3 | 1 | 3 | 0 | 0 | 1 | 2 | 1 | 3 | 1 | 0 | 7 |
- *REZ12 was diagnosed with acute humoral rejection, with prominent features of acute tubular necrosis, peritubular capillaritis, and C4d deposition in the allograft.
- The combined score per group (bolded numbers) was determined by adding the scoring for glomerulitis, allograft glomerulopathy, mesangial matrix increase, peritubular capillaritis and C4d.
Figures 4C and D depict morphologic changes observed in the IT + tacrolimus + alefacept group. These include transplant glomerulopathy, subendothelial accumulation of ground substance (arteriolar endothelial injury) and C4d deposition (Figure 4C). Glomerular basement membrane thickening and duplication were noted on electron microscopy (Figure 4D). While donor-specific antibodies were present in all four animals in this treatment group, their graft immunohistochemistry reflected weak and varying intensities of C4d staining. This could be due to the time course of antibody-mediated injury. The average graft survival in this group was 65 days, which is shorter than the onset of C4d deposition detected by Smith et al.(51); furthermore, they report alloantibodies as the first to appear in the progression of chronic humoral rejection. Einecke et al. also underscored the importance of microcirculation changes and HLA antibodies in defining antibody-mediated rejection, as they preceded late kidney failures regardless of C4d+ status (52).
Alefacept increases CD4+ effector memory T cell proliferationin vitro
As de novo alloantibodies were consistently detected in the animals treated with IT + tacrolimus + alefacept, we investigated the effects of alefacept on lymphocyte proliferation and memory phenotypes in vitro. Alefacept was titrated at 0, 100 and 500 μg/mL in CFSE-mixed lymphocyte culture. Increased doses of alefacept corresponded to significantly increased CD3+ lymphocyte proliferation (Figure 5A); no difference was seen in CD20+ proliferation (Figure 5B). Specifically, CD4+ effector memory T cells exhibited the highest increase in proliferation with increased alefacept doses (Figures 5C and D). This phenomenon was not identified in vivo, as CD4+ effector memory cells are minimally detected in peripheral blood. They were, however, much more prevalent in rejected grafts (Figure 5E), suggesting that alefacept-driven proliferation of CD4+ effector memory cells occurs locally at the site of the alloantigen presentation.
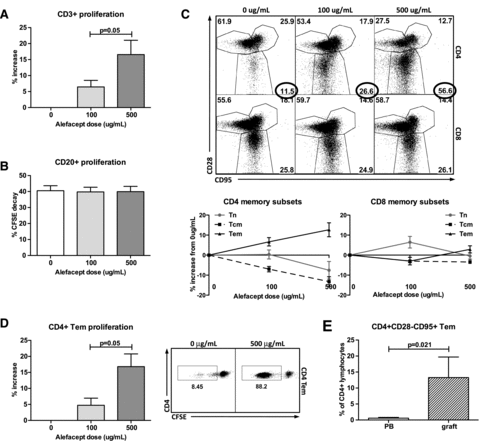
Alefacept increases CD4+ effector memory cell proliferation in vitro. Nine mixed lymphocyte cultures were incubated for 5 days with alefacept in 0, 100 and 500 μg/mL groups. (A) Increased proliferation (by CFSE decay) was observed in CD3+ cells with increasing doses of alefacept (p = 0.05, normalized at 0 ug/mL for variable rates of reactivity among rhesus pairs). (B) CD20+ proliferation was unchanged across different doses of alefacept. (C) Analysis of memory subsets revealed a proportional increase in CD4+ effector memory cells. CD4+ central memory cells decreased with increasing alefacept; other subset populations were relatively unchanged. (D) In addition to a proportional increase, CD4+ effector memory cells also had increased proliferation with increasing alefacept doses. (E) While CD4+ effector memory populations are minimally detected in peripheral blood in vivo, rejected grafts show increased sequestration of CD4+ Tem, suggesting a localized effect that may be difficult to monitor in peripheral blood in vivo.
Discussion
Chronic rejection is a major barrier to transplant tolerance, and there is a clear need for better characterization of this multifactorial entity. In this study, anti-CD3 immunotoxin was unable to achieve long-term graft survival, likely due to homeostatic repopulation of memory T cells. We hypothesized that adjuvant immunosuppression using tacrolimus +/− alefacept would target and mitigate the activation of memory T cells, allowing for prolonged graft survival. Three of four allografts treated with IT + tacrolimus succumbed to acute cellular rejection by 1 month; however, all four animals treated with IT + tacrolimus + alefacept had donor-specific alloantibody production as well as histological and ultrastructural evidence of antibody-mediated injury. As DSA was detected 2–4 weeks after transplantation, the cascade of events leading to their production must have occurred early in the postoperative period.
The postdepletion lymphocyte repertoire in IT + tacrolimus +/− alefacept groups showed a skewing toward a CD4+ cell dominant environment, particularly in the first 2 weeks after transplantation. Furthermore, the animals experienced increased CD4+ cell proliferation with the addition of alefacept, despite reports in the psoriasis literature documenting reduction of CD4+ and CD8+ memory T cells with alefacept (53–55). Combined with the in vitro data associating alefacept with CD4 effector memory proliferation, these observations suggest a role of CD4 memory cells in contributing to the early and consistent production of donor-specific alloantibodies. During this time, these homeostatically proliferating helper T cells provide fertile ground for B cell activation and alloimmunity. CD8+ effector cells—the highest expressers of CD2+ and thus most susceptible to alefacept (46)—are effectively suppressed. Our in vitro observation of increased CD4 effector memory proliferation was different from observations by Weaver et al. In their in vitro experiments with LFA-3Ig and CTLA4-Ig in mixed lymphocyte culture, a dose-dependent decrease in bulk CD4 and CD8 T cell proliferation was noted (memory subsets not shown). Our dosing (0, 100, 500 mcg/mL) was higher than that used in Weaver et al. (0.1, 1, 10, 50, 100 mcg/mL); however, this likely does not explain the discrepancy in findings. We did observe a decrease in CD4 central memory and naive populations, and no significant change in CD8 memory subsets.
While early graft loss precluded the IT + tacrolimus + alefacept treated animals from developing the full spectrum of morphologic changes associated with chronic AMR, early changes of antibody-mediated rejection were consistently produced, including DSA and endothelial injury leading to glomerulopathy and basement membrane thickening/duplication. Three of four animals treated with alefacept maintained graft survival until the withdrawal of alefacept at 2 months; perhaps prolonging alefacept therapy may extend graft survival enough to develop chronic AMR. Another consideration is that alefacept provided sufficient maintenance in this combination regimen until an overwhelming humoral and often accompanying cellular rejection response occurred. Ng et al. recently reported that B cells promote differentiation and enhance proliferation and survival of alloreactive T cells to become long-lived memory T cells (56). The detection of alloantibodies could herald a concerted and potentially more aggressive alloimmune response, which supports our findings of antibody-mediated injury mixed with acute cellular rejection.
The combination of immunosuppressive agents in this study successfully promoted alloantibody production in all alefacept-treated animals, but the question of clinical applicability must be addressed. Despite achieving profound peripheral depletion, IT-treated animals experienced short graft survival. Additionally, a recent study using IT in a xenotransplant model reported adverse effects in two of four animals, specifically death from pneumonia or cardiac failure (57). Although the IT is likely unsuitable for human clinical trials, it merits use in NHP models for the following reasons. First, it reproduces biological phenomena associated with depleting agents in humans, namely sustained peripheral T cell depletion, homeostatic repopulation of memory T cells and a high incidence of DSA production. Secondly, the FN18-CRM9 immunotoxin previously used in our lab exhibited a high incidence of donor-specific antibodies (73%), but the drug was replaced with a new recombinant IT to overcome the production/yield difficulty, linkage heterogeneity and potential immunogenicity of its chemically conjugated predecessor (58–60), making it unavailable for testing of B cell therapeutics. Lastly, alemtuzumab would be clinically most relevant, but hemolytic complications prohibited its use in our rhesus macaques, as most old world monkeys ubiquitously expressed alemtuzumab's target molecule CD52, including on erythrocytes (61). As mentioned above, tacrolimus suppresses rapid repopulation of memory T cells after depletion. Given that 94% of kidney transplant recipients are discharged with a CNI-based regimen (62), the use of tacrolimus in our model was clinically most appropriate. While alefacept is not available for human kidney transplantation, its addition in this model prolonged graft survival long enough for donor-specific antibodies and subsequent tissue injury to be detected.
In conclusion, CD3 immunotoxin induced profound peripheral depletion but short graft survival. When given with tacrolimus and alefacept, however, homeostatic repopulation of CD4+ helper T cells was followed by early alloantibody production. This immunosuppressive regimen provides a model of de novo alloantibody production and antibody-mediated rejection, which may prove valuable to study mechanisms contributing to the development of chronic rejection.
Acknowledgments
This work was supported by the National Institute of Health (1U01AI074635) and collaboration with the Yerkes National Primate Center (Yerkes base grant (P51RR-00065). We would like to thank Hong Yi for obtaining electron microscopy images, Kelly Hamby for assisting in animal surgeries and the Yerkes veterinary staff for their dedication to the care of our animals.
Funding sources
This work was supported by the National Institute of Health (1U01AI074635) and collaboration with the Yerkes National Primate Center (Yerkes base grant (P51RR-00065).
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




