B-Cell Depletion Extends the Survival of GTKO.hCD46Tg Pig Heart Xenografts in Baboons for up to 8 Months
Abstract
Xenotransplantation of genetically modified pig organs offers great potential to address the shortage of human organs for allotransplantation. Rejection in Gal knockout (GTKO) pigs due to elicited non-Gal antibody response required further genetic modifications of donor pigs and better control of the B-cell response to xenoantigens. We report significant prolongation of heterotopic alpha Galactosyl transferase “knock-out” and human CD46 transgenic (GTKO.hCD46Tg) pig cardiac xenografts survival in specific pathogen free baboons. Peritransplant B-cell depletion using 4 weekly doses of anti-CD20 antibody in the context of an established ATG, anti-CD154 and MMF-based immunosuppressive regimen prolonged GTKO.hCD46Tg graft survival for up to 236 days (n = 9, median survival 71 days and mean survival 94 days). B-cell depletion persisted for over 2 months, and elicited anti-non-Gal antibody production remained suppressed for the duration of graft follow-up. This result identifies a critical role for B cells in the mechanisms of elicited anti-non-Gal antibody and delayed xenograft rejection. Model-related morbidity due to variety of causes was seen in these experiments, suggesting that further therapeutic interventions, including candidate genetic modifications of donor pigs, may be necessary to reduce late morbidity in this model to a clinically manageable level.
Abbreviations:
-
- CC
-
- consumptive coagulopathy
-
- CUF
-
- cobra venom factor
-
- DXR
-
- delayed xenograft rejection
-
- GTKO
-
- glycosyl transferase knockout
-
- HAR
-
- hyperacute rejection
-
- LVP
-
- left ventricular pressure
-
- SPF
-
- specific pathogen free
-
- TM
-
- thrombotic microangiopalsy
Introduction
Thousands of patients with end-stage kidney, heart, liver and lung disease succumb while waiting for a transplant due to the critical shortage of donor organs (1). Even the best available mechanical alternatives to transplantation, such as dialysis, mechanical circulatory support and extracorporeal membrane oxygenation, are constrained by cost and technical limitations, have generally unfavorable impacts on quality of life, and do not adequately address the organ shortage (2). Despite significant progress, tissue engineering remains far from clinical application.
Xenotransplantation, the use of organ transplants from other species, could potentially address the unmet clinical need (3–5). Infectious and ethical concerns associated with xenotransplantation are considered manageable (6, 7). “Hyperacute rejection (HAR)” of pig organs usually occurs in nonhuman primate models, as in man (8) but can be significantly attenuated by genetic modifications to the donor pig, including removal of the galactose α-1,3 galactose carbohydrate epitope (α-Gal) by galactosyl transferase gene knockout (GTKO) (4,9–11) or expression of human complement pathway regulatory proteins (hCRP) (12–14). Expression of human CD46 “transgene” on GTKO organs (GTKO.hCD46Tg) has been proposed to address the problem of complement injury driven by pre-existing or elicited antipig antibodies directed at targets other than α-Gal (anti-non-Gal antibodies) (15). Further, evidence is accumulating that elicited “anti-non-Gal” antibodies play a major role in posttransplant thrombotic microangiopathy (TM), consumptive coagulopathy (CC) and delayed xenograft rejection (DXR) (16).
In this study, we hypothesized that B cells, as antigen-presenting cells and the dominant source of elicited antipig antibodies, are the principle trigger for graft injury in baboon heart xenograft recipients. We report that efficient depletion of B cells by anti-CD20 antibody at the time of transplant prevents detectable elicited antipig immune response and significantly delays associated graft injury.
Material and Methods
Animal
Specific pathogen free (SPF) baboons weighing 7–15 kg from University of Oklahoma (Norman, OK, USA) were housed in a clean pathogen free facility. GTKO.hCD46 pigs (Revivicor Inc., Blacksburg, VA, USA) were used at 3–8 weeks old with a maximum weight of 12 kg. The origin of the CD46 transgene was same as described before (12). This line of CD46 pigs was made using CD46 minigene that demonstrated high-level expression of human anti-CD46 in all tissues of these pigs, including in heart. This line of CD46 pigs was cross-bred with Revivicor GTKO pigs over more than four generations. The transgene is stable at genomic level, and expression of hCD46 protein is consistent and high level across all GTKO/CD46 pigs, as in the CD46-only lines. All animals were used in compliance with guidelines provided by the National Heart, Lung and Blood Institute (NHLBI) Animal Care and Use Committee (ACUC).
Flow cytometric analyses
Gal 1–3 α-Gal and CD46 expression were measured on porcine peripheral blood mononuclear cells (PBMC) by exposure to 100 μg/mL Isolectin B4 (FL-1201, Vector labs) and antihuman CD46 antibody (MCA 2113PE, AbD Serotec), and events recorded by flow cytometry.
B- and T-lymphocytes were quantified in peripheral blood were labeled with mouse anti-human CD3 (Becton Dickinson) and anti-CD19 (Becton Dickinson, San Diego) and were analyzed on FACS Calibur (Becton Dickinson).
Transplant procedure
All transplant procedures were performed at a NHLBI core surgical facility. The heterotopic transplant procedure has previously been described (14). Briefly, the recipient baboon's infrarenal aorta and inferior vena cava are exposed through a midline abdominal incision. Side-biting clamps are applied; an aortotomy and venotomy made, and the end-to-side anastomosis are performed between donor and recipient aorta and donor pulmonary artery with recipient inferior vena cava.
Telemetry implantation and methods of evaluating graft function
A telemetry device (Konigsberg Instruments, Inc., Pasadena, CA, USA) was implanted into the recipient to monitor graft left ventricular pressure and electrocardiogram (EKG), as well as, the recipient's temperature. The telemetry device data are transmitted wirelessly to a receiver attached to the animal's cage (RMISS, Wilmington, DE, USA). The parameters recorded include peak systolic pressure (PEAK), end diastolic pressure (END), left ventricular pressure (PEAK-END) (LVP), heart rate based on LVP (LVPHR), EKG, heart rate based on EKG (EKGHR) and recipient's body temperature (TEMP). Heart function was continuously evaluated by telemetry and drop in LVP below 60 mmHg signified the time point at which the rejection process starts to affect the graft contractility, and a pressure below 10 mmHg was an indication of complete cessation of graft contractility. When the recipient was sedated, palpation (scored as ++++ (fully functional) to 0 (rejection) and/or ultrasound was used to confirm the graft status. Final diagnosis of rejection was made by histopathology.
Experimental groups and immunosuppressive regimen
The immunosuppressive regimen is described in detail in Table 1. In brief, two baboons (group A) received no immunosuppression. Two baboon (group B) received induction regimen (previously described (17) of 40–50 mg/kg antithymoglobulin (ATG) (Genzyme, Cambridge MA, USA) on days −2 and −1; 50–100 U/kg of cobra venom factor (CVF) (Quidel, San Diego, CA, USA) on days −1, 0 and 1; and 25 mg/kg of anti-CD154 antibody (Beth Israel Medical Center, Boston, MA, USA) on days −1 and 0. Nine baboons (group C) in addition to group B induction regimen also received 19 mg/kg of anti-CD20 antibody (Rituxan; Genetech, South San Francisco, CA, USA) on days −7, 0, 7 and 14. Immunosuppression in groups B and C was maintained with daily dose of 20 mg/kg of Mycophenolate Mofetil (MMF) (Genzyme); anti-CD 154 antibody (25 mg/kg) and Ketorolac (15 mg) on days 3, 4, 7, 10, 14, 19 and then once weekly thereafter with tapering dose of methyl prednisolone (starting doze 2 mg/kg) for 7 weeks. Supportive treatment included continuous heparin infusion to keep the activated clotting time (ACT) level twice the baseline, Ganciclovir (Roche, Nutley NJ, USA) daily to prevent the viral infections, Epogen 200 U/kg daily from days −7 to 7 and Cephazolin 250 mg twice a day for 7 days.
| Drugs | Source | Dose | Timing |
|---|---|---|---|
| Induction | |||
| Anti-CD20 | Genetech, San Francisco, CA | 19 mg/kg | Preop days −7, 0, 7 and 14 |
| ATG | Genzyme, Cambridge, MA | 40–50 mg/kg | Preop days −2 and −1 |
| Anti-CD154 | NHP Reagent Resource, Boston, MA | 25 mg/kg | Preop days −1 and 0 |
| CVF | Quidel, San Diego, CA | 50–100 U/kg | Preop days −1, 0 and 1 |
| Maintenance | |||
| Anti-CD 154 | 25 mg/kg | Postop days 3, 7, 10, 14, 19, q weekly | |
| MMF | Genzyme, Cambridge, MA | 20 mg/kg/2-hour IV infusion | BID daily |
| Solu-Medrol | Pfizer, New York, NY | 2 mg/kg | BID, tapered off in 7 weeks |
| Aspirin | Major Phama, Livonia, MI | 81 mg | Daily |
| Heparin | Hospira, Lake Forest, IL | Maintain ACT 2 × baseline | Continuous infusion |
| Supportive | |||
| Ganciclovir | Roche, Nutley, NJ | 5 mg/kg/day | Daily |
| Cefazolin | Hospira, Lake Forest, IL | 250 mg | BID for 7 days |
| Epogen | Amgen, Thousand Oaks, CA | 200 U/kg | Daily from day −7 to 7 then weekly |
| Ketorolac | Hospira, Lake Forest, IL | 15 mg | Just before anti-CD154 |
Histology and immunohistochemistry
Paraffin sections from biopsies and explanted xenografts were stained with hematoxylin and eosin for light microscopy. Sections were analyzed semi-quantitatively for the presence of hemorrhage, necrosis, thrombosis and cellular infiltrates. Rejection was scored from 0 to 4 based on the percentage of graft affected (0 = none, 1 =≤20%, 2 =≤50%, 3 =≤75%, 4 =≥75%). Frozen tissue sections from explanted xenografts were assessed by immunohistochemistry staining as described previously (18). Staining intensity was assigned ‘‘− to +++,’’ taking into account the extent and intensity of staining relative to positive and negative control sections (hyperacutely rejected pig heart and normal pig heart, respectively).
Detection of non-Gal antibodies
Porcine aortic endothelial cells (PAEC) were isolated and cultured from GTKO pigs. The method of staining has been described previously (14).
Treatment of rejection
Upon detecting rejection by telemetry, rescue therapy was initiated with IV methyl prednisolone (10–15 mg/kg) for 6 days. Heparin dose was also increased to prevent thrombus formation, whenever a drop in ACT below the target value was observed.
Results
Confirmation of GTKO.hCD46 pig phenotype
Cloned or bred GTKO.hCD46Tg donor piglets lacked the Gal epitope on the cells’ surface as measured by flow cytometry using IB4 Lectin (Figure 1A a). Peripheral blood lymphocytes (by FACS, Figure 1A b), endothelial cells, and heart tissue (by immunohistochemistry, IHC, Figure 1A c) from GTKO.hCD46 pigs expressed high levels of hCD46.

(A) Expression of Gal epitope and hCD46. Peripheral blood lymphocytes of pigs with modified genetics were tested by flow cytometry for the absence of Gal epitope using IB4 lectin (A) and transgenic expression of human CD46 (B). Expression of hCD46 was confirmed by immunohistochemical staining of heart vessels (C). (D) Non-Gal antibody levels in SPF baboons and human: PAEC from GTKO pigs were isolated and mixed with baboon sera (1:10 dilution). After incubation, the cells were further stained with PE-goat antihuman IgG/IgM or 20 μL of 1:10 FITC/PE. The mean fluorescence intensity of the cells for each test and control serum was measured for the estimation of non-Gal antibodies.
Anti-non-Gal antibody levels in SPF baboons
Recipient baboons bred in a specific pathogen free (SPF) facility have lower serum levels of anti-non-Gal IgM antibodies (similar to levels in human) relative to conventional non-SPF baboons (Figure 1D ). Differences in IgG levels were less pronounced between these baboon populations.
Determination of xenograft function by continuous telemetry monitoring
The telemetry provided accurate evaluation of graft function by continuous measurement of LVP, EKG and recipients’ temperature. Representative tracing of LVP heart rate, peak systolic pressure and end diastolic pressure are shown in Figure 2A (long-term surviving graft) and Figure 2B (graft rejecting in 75 days). At any significant spike in temperature the recipient baboon was immediately evaluated for leuckocytosis, cultured and treated with antibiotics and in most cases infections were effectively controlled. The arrow in Figure 2B indicates institution of rescue therapy with steroids, which was not found to be effective in preventing rejection.

Role of anti-CD20 in cardiac xenograft survival
Cardiac xenograft and recipient baboon survival from all three groups are summarized in Table 2.
| Group | Baboon ID | Mean survival time (days) | Median survival time (days) | Survival time (days) | LV pressure (mmHg)3 | Contractility score4 | Rescue therapy (post-op day/ Rx period)6 | Rejection score on histology 5 | Complications | Recipient outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| A | FA77 | <1 | <1 | <1 | <10 | 0 | N/A | 4 | N/A | Euthanized1 |
| FC40 | <1 | <10 | 0 | N/A | 4 | N/A | Euthanized1 | |||
| B | 8907 | 8 | 8 | 8 | <10 | 0 | 7 | 4 | None | Alive after explant |
| 9607 | 8 | <10 | 0 | 7 | 4 | none | Alive after explant | |||
| C | 7404 | 94 ± 69 | 71 | >179 | >40 | ++ | None | 2 | Wound infection/systemic infection | Died |
| 404 | >58 | >40 | ++ | 57 /1 | 2 | Abdominal bleeding (cause unknown) | Died | |||
| 2004 | >42 | >60 | +++ | None | 2 | None | Euthanized1 | |||
| 1107 | 75 | <10 | 0 | 71/4 | 4 | None | Euthanized1 | |||
| 1207 | >236 | N/A2 | ++ | 230 /6 | 3 | None | Euthanized | |||
| Intestinal rupture/intra-abdominal | ||||||||||
| 2707 | >115 | >50 | +++ | 110 /5 | 3 | Bleeding | Died | |||
| 2807 | >39 | >60 | ++++ | None | 0 | Gl bleeding | Died | |||
| 8007 | >36 | >60 | ++++ | None | 1 | Ventricular fibrillation/IVC thrombosis | Euthanized | |||
| 11308 | >71 | >40 | ++ | 70 /1 | 2 | Neurologic/anesthetic complication | Euthanized |
- Group A = no immunosuppression; group B = ATG, CVF, anti-CD154, MMF and steroid; and group C = group B regimen + anti-CD20.
- 1ACUC-mandated euthanazia after laparotomy.
- 2 Telemetry implant malfunctioned and was taken out.
- 3LV pressure at the time of explant.
- 40 = nocontractility, ++++= normal contractility.
- 5Percent graft affected 0 = none, 1 = <20%, 2 = <50 %, 3 = <75%, 4 = >75%.
- 6 Rescue therapy initiated only once in each case.
Untreated baboons (group A; n = 2) rejected GTKO.hCD46Tg heart xenografts in an “acute” fashion within 24 hours. Group B baboons (n = 2) had an 8-day median graft survival (p = 0.02 vs. untreated). A similar 10-day median survival (n = 6) was observed at University of Maryland (UMD) by our co-authors using non-SPF baboons (unpublished results, personal communication). No group B grafts exhibited normal function at the time of explantation. Addition of depleting humanized anti-CD20 antibody (Rituxan) to the group B regimen (group C; n = 9) was associated with graft prolongation up to 236 days, with a median graft survival time of 71 days and mean graft survival of 94 days (p<0.005 vs. group B). Xenograft survival for 236 days achieved in our laboratory is the longest survival time in a pig to primate solid-organ xenotransplantation model reported to date. B-cell depletion by the anti-CD20 antibody was very effective and sustained, with no circulating B cells detected in treated baboons for at least 60 days post-transplantation (Figures 3A a and b). Depletion of circulating B cells was not complete in group B animals (Figure 3A a). A low white blood cell count was maintained throughout the graft survival period and minor infections were promptly treated with antibiotics (Figure 3B a). As shown in Figures 3B c and d, a fluctuation in the value of platelet and hemoglobin was appreciated during the first 30 days after surgery which warranted transfusion in some animals (n = 4) with hemoglobin drop below 10 gm/dL and platelet level of less than 70 000/mm3. The platelet numbers stabilized in long-term animals with small fluctuations within the normal range (range 200–350 000/mm3).
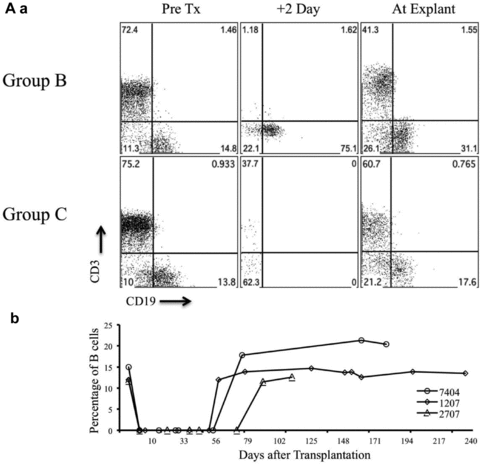
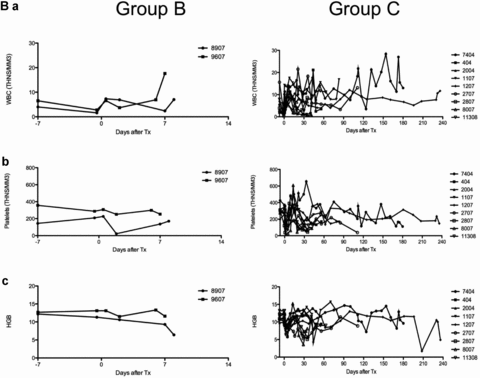
(A) Suppression of B and T cells by the immunosuppression regimen. Analyses of B-cell suppression in representative baboons in groups B and C, at different time points, by flow cytometery is shown (CD19) (3A, a and b) along with suppression of T cells (CD3) by other agents in the immunosuppressive regimen (3A a). Complete circulating B-cell depletion was observed for 60 days with the last dose of anti-CD20 given on day 14 posttransplantation. (B) White blood cell (WBC) and platelet numbers, and hemoglobin levels in experimental baboons: total WBC count in group B and C baboons is shown in Figure 3B a. The comparison of platelet numbers is shown in 3B b and hemoglobin in 3B c.
Impact of anti-CD20 induction on elicited anti-non-Gal antibody response
There was no discernable increase in the level of circulating non-Gal antibodies (both IgM and IgG) in group C compared to that in groups A and B (Figures 4A and B). However, a marked increase of antibody production was observed in group B animals, which was also closely associated with delayed xenograft rejection (DXR). This striking finding demonstrates efficient control of elicited xenoimmunity in group C by eliminating B-cell response.
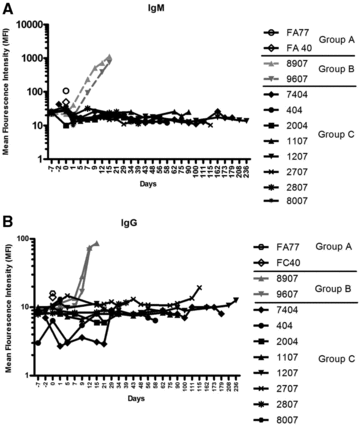
Comparison of non-Gal antibody levels in all groups. Mean fluorescence intensity (MFI) measurement of non-Gal (IgM (4A) and IgG (4B)) antibody in all experimental groups; without immunosuppression (group A), immunosuppression without anti-CD20 (group B), and with full immunosuppression including anti-CD20 (group C).
Complications limiting sustained survival of recipient baboons
Complications are summarized in Table 2. One baboon developed septicemia, which led to his demise after self-inflicted reopening of a groin incision. Three baboons died after abdominal bleeding due to severe gastritis possibly due to aspirin and anticoagulant use, rupture of intestine at the duodeno-jujenal junction and an unknown cause. Three baboons were euthanized either due to restriction imposed by ACUC (in early experiments that mandated euthanasia after exploratory laparotomy) or due to surgical complications during laparotomy. Laparotomies were performed for ventricular fibrillation, to perform the biopsy and to confirm the function of the xenograft. However, graft was functional in all these cases. Most of these complications have been previously described by our group and are inherent to the heterotopic location of the graft (19).
Histology and immunohistochemistry (IHC) of transplanted GTKO.hCD46Tg pig hearts
Histology and IHC analyses were performed on all explanted xenografts and representative images from all three groups are shown in Figure 5. Group A hearts demonstrated histolopathalogical findings of acute interstitial hemorrhage, diffuse coagulative myocytes necrosis and TM (Figures 5A a and b) and IHC demonstration of antibody (both IgM and IgG) deposition (Figures 5B a and b). Group B graft histopathology showed signs of delayed xenograft rejection, including TM with microvascular thrombosis, interstitial hemorrhage and ischemic myocyte necrosis (Figures 5A c and d). Antibody deposition similar to that in group A was seen on IHC in this group (Figures 5B c and d). Myocardial morphology was normal in group C animals except for some patchy interstitial fibrosis with TM in areas of grafts that survived more than 100 days (Figures 5A e and f). Antibody deposition on IHC was similar to that in groups B and C (Figures 5B e and f). The absence of cellular infiltration in explanted grafts (Figures 5A e and f) and adequate suppression of circulating T cells in peripheral blood (Figure 3A a) indicates that the T-cell response to xenoantigens was also effectively suppressed by this regimen. A trace of complement deposition was also seen in groups A and C.

Histological and IHC evaluation of the cardiac xenograft. Graft histology and IHC was performed at the time of explantation and representative results from each group are shown.(A) Histology: group A—section of right ventricle with extensive acute interstitial hemorrhage and arterial thrombus (200×) (a). Section of interventricular septum with diffuse coagulative necrosis and acute interstitial hemorrhage (400×) (b). Group B—section of left ventricle with focal dystrophic mineralization of myofibers (200×) (c). Section of left ventricular with venular thrombus, coagulative necrosis of adjacent cardiac myocytes and acute interstitial hemorrhage (400×) (d). Group C—section of left atrium with coagulative necrosis of myocytes, loss of myocytes and replacement of myocytes with moderate interstitial fibrosis with increased numbers of fibroblasts and adjacent collagen (200×) (e). Section of interventricular septum demonstrating an area of relatively normal cardiac myocytes (400×) (f). (B) Immunohistochemical staining of graft for non-Gal antibody deposition for both IgM and IgG antibodies in all three groups.
Discussion
Substantial progress has been made over the past two decades to overcome pig heart xenograft rejection. An immunosuppressive regimen based on targeting CD154 appears to prevent detectable cellular and humoral immune responses to porcine antigens in baboons preselected for low anti-non-Gal antibody titers. With this regimen, TM and/or CC in the recipient emerged as the principal limits to long-term graft function (20).
Our current data strongly support the hypothesis that elicited antibody to non-Gal antigens, and perhaps B-cell-dependent cellular immune mechanisms contribute significantly to the pathogenesis of DXR in baboons with pre-existing anti-non-Gal antibody titers similar to those found in humans. The importance of anti-non-Gal antibody is inferred from the association between appearance of antibody in association with GTKO (14,17), hCPRP and GTKO.hCD46 (current report) graft demise in baboons treated with the backbone anti-CD154-based regimen. A role for elicited anti-non-Gal antibody in TM, CC and DXR remains speculative in the context of added B-cell depletion since increasing titers remain undetectable in serum even in the setting of late graft failure of variable degrees seen in this experiment.
Other investigators have previously tried CD20 antibody to suppress anti-Gal and non-Gal antibody response (21). All the studies had variable graft survival with somewhat incomplete suppression of B-cell response but the striking results described in this study demonstrate efficient control of elicited xenoimmunity in the group receiving GTKO.hCD46Tg cardiac graft and anti-CD20 antibody. Whether removing a pivotal APC population or incidentally depleting the antipig precursor circulating B-cell population, induction depletion of circulating B-cell represents a major advance to control elicited antipig immunity and delay TM and/or CC onset toward safe clinical application of pig xenografts in man. Our study also demonstrates that four doses of anti-CD20 were sufficient to suppress the circulating B-cell response for more than a 6-month period and extend graft survival. Improved graft survival over the other studies that utilized anti-CD20 described by Ekser et al. could be multifactorial and include the pig genetics, effective costimulation blockade, the immunosuppressive regimen, surgical technique and also post-operative management of the baboons. All these factors may have augmented the effect of anti-CD20. However it is unclear whether the use of SPF baboons with low “human-like” levels of anti-non-Gal antibodies played any significant role in graft prolongation. In addition to the suppression of B-cell numbers, the T cells were efficiently depleted in peripheral blood by induction treatment with ATG and maintenance with MMF and anti-CD154. Acute rejection of group A grafts within 24 h was unexpected and we speculate that protection of graft from complement mediated injury was not complete even with CD46 transgene expression and that the amount of pre-existing non-Gal antibodies present in the SPF baboons is enough to induce acute graft rejection. This is also evident by slight prolongation of grafts and prevention of acute rejection in group B where CVF was used. While we have observed long-term survival using this regimen up to 236 days, the mean graft survival in group B grafts was shorter than the survival reported by Kuwaki et al. (17). This could be due to many reasons and attributed to the use of pigs from a different source, preselection of baboons with very low non-Gal antibody titers and additional manipulations to the recipient like thymic irradiation and loCD2b treatment by this group. While transgenic pigs with GTKO alone (17), or overexpression of the hCD46 transgene alone (12), have shown benefits in prolonging xenograft survival, this study used only the GTKO/CD46 combination, and thus it is difficult to comment on whether the combination of both genetic modifications has a beneficial role in long-term survival in the context of this anti-CD20-supplemented immunosuppression.
The use of telemetry in this experiment provided accurate monitoring of graft function and helped in managing complications. Temperature rise as a harbinger of infection led to early diagnosis and treatment. Telemetric monitoring of graft EKG and LV pressure had a major advantage over other methods for evaluating graft function, e.g. palpation and echocardiography, both of which require sedation of recipient baboon. We periodically use these traditional methods to confirm our telemetry findings and found telemetry to be most accurate.
Detection of anti-pig non-Gal antibody in grafts from all groups is not surprising, since CD20 is not expressed on plasma cells and therefore anti-CD20 treatment was not expected to significantly alter elaboration of pre-existing anti-non-Gal by plasma cells. If preformed anti-non-Gal antibody contributes to the pathogenesis of TM or/and CC, depleting plasma cells may delay or prevent both of these conditions. Targeting the plasma cell population could be done either selectively, perhaps through an antigen-specific immune targeting approach based on identification of dominant anti-non-Gal antibody targets, or nonspecifically, by addition of a proteasome inhibitor to the group C regimen. Expression of coagulation pathway regulatory molecules on the endothelium of GTKO.hCD46Tg pig organs will reveal whether TM and/or CC are primarily driven by coagulation pathway dysregulation, and whether these complications can be safely and more reliably prevented without further suppression of recipient immunity. The humoral pattern of rejection and presence of complement deposits in the long-term graft may indicate failure of protection from complement over a period of time. A personal communication with our co-authors from UMD indicates that biopsies performed by them at an earlier time point does not reveal complement deposition indicating that somehow the complement reappears over a period of time. This activation of complement indicates the loss of capacity of CD46 molecule to down regulate the complement affectivity, perhaps driven by previously described platelet and coagulation pathway dysregulation, perhaps amplified by effects of elicited anti-non-Gal antibody.
Model-related complications posed a nonimmunologic hurdle to long-term success following heterotopic heart xenotransplantation, similar to those encountered by Kuwaki, McGregor and others (13,17,21).
The morbidity and mortality observed in group C was not primarily immunologic. Multiple complications resulted in animal's mortality while the graft was still functional. Some of the complications encountered were due to the location of heterotopic heart and donor–recipient size discrepancy. This scenario contributed to intestinal obstruction and rupture, intra-abdominal bleeding, and thrombosis of inferior vena cava transient thrombocytopenia and drop in hematocrit was seen in almost all the baboons with platelet numbers dropping to even below 70 000 in some cases. This condition mostly occurred first immediately after transplantation and in some cases reoccurred within 1 month of transplantation probably due to more frequent use of anti-CD154 during this period. In the initial experiments due to limited availability of blood, only small amounts of packed red cells were transfused with transient effect but in most recent cases, larger amounts of packed cells resulted in a remarkable long-lasting recovery of hemoglobin. The platelet number also improved over a period of time with the transfusion of packed red cells. Infections did occur but were controlled with prompt antibiotic therapy instituted at the onset of fever. With the knowledge that anti-CD154 is prothrombotic, attempts are being made in our laboratory to replace it with anti-CD40 based on the recent demonstration by the Emory group (22).
The previous longest survival of a cardiac xenograft in a baboon was 179 days (17). This study demonstrates for the first time that xenograft rejection can be avoided for at least 236 days using GTKO.hCD46Tg pigs with peritransplant recipient B-cell depletion. The limiting factors are various nonimmune, technical complications, many of which may be preventable or more readily treated in clinical application. This experiment paves the way for testing orthotopic transplantation in this model and assessing the ability of the transplanted heart to sustain life.
Acknowledgments
We acknowledge the staff of Laboratory of Animal Medicine and Surgery (LAMS) and Division of Veterinary Resources (DVR) for their support in surgical procedures and care of animals. Flow cytometry core of NHLBI for their help in FACS analyses. Patricia Jackson for administrative help. Dr. Keith Reimann at Beth Israel Deaconess Medical Center, Boston, MA and ATCC for providing the CD154 antibody.
Disclosure
Two authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. D.A. is the CEO and President of Revivicor, Inc. R.P. serves on the scientific advisory board of Revivicor, Inc.




