Living Kidney Donors: Impact of Age on Long-Term Safety
Abstract
The safety of older live kidney donors, especially the decline in glomerular filtration rate (GFR) after donation, has been debated. In this study we evaluated long-term renal outcome in older live kidney donors. From 1994 to 2006 follow-up data of 539 consecutive live kidney donations were prospectively collected, during yearly visits to the outpatient clinic. Donors were categorized into two groups, based on age: <60 (n = 422) and ≥60 (n = 117). Elderly had lower GFR predonation (80 vs. 96 mL/min respectively, p < 0.001). During median follow-up of 5.5 years, maximum decline in eGFR was 38%± 9% and the percentage maximum decline was not different in both groups. On long-term follow-up, significantly more elderly had an eGFR <60 mL/min (131 (80%) vs. 94 (31%), p < 0.001). However, renal function was stable and no eGFR of less than 30 mL/min was seen. In multivariate analysis higher body mass index (HR 1.09, 95%CI 1.03–1.14) and more HLA mismatches (HR 1.17, 95%CI 1.03–1.34) were significantly correlated with worse graft survival. Donor age did not influence graft survival. After kidney donation decline in eGFR is similar in younger and older donors. As kidney function does not progressively decline, live kidney donation by elderly is considered safe.
Abbreviations:
-
- LDN
-
- laparoscopic donor nephrectomy
-
- MIDN
-
- mini-incision open donor nephrectomy
-
- GFR
-
- glomerular filtration rate
-
- HLA
-
- human leukocyte antigen
-
- BMI
-
- body mass index
Introduction
The outcomes of transplantation of kidneys derived from live kidney donors are superior with regard to early function and survival, as compared to transplants derived from deceased donors (1–3). Live kidney donation is now generally accepted because the operation is safe, and donation does not lead to increased mortality rates at long term (4). Due to the worsening shortage of deceased kidney donors we are trying to expand and maximize our live donor pool, reconsidering the contra-indications for donation.
Nowadays, older live donors, obese donors and donors with minor comorbidity may be candidates for kidney donation. Certainly, they would not have been selected in the past. There is an ongoing shift toward the acceptance of these donors in order to bridge the gap between demand and supply of kidney transplants (5,6). Since a few years the percentage of older live kidney donors has also increased in our center (Figure 1). Controversy remains, as age-related changes in the kidney may result in a decline in renal function over the years, and the combination of aging and a donor nephrectomy is not properly investigated. Therefore questions have risen about the outcome of older live kidney donors and especially the decline in glomerular filtration rate (GFR) after donation.
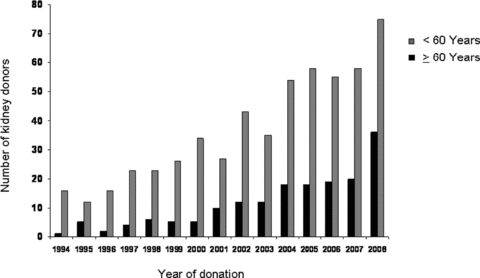
Number of live kidney donors in two age groups over the years 1994–2008.
Older donors may also have an increased risk of perioperative complications. They often have a higher ‘American Society of Anesthesiologist score’ (ASA-score), a higher incidence of hypertension and a higher body mass index (BMI), all possibly contributing to a higher risk of perioperative problems. Therefore, the aim of this study was to evaluate short-term and long-term renal outcome after live kidney donation of older live kidney donors in comparison to the outcome in younger donors.
Methods
Study population and data collection
In this study we included all 539 consecutive live kidney donors who underwent live donor nephrectomy at our center from 1994 to 2006. Data of these donors and corresponding recipients were prospectively collected. Observation was until May 2010. All donors were preoperatively screened by a nephrologist, and subsequently by a medical psychologist, an anesthesiologist, and a cardiologist if indicated. Obese donors (BMI>30 kg/m2) and donors with multiple arteries on both sides were not excluded from donating. GFR was estimated by use of the modification of diet in renal disease formula (MDRD), which estimates GFR using three variables: serum creatinine, age and gender (7). The donor was discharged when a normal diet was tolerated and mobilization was adequate. Visits to the outpatient clinic were scheduled at 3 weeks, 2 months and 1 year following donor nephrectomy. Standard yearly follow-up consisted of blood analysis, blood pressure measurements and urine analysis. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or the use of antihypertensive medication, according to the American Heart Association guidelines. Proteinuria was defined as ≥0.3 g/L. In this study donors were categorized into two age groups: <60 years and ≥60 years. This cut-off point was based on other articles (8–12). Serum creatinine of the recipient was recorded preoperatively, during the first 14 days, day 21, 28 and every 3 months thereafter. Donor, graft and recipient survival were also recorded. The institutional review board approved the study.
Operative techniques
Open (until 1998), mini-incision open (from 2001 to 2006) and laparoscopic donor nephrectomy (from 1997) were performed during this era. These techniques have been described previously (13,14). The donor and the corresponding recipient were operated on by the same team. After nephrectomy all kidneys were perfused with Eurocollins (Fresenius, Bad Homburg, Germany). Skin to skin time was defined as the time from the first incision until closure of the last incision. Warm ischemia time was defined as the time from closing the stapling device until backtable perfusion of the kidney.
Statistical analysis
Categorical variables are presented as a number (percentage). Continuous variables are presented as a median (range). Categorical variables were compared with the chi-square test; continuous variables were compared with the Mann–Whitney U-test. Death-censored graft survival and recipient survival was analyzed by Kaplan–Meier analyses and compared using the log-rank test. In a multivariate analysis, with backward elimination, we assessed the independent effects of donor and recipient variables on graft survival. We corrected for donor and recipient age, donor and recipient gender, donor BMI, number of arteries, number of previous transplants, mismatch-total, mismatch-DR, PRA, previous dialysis treatment. All analyses were conducted using SPSS (version 15, SPSS Inc., Chicago, IL, USA). A p-value <0.05 (two sided) was considered statistically significant.
Results
Baseline characteristics (Table 1)
| <60 (n = 422) | ≥60 (n = 117) | p-Value | |
|---|---|---|---|
| Gender: female | 235 (56%) | 69 (59%) | 0.526 |
| Age (years) | 46 (18–59) | 65 (60–90) | – |
| BMI1 (kg/m2) | 25 (14–41) | 26 (18–34) | 0.035 |
| GFR2 (mL/min) | 96 (54–173) | 80 (54–146) | <0.001 |
| ASA-classification3 >1 | 69 (16%) | 38 (33%) | <0.001 |
| Operation: LDN4 | 264 (63%) | 68 (58%) | 0.151 |
| Recipient | |||
| Gender: female | 163 (39%) | 52 (44%) | 0.263 |
| Age | 46 (8–76) | 48 (19–81) | <0.001 |
- 1Body mass index; 2glomerular filtration rate; 3American Association of Anesthesiologists; 4laparoscopic donor nephrectomy.
Four hundred and twenty-two donors were younger than 60 years and 117 donors were 60 years or older. Older donors had a lower eGFR predonation, a higher BMI, and a higher American Society of Anaesthesiologists-classification (ASA-classification) as compared to younger donors.
Intraoperative data (Table 2)
| < 60 (n = 422) | ≥60 (n = 117) | p-Value | |
|---|---|---|---|
| Skin to skin time (min) | 192 (84–420) | 185 (94–395) | 0.444 |
| Warm ischemia time (min) | 5 (1–20) | 4 (1–13) | 0.024 |
| Blood loss (mL) | 180 (0–3000) | 230 (0–1285) | 0.011 |
| Conversion1 | 17 (6%) | 3 (4%) | 0.588 |
| Complications minor | 20 (5%) | 10 (9%) | 0.115 |
| Complications major | 6 (1%) | 2 (2%) | 0.820 |
| Hospital stay (days) | 3 (1–31) | 4 (2–15) | 0.012 |
- 1As a percentage of the donors who underwent LDN.
Skin to skin time did not differ between the groups. Blood loss was significantly more in the oldest group and warm ischemia time was significantly shorter. Rates of minor and major intraoperative complications did not significantly differ. Major complications occurred in six younger donors (1%) including two bleedings, one of the stapled artery, which necessitated conversion and blood transfusion with three packed cells and two fresh frozen plasma's, and one diffuse bleeding, which resulted in a laparotomy 5 h after donation to control bleeding. One splenic lesion occurred, which needed conversion and splenectomy. Three intestinal lesions occurred, two needed resection of a small bowel segment and one serosal lesion of the colon was sutured. In the youngest group there were 17 conversions (4%), 2 conversions are mentioned above. Bleedings (n = 9), lack of overview due to intra-abdominal fat (n = 3) and technical problems (n = 2) necessitated the other conversions.
In the oldest group there were two major complications (2%), a splenic lesion, which necessitated splenectomy, and a bleeding from the rectus abdominis, which needed a reoperation the next day. There were three conversions (3%) in the oldest group; two resulted in an open procedure: one due to bleeding of the caval vein and one due to inadequate overview caused by intra-abdominal fat. In one procedure there was a conversion to a hand-assisted procedure, due to bleeding of the renal vein.
Postoperative data and long-term follow-up
The median follow-up was 5.5 years. Donors in the older group had a significantly longer hospital stay. Postoperative complications did not significantly differ between groups (3.8% and 4.3%). In the youngest group five donors developed pneumonia. These were successfully treated with antibiotics. One donor developed a pneumothorax which could be treated conservatively. Two donors developed urinary tract infections, which were treated successfully with antibiotics; four donors developed incisional hernias, which needed mesh placement. Two donors developed wound infections of the pfannenstiel incision, which were treated conservatively, one donor had an exacerbation of his known asthma and one donor developed an infection of the mesh that was used to correct an incisional hernia from an earlier appendectomy. In the oldest group one donor developed a pneumonia, which was successfully treated with antibiotics; two donors had a wound infection of the pfannenstiel incision, treated effectively with antibiotics. Two donors developed an incisional hernia, one of the pfannenstiel incision and one of the subcostal incision, both repaired with a mesh.
Older donors had a lower GFR before donation, but there were no differences in the mean maximum decline (Figures 2 and 3). The mean maximum decline in eGFR was 38%± 9%.
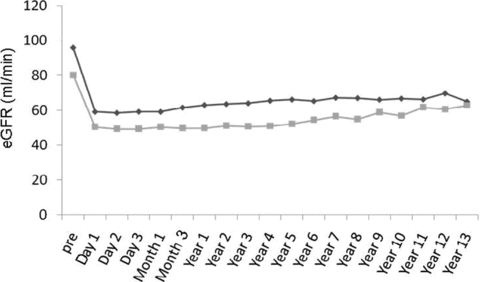
Median estimated glomerular filtration rate (eGFR) of 539 live kidney donors divided into two age groups. (Black line indicates <60; gray line indicates ≥ 60.)
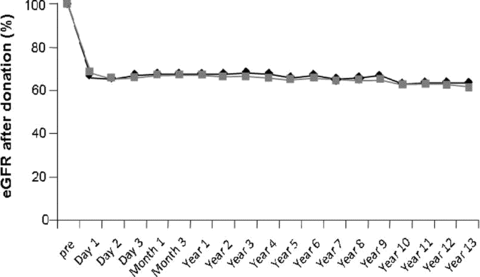
Percentual difference in estimated glomerular filtration rate (eGFR) of 539 live kidney donors divided into two age groups. The baseline value is 100%. (Black line indicates <60; gray line indicates ≥ 60.)
At 5 years after donation, significantly more older donors had a GFR < 60 mL/min compared to younger donors (131 [80%] vs. 94 [31%], p <0.001). The renal function stabilized during follow-up and there were no donors with a GFR of less than 30 mL/min during follow-up. After donation 12 (10%) elderly developed hypertension versus 25 (6%) of the younger donors (p = 0.56).
Proteinuria was seen in four older donors after 1 year (n = 98 [4.1%]), in three donors after 5 years (n = 64 [4.7%]) and no donors showed proteinuria after 10 years (n = 15). Proteinuria was seen in 12 younger donors after 1 year (n = 354 [3.4%]), in eight donors after 5 years (n = 206 [3.9%], and in six donors after 10 years (n = 94 [6.4%]). There are no significant differences between the groups at these time points (p-values: 0.91, 0.78 and 0.32).
Graft and patient survival
One and 3 year death-censored graft survival was 97% resp. 94% for kidneys derived from younger live donors and 91% resp. 89% for those derived from older live donors (p = 0.011 resp. p = 0.008). There were 76 graft failures in the period studied. A higher donor BMI (p = 0.003, hazard ratio 1.085) and a higher mismatch-total (p = 0.020, hazard ratio 1.072) were independently associated with a shorter graft survival (Table 3). Recipient survival did not differ between transplants derived from younger and older donors (p = 0.072). Three older donors died during follow-up, one due to cancer, one due to a cardiac arrest and one unknown cause. One younger donor died 6 days after donor nephrectomy from thrombotic thrombocytopenic purpura. Eight younger donors died during follow-up, three due to cancer, one in a car accident and four by an unknown cause.
| Hazard ratio | 95%CI | p-Value | |
|---|---|---|---|
| BMI donor | 1.085 | 1.029–1.144 | 0.003 |
| Mismatch-total | 1.172 | 1.025–1.340 | 0.020 |
| Age donor | 1.014 | 0.995–1.034 | 0.169 |
| Age recipient | 0.988 | 0.917–1.005 | 0.210 |
| Gender donor | 0.895 | 0.563–1.423 | 0.672 |
| Gender recipient | 0.906 | 0.565–1.452 | 0.810 |
| Number of arteries | 0.477 | 0.917–2.379 | 0.107 |
| Number of previous transplants | 0.881 | 0.539–1.438 | 0.894 |
| Mismatch-DR | 1.039 | 0.559–1.931 | 0.932 |
| PRA | 1.004 | 0.989–1.019 | 0.656 |
| Previous dialysis | 0.958 | 0.546–1.682 | 0.812 |
Data on donors older than 70 years
There were 25 (5%) donors of 70 years or older. The mean age in this group was 74 (70–90) years. We did not observe any significant differences in operative time, complications, conversions or development of hypertension in comparison to the group younger than 70 years. Hospital stay was significantly higher for donors of 70 years or older (5 vs. 4 days, p <0.001), possibly explained by the social conditions needed to offer these donors adequate care in the home situation.
Discussion
Survival of live kidney donors in the years following nephrectomy has recently been reported favorable as compared to age-matched controls (4) We add that surgical morbidity is acceptable, postoperative renal function is stable and the risk of hypertension is not higher than in the general population.
The Western world is aging. In Europe 17% of the inhabitants were 65 years or older in 2008. This percentage will rise to approximately 29% in 2050 (15). On the one hand this will lead to an increasing number of patients suffering from renal insufficiency, but also to an increasing number of older persons willing to donate. We provide evidence that healthy individuals in the older age category may undergo live kidney donation with good results for the donor as well as the recipient. Thus, live donation by older donors may offer an attractive option to further stabilize waiting lists for kidney transplantation.
More than 20% of the live donors in this study were 60 years or older. In our center the mean donor age has increased over the last 15 years from 43 in the 1990s to 50 nowadays. We even included a group of 25 donors older than 70 years. The average age in this study is relatively high, in particular when compared to American studies (16–18). Results of the present study may encourage other centers to include healthy older donors.
Several concerns have made doctors cautious of accepting older donors. These include possibly higher perioperative and postoperative complication rates, due to an increase in comorbidity related to aging. Risk factors for renal and cardiovascular damage, such as hypertension and overweight, are more prevalent in the elderly. In concordance with the literature we do not report a difference in perioperative complication rate between younger and older donors (16,19–21). This may be the result of the selection process including donors with no or minor comorbidity only.
Furthermore, information regarding the long-term renal consequences of reduced renal mass in healthy humans has mainly come from studies of veterans who lost a kidney as a result of trauma (22). However, these veterans lost their kidney at relatively young age. GFR slowly declines over the years, with 5–10 mL/min per decade, leading to a further reduction of the residual capacity in donors (23). In accordance with the literature, our data indicate that after an initial drop in kidney function there is no accelerated decline after donation, neither in young donors, nor in older donors (20,24–25). None of the donations led to a GFR of less than 30 mL/min during follow-up, and the prevalence of hypertension was lower in comparison to the normal population. Proteinuria was rare in our study and did not differ between younger and older donors. Although isolated cases of renal failure have been described, no large study has shown evidence of progressive deterioration of renal function after live kidney donation (25–29). We would like to extrapolate this for the older donors: live donation alone will not lead to renal insufficiency.
Some worrisome reports have been published on whether renal function of the transplanted graft may be compromised due to older age of the donor. The association of older donor age, and higher rejection rates and unfavorable graft survival has been debated (9,30). We did not assess an association between higher age and lower graft survival in the multivariate analysis. This is in concordance with recent literature (9–10,12,31–34). However, higher donor BMI and higher mismatch-total were independently associated with shorter graft survival. The latter association has been reported in the literature (35). Others did report BMI as a risk factor for graft failure, but the precise underlying mechanisms are not known (36–38). Possibly, obesity-induced hyperfiltration and glomerular hypertension lead to renal damage and sclerosing glomerulopathy (38). However, from the perspective of the recipient, transplantation from an older donor or with a higher BMI is probably nearly always preferred to both dialysis and transplantation from a deceased donor (1–3).
Our study comprises a unique group, with a large group of older live kidney donors. We have described a cohort study with regular, yearly follow-up. One drawback is the time-frame of 13 years in which these donors are included. It should be noted that in this period there have been changes in all aspects of the live kidney donation and transplantation including a shift from related to unrelated donors, major changes in surgical technique and different immunosuppressive regimens in the recipient. Further follow-up is indicated to evaluate the outcome of this shift in the near future.
In conclusion, live kidney donation by older donors may be considered safe as morbidity of the operation is limited, GFR does not progressively decline, and graft-survival is acceptable. We encourage accepting carefully selected older donors in living kidney donation programs.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
The authors have no conflict of interest.




