Renal Transplantation for Fibromuscular Dysplasia
Abstract
This is the first report that presents renal transplantation after bilateral nephrectomy as the final treatment for severe renovascular hypertension due to fibromuscular dysplasia (FMD). We describe the history of a 1-year-old girl who suffered from renovascular hypertension due to FMD. Imaging revealed multiple bilateral stenoses of the renal artery extending into the distal branches. The hypertension proved unresponsive to pharmacologic treatment and the intrarenal peripherally located stenoses rendered a conventional approach such as transluminal or surgical angioplasty not feasible. At the age of 5 years, a unilateral nephrectomy of the most affected kidney was performed, but she remained hypertensive and developed progressive cardiomyopathy and retinopathy. At the age of 6 years the remaining kidney was removed, followed by a living related renal transplantation with a kidney donated by her mother. Posttransplantation, she developed mild hypertension due to a postanastomotic stenosis, which was easily controlled with antihypertensives. Now 8 years after transplantation, she has experienced no further blood pressure related problems. Although there is a risk of recurrence of FMD after performing a living related transplantation, our report suggests that this procedure is relatively safe, provided appropriate preoperative evaluation and follow up is performed.
Abbreviations:
-
- FMD
-
- fibromuscular dysplasia
-
- PTA
-
- percutaneous transluminal angioplasty
-
- MRA
-
- magnetic resonance angiography
-
- DSA
-
- digital subtraction angiography
Introduction
Fibromuscular dysplasia (FMD) is a vasculopathy associated with stenosis, dissection, and aneurysm formation. The pathogenesis is not completely understood, and it is therefore commonly described as a nonatherosclerotic, noninflammatory vascular disease. FMD is histologically characterized by hyperplasia of connective tissue within the vessel wall and is classified according to the arterial layer in which it predominates. The prevalence of symptomatic FMD is estimated at 0.4%. However, angiographic signs of FMD are encountered in 3–4% of asymptomatic potential living kidney donors (1–3). The renal and carotid arteries are most frequently involved (respectively 60–75% and 25–30% of cases) but FMD can manifest in almost every blood vessel (4). In pediatrics, FMD is reported to be the most important cause of renovascular hypertension, but the prevalence is not precisely known. In a cohort of Turkish children with renovascular hypertension, FMD was found in 31% (5).
The clinical presentation of FMD depends on the afflicted arterial bed. In most cases it is the resultant of renovascular hypertension with complaints of a headache, visual blurring, epistaxis, Bell's palsy and seizures. Other symptoms, not necessarily linked to hypertension, include stroke, abdominal pain and weight loss (6).
Possible strategies in children for establishing the presence of renovascular hypertension and its underlying cause have been described earlier (6). Since pathology of the involved vessels is often not readily available, the diagnosis of FMD is generally based upon the typical appearance on angiography. Medial fibroplasia, the most common form of FMD, is classically characterized by alternating segments of stenosis and dilatation, resembling a ‘string of beads’ on an angiogram. Patients with hypertension should also undergo fundoscopy and echocardiography for the assessment of end organ damage.
The initial treatment of renovascular hypertension consists of antihypertensive medication. Drugs affecting the renin–angiotensin axis have to be used with caution due to the acute and severe renal failure sometimes observed in children (6). When the blood pressure is under insufficient control despite maximum tolerable pharmacological treatment or when progression of ischemic renal damage is found, strategies for revascularization must be considered. Percutaneous transluminal angioplasty (PTA) has become the most applied technique; alternatively the lesion can be corrected surgically. A recent meta-analysis reported a success rate of 46% after PTA versus 58% after surgical revascularization in adult patients with FMD. In children the success rate for both interventions appears to be better (60–100%; Ref. 7). When the aforementioned ‘kidney-sparing’ strategies fail, one can perform a partial or total nephrectomy of the affected kidney. The strategy followed in this case report goes one step further.
Case Report
A 13-month-old girl was presented at the pediatric outpatient clinic because of a cardiac murmur, discovered at the bureau for infant health care. Her prior and family history was unremarkable (in particular with regard to cardiovascular and cerebrovascular disease). Physical examination demonstrated a height of 73 cm (10th percentile height-for-age) and weight of 8150 g (5th percentile weight-for-age). She was in a good general condition but had a systolic murmur grade IV over the 4th left intercostal space, and a blood pressure of 140/113 mmHg (95th percentile-for-age: 101/57). Laboratory investigations revealed a normal serum creatinine, but significant proteinuria (3 gr/L). Her electrolytes were normal, plasma renin was slightly elevated (10.4 nmol/L/h; normal for age < 10). Cardiologic evaluation revealed left ventricular hypertrophy with a tricuspid and mitral valve insufficiency. Renal ultrasound demonstrated a small right kidney with loss of corticomedullary differentiation and a normal left kidney. An angiogram revealed multiple stenoses in both renal arteries (several segments with a ‘string of beads’ appearance), extending into the distal branches with localized perfusion defects. There were no stenoses in the superior mesenteric artery (Figure 1). The general appearance of the renovascular defects was in accordance with the diagnosis of FMD. The differential diagnosis includes: previous renal artery trauma (after catheter/surgery), inflammatory arterial disease, pheochromocytoma, Marfan’s-, Williams,- or Ehlers-Danlos syndrome, and type 1 neurofibromatosis (4,8). There had been no invasive medical interventions in the past. There were no laboratory results pointing to systemic inflammation or excessive catecholamine production, nor did she have specific clinical manifestations of the aforementioned diseases or syndromal diagnoses (therefore no specific genetic analysis was performed). The right kidney demonstrated a significant loss of parenchyma and had a differential function of 27% on a MAG3 scan. The bilateral stenoses in the distal branches of the renal arteries were deemed inaccessible for PTA and antihypertensive medication was started. Unfortunately, the blood pressure could not be controlled despite the combination of a beta-blocker, calcium channel antagonist, angiontensin II receptor blocker and thiazide (all agents: maximum dose for bodyweight). She developed progressive left ventricular hypertrophy together with complaints of fatigue, abdominal pain and headache. In addition, we were afraid of cerebrovascular complications, in specific intracranial hemorrhages, which are reported in several cases of FMD and renovascular hypertension (9,10). To get a better control over her blood pressure, we decided to perform a nephrectomy of the most affected (right) kidney when she was 5.5 years old. Histological investigation confirmed the diagnosis of medial FMD in the renal artery and its smaller branches. Blood pressure improved after the nephrectomy, but 6 months later again worsened (160/90 mmHg; 95th percentile-for-age: 111/73 mmHg). Renewed intensification of her antihypertensive medication had no effect and complaints reappeared. The cardiomyopathy proved progressive, and she developed a grade I hypertensive retinopathy. Her estimated GFR was 35–40 mL/min/1.73 m2. Because her condition was deteriorating, we considered taking out the remaining kidney and start renal replacement therapy. This was discussed with the parents who agreed and opted for a kidney donation. After medical screening, her mother (age: 31 yr) was found suitable for donation. She had a supine office blood pressure of 130/72 mmHg. A magnetic resonance angiography (MRA) of the donor's renal arteries revealed no signs of FMD. In the recipient, additional screening before transplantation revealed further severe stenoses of both carotid arteries (‘string of beads’ appearance) and signs of collateral redistribution via the posterior arteria communicans. Since the patient had no related complaints or symptoms, we performed no specific interventions.
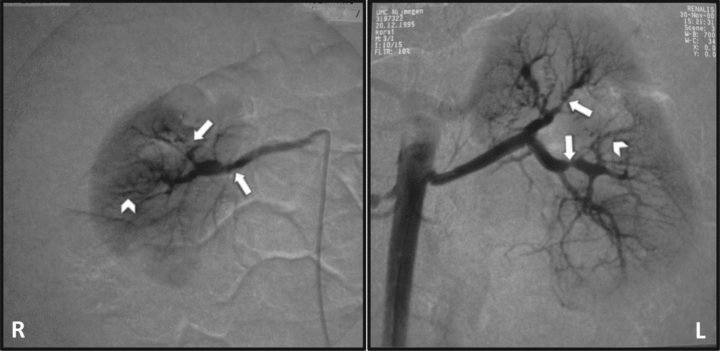
Renal angiography showing severe stenosis of the right main renal artery and multiple stenoses in the peripheral branches (arrows). Left renal artery demonstrating mild irregularities in the main stem and again multiple stenoses in the peripheral branches (arrows). In both kidneys, segments with a ‘string of beads’ appearance can be seen (arrow heads). Furthermore, bilateral perfusion defects were observed with signs of parenchymal loss of the right underpole. Normal aspect of the aorta.
When the girl was 6 years old, a nephrectomy of the remaining kidney was performed followed by a living related transplantation procedure. No perioperative complications occurred. In the first week the flow in the renal artery was normal. One month later, physical examination revealed a bruit over the graft. Doppler ultrasound demonstrated a stenosis of the transplant artery, located just behind the anastomosis, with a reduction in diameter of less than 75% and a resistive index of 0.8. Her blood pressure was 134/62 mmHg and atenolol was started. We performed no additional angiogram, because the blood pressure responded to this single agent and her allograft function remained stable. She experienced no complaints or symptoms (in particular neurological) after her blood pressure normalized. At the time of writing this report she is 14 years old. Her immunosuppressive regime consists of prednisone and mycophenolate mofetil. She has an estimated GFR of 62 mL/min/1.73 m2. Her last blood pressure was 110/62 mmHg (95th percentile for age: 125/81) with solely enalapril 2.5 mg. Her mother currently has an estimated GFR of 65 mL/min/1.73 m2, no proteinuria and a normal blood pressure (120/70 mmHg).
Discussion
To the best of our knowledge we are the first to present renal transplantation after a bilateral nephrectomy as the final treatment for severe renovascular hypertension due to FMD. The strategy, which involved a living related donor, immediately relieved the patient's hypertension, and furthermore prevented time on dialysis.
Living donation has become an important modality in kidney transplantation with a significant positive impact on the outcome of patients with ESRD. However, its success also depends on the outcome of the donors, which should therefore be carefully selected and followed up to minimize the risk for ‘donation-related’ health problems (11,12). In related donors, special attention should be given to the presence of a familial disease that could (re)occur in both donor and recipient. Rushton et al found in 12 of 20 families of patients with FMD additional subjects who had a history of hypertension, stroke, myocardial infarction or claudication before the age of 50. The authors considered this suggestive for familial FMD in 60% of cases (13). However, family members were not examined physically, risk factors for atherosclerosis were not stated and no imaging studies were performed. Therefore the true prevalence of FMD within these families was probably overestimated. A more recent cross sectional study in families of 100 FMD patients did perform imaging and found 11 angiographically confirmed cases of FMD in 1st degree relatives (11%). This is probably an underestimation of the true prevalence since only a few relatives were screened with an angiogram. Interestingly, all the familial cases involved women with multifocal lesions. Furthermore, extra-renal manifestations were two to three times more common in familial cases (14). The increased risk for the presence of FMD in the donor poses a dilemma for living related donation within these families and could have important consequences for both recipient and donor. In our report the donor appeared to have a higher risk for familial FMD (female sex, index patient with multiple localizations), but she had no hypertension or angiographic signs of renovascular disease, so we proceeded with the living donation.
FMD can occur in both recipient and donor after transplantation, despite the absence of radiographic signs at the time of donor evaluation. This is illustrated by a report on a living related donation in which a stenosis attributed to FMD was found in the lower renal artery during donor evaluation. The stenosis was surgically corrected during transplantation. Six months later a new and significant stenosis in the upper artery was found (15). Our patient also developed a stenosis in the renal artery after transplantation. The described location was not typical for ‘posttransplant renal artery stenosis’, which usually is located at the anastomosis. Therefore, we cannot exclude the possibility of FMD in the donor artery. Because the concurrent hypertension was relatively easy to control with medication, we performed no additional angiogram. Parasuman et al described a 41-year-old female donor who developed medial dysplasia after she donated a kidney to her father. She appeared to have no abnormalities on the CT angiogram performed before donation. One year after the transplantation, she developed severe hypertension with a typical string of beads appearance in the renal artery of the remaining kidney on a conventional angiogram. After a follow up of 3 years the recipient had still not developed what the authors called ‘signs of renovascular disease’ (16). We recommend that especially in potential donors with an increased risk for FMD, such as family members of patients with FMD, screening for renovascular lesions should be performed with DSA to minimize the risk of missing existing lesions. Furthermore, cerebrovascular lesions are demonstrated in almost a third of all patients with FMD and were found in 11% of patients with renal artery FMD (8). Asymptomatic cerebrovascular FMD is generally benign but its importance in the setting of living kidney donation is not known. The presence of cervicocranial stenoses or aneurysms can be of relevance during the donor operation and follow up. We therefore recommend adding a MRA of the head as part of donor screening in case of a bruit over the carotid arteries, neurological symptoms or the presence of renovascular FMD.
FMD in the donor renal artery is not regarded as an absolute contraindication for performing kidney transplantations and published cases are summarized in Table 1 (15,17–21). Kolettis et al described 36 living donor transplantations with kidneys classified as having mild (irregularity without significant stenosis) or moderate renovascular FMD (irregularity with less than 50% stenosis). After a median follow up of 37 months, none of the recipients were reported to have vascular complications, but 24 out of the remaining 30 recipients (80%) required antihypertensives of whom 9 needed 3 or more agents (22).
| Author | Donor sex | Donor Age (years) | Possible signs of FMD | Preop. angiography | Periop. surgical angioplasty | Hypertension during follow up | Follow up (months) | Postop. angiography |
|---|---|---|---|---|---|---|---|---|
| FMD in renal allografts after living donation | ||||||||
| Linder (18) | F | 50 | No | Slight narrowing | Yes | No | 24 | no |
| Serrano (20) | F | 53 | No | Bands of stenosis | Yes | No | 26 | no |
| Nahas (19) | F | 41 | No | nm | Yes | No | 27 | no |
| F | 56 | No | nm | Yes | No | 21 | no | |
| M | 45 | No | nm | Yes | No | 115 | no | |
| Pfeiffer (17) | F | 56 | No | String of beads | Yes | No | 27 | no |
| F | 63 | No | String of beads | Yes | No | 23 | no | |
| Wolters (21) | F | 70 | No | String of beads | No | Decreased GFR | After 5 days onset fall graft function | Postanastomotic bandlike stenosis |
| Kolettis (22) (n = 36) | F: 29 vs. M: 7 | 51 (median) | No | Mild to moderate FMD | No | 6: no 15: ≤2 BPM 9: ≥ 3 BPM (6 LTFU) | 37 (median) | nm |
| Benavides (15) | F | 35 | No | Focal stenosis lower pole artery | Yes | Yes | After 6 months onset hypertension | Bandlike stenosis in upper pole artery |
| FMD in renal allografts after deceased donation | Onset hypertension (months) | |||||||
| Nghiem (25) | F | 40 | ICH, aneurysm contralat. a. renalis | No | No | Yes | 9 | String of beads |
| Linder (18) | F* | 53 | ICH | No | Yes | Yes | 4,5 | Multiple stenoses |
| F* | 53 | ICH | No | Yes | No | – | Dysplasia after 12 months | |
| Campieri (26) | F | 49 | ICH | No | No | Yes | 30 | String of beads |
| Verove (23) | F | 46 | No | No | No | Yes | 0,5 | String of beads |
| Sevastos (24) | F | 25 | nm | No | No | Yes | 120 | String of beads |
- BPM = blood pressure medication; LTFU = lost to follow up; nm = not mentioned; ICH = intracrebral hemorrhage.
- *Same donor.
We found five reports concerning grafts from deceased donors without a history of hypertension in which significant postoperative stenosis attributed to FMD occurred (Table 1; Refs. 18,23–26). In these recipients hypertension could be controlled with conventional methods. Remarkably, all donors were female, half of whom died due to a cerebrovascular bleeding. In retrospect, these donors probably suffered from concomitant cerebrovascular localizations of FMD. Hypertension was frequent. In at least 30 of the 51 recipients mentioned in Table 1, high blood pressure was found within a relatively short follow-up-period. Perioperative correction appeared effective because in 8 of 10 cases in which the affected segment of the renal artery was removed, no hypertension was described during follow up. We found no reports of graft lost due to FMD. Unfortunately, the follow-up of the living donors was not mentioned in these reports. Indudhara et al described the follow-up of 37 asymptomatic donors who were found to have renovascular FMD during pretransplant evaluation. 19 were selected based on FMD severity and proceeded to donate their (most) affected kidney. After 4.5 years none had hypertension, proteinuria or an abnormal serum creatinine, indicating that patients with FMD may be suitable as donors (3).
In conclusion, this report demonstrates that renal allograft transplantation after bilateral nephrectomy can be considered for therapy-resistant renovascular hypertension due to FMD. When confronted with the option for a living related donation procedure the possibility of FMD in the donor should be taken into account and investigated by means of DSA. The presence of angiographic signs of FMD in the donor artery does not pose an absolute contraindication for transplantation since successful transplantations have been performed with these kidneys, but appropriate precautions should be undertaken. Both recipients and donors with signs of FMD deserve a careful follow-up after transplantation since symptomatic FMD can reoccur.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. Furthermore, no commercial organization took part in its preparation or funding.




