Essential Role of PDL1 Expression on Nonhematopoietic Donor Cells in Acquired Tolerance to Vascularized Cardiac Allografts
Abstract
The PD1:PDL1 pathway is an essential negative costimulatory pathway that plays a key role in regulating the alloimune response. PDL1 is expressed not only on antigen-presenting cells (APCs) but also cardiac endothelium. In this study, we investigated the importance of PDL1 expression on donor cardiac allograft in acquired transplantation tolerance in a fully MHC-mismatched model. We generated PDL1 chimeric mice on B6 background that expressed PDL1 on either hematopoietic cells or nonhematopoietic cells of the heart. Sham animals were used as controls. These hearts were then transplanted into BALB/c recipients and treated with CTLA4-Ig to induce tolerance. Cardiac endothelium showed significant expression of PDL1, which was upregulated upon transplantation. While the absence of PDL1 on hematopoietic cells of the heart resulted in delayed rejection and prevented long-term tolerance in most but not all recipients, we observed an accelerated and early graft rejection of all donor allografts that lacked PDL1 on the endothelium. Moreover, PDL1-deficient endothelium hearts had significant higher frequency of IFN-γ-producing alloreactive cells as well as higher frequency of CD8+ effector T cells. These findings demonstrate that PDL1 expression mainly on donor endothelium is functionally important in a fully allogeneic mismatched model for the induction of cardiac allograft tolerance.
Abbreviations:
-
- APC
-
- antigen presenting cell
-
- BM
-
- bone marrow
-
- PDL-1
-
- programmed death ligand-1
Introduction
T cells play a critical role in acute and chronic allograft rejection (1). In order to fully activate naive T cells, a second signal delivered by positive costimulatory molecules on antigen-presenting cells (APCs) is required. APCs also express negative costimulatory molecules that are capable of inhibiting T-cell activation. It is now clear that the integration of positive and negative costimulatory signals by T cells will ultimately determine the fate and functional status of the T-cell response (1), generating important potential targets to induce tolerance in organ transplantation.
Among the negative pathways, the programmed-death 1 (PD1) receptor and its ligands PDL1 (B7-H1) and PDL2 (B7-DC) have been well characterized. While PD1 is predominantly expressed on activated peripheral T cells and B cells, PDL1 and PDL2 are both expressed on APCs (2,3). Moreover, PDL1 is constitutively expressed by a variety of parenchymal cells, including heart, lung, kidney, pancreas and placenta, and can be induced on endothelial cells (2,4). The expression of PDL1 on nonhematopoietic cells suggests that PDL1 might be important in the regulation of autoreactive T and B cell responses in peripheral tissues and/or may regulate inflammatory responses in the target organs (4,5).
Since targeting negative costimulatory pathways might represent a physiologic means of dampening the alloimmune response and inducing long-term allograft survival, great interest has developed in the role of the PD1: PDL1 pathway in transplantation tolerance. Our group has shown that a blocking anti-PDL1 Ab abrogates tolerance induced by CTLA4-Ig in an established model of heart transplantation tolerance, demonstrating a critical role of PDL1 in allograft acceptance (6). In this study, we dissect the role of PDL1 expression on donor APCs and/or endothelial cells of donor hearts and demonstrate for the first time the critical role of PDL1 on donor endothelial cells for tolerance induction.
Material and Methods
Mice
C57BL/6 (H2b, B6) and BALB/c (H-2d) mice were purchased from Jackson Laboratory. PDL1−/− mice on the B6 background were maintained as a breeding colony in our animal facility. All mice were 8–12 weeks of age and housed in accordance with institutional and National Institutes of Health guidelines.
Bone marrow chimeric mice
Bone marrow (BM) cells were isolated from B6 wild-type and PDL1−/− donor mice. Four-week-old recipients WT and PDL1−/− mice were lethally irradiated with a single dose of 1100 rad. Twenty-four hours after irradiation, the recipients were infused intravenously with 2 × 107 BM cells suspension. The PDL1−/− recipients were infused with WT (W-P), and WT recipients were infused with PDL1−/− BM cells (P-W). In addition to that, B6 sham animals were created in which irradiated B6 WT mice were infused with BM cells from B6 WT mice (W-W) and PDL1−/− sham mice were generated by infusing PDL1−/− cells into irradiated PDL1−/− recipients (P-P). Six weeks after irradiation and BM reconstitution, the mice were euthanized and the hearts were transplanted into BALB/c recipients. We confirmed chimerism by analyzing the percentage of PDL1 expression on dentritic cells, macrophages, B cells, CD4+ T cells and CD8+ T cells in blood and peripheral lymphoid organs.
Heterotopic heart transplantation
Vascularized heart grafts were placed in an intra-abdominal location (7). Graft function was assessed by palpation of the heartbeat. Rejection was determined by complete cessation of palpable heartbeat and was confirmed by direct visualization after laparotomy. Graft survival is shown as the median survival time (MST) in days.
Antibodies and in vivo treatment protocol
CTLA4-Ig is a fusion protein composed of a human IgG1 fused to the extracellular domain of CTLA4, which was purchased from Bristol-Myers-Squibb. Cardiac allograft recipients were treated with CTLA4-Ig i.p. according to the following protocol: 0.5 mg of mAb on the day of transplantation and 0.25 mg on days 2, 4 and 6 after transplantation (6).
ELISPOT assay
Splenocytes recovered at 7 days after transplantation from BALB/c recipients of chimera or sham heart allografts were restimulated by irradiated donor-type splenocytes. The ELISPOT assay (R&D Systems) was adapted to measure the frequency of alloreactive T cells producing IFN-γ, IL-4 and IL-6, as reported previously (7). The frequencies of cytokine-secreting alloreactive cells were expressed as the number of cytokine-producing cells per 0.5 × 106 responder cells. All samples were tested in triplicate wells.
Flow cytometry
Splenocytes from BALB/c recipients of chimeric hearts at 7 days after transplantation were stained with fluorochrome-labeled mAbs against CD4, CD8, L-selectin (CD62L), CD44, CD25 and FoxP3 (BD Biosciences, San Jose, CA). Intracellular FoxP3 staining was performed using the Cytofix/Cytoperm intracellular staining kit. Flow cytometry was performed with a FACSCalibur system (BD Biosciences) and analyzed using FlowJo software, assessing regulatory T cells (CD4+CD25+FoxP3+) as well as CD4+ and CD8+ effector memory (CD44high CD62Llow phenotype) and central memory (CD44high CD62Lhigh phenotype) cells. For endothelial cell phenotyping, hearts were excised, minced and digested with ∼500 U/mL collagenase (Worthington Biochemical) for 30 min at 37°C. Cells were mashed through 70 μm filters and red blood cells were lysed. Cells were blocked with anti-CD16/CD32 (eBioscience) and incubated with fluorophore-conjugated anti-PDL1 (MIH5), anti-CD105 (MJ7/18), anti-CD31 (MEC13.3). After being washed, stained cells were analyzed on an LSRII flow cytometer and further analyzed using FlowJo software. Endothelial cells were identified as CD105+CD31+ cells.
Morphology
Cardiac graft samples from transplanted mice were recovered from rejecting (cessation of heartbeat by palpation) and long-term survivors (>100 days) as well as at 7–10 days after transplantation, then fixed in 10% formalin, embedded in paraffin, coronally sectioned, and stained with hematoxylin and eosin for evaluation of the degree of rejection according to International Society of Heart and Lung Transplantation (ISHLT) guidelines and for percentage of myocyte loss by light microscopy. An examiner blinded to the groups read all the samples.
Immunohistochemical and immunofluorescence staining
Immunohistochemical staining was performed on frozen sections of heart tissue as described previously (8). Briefly, 9 μm sections were cut from O.C.T. embedded tissues and fixed in acetone. Sections were blocked with normal goat serum and stained with anti-PDL1 (10F.9G2), then washed and stained with antimouse Alexa 546, Alexa-488 conjugated ICAM-1 (YN1/1) and Alexa-647 conjugated CD31 (MEC13.3). Isotype-matched antibodies were used as controls. Immunofluorescence was examined by confocal microscopy (Olympus FV1000 or Zeiss LSM510).
Statistics
Graft survival was expressed graphically using the Kaplan-Meier method, and statistical differences in survival between the groups were assessed by the log-rank test. Mann-Whitney nonparametric test was used for comparison of means between chimeric groups in our in vitro experiments. A p < 0.05 was considered statistically significant.
Results
Generation and characterization of chimeric PDL1−/− mice
We created chimeric mice by reconstituting PDL1-deficient mice with BM cells from wild-type mice (W-P) and wild-type mice with BM cells from PDL1-deficient mice (P-W) as previously described (4) (Figure 1A). Six weeks after BM reconstitution, PDL1 expression on different cell populations was determined in the chimeric animals by flow cytometry and immunohistochemistry. In the W-P mice, PDL1 expression was present on hematopoietic cells with predominance on APCs in both blood and spleens (Figure 1B), while no PDL1 was visualized on the endothelium of the hearts (Figure 2A). In contrast, PDL1 expression on P-W was minimal on APCs (Figure 1B) and was predominant on the endothelium of the hearts (Figure 2B). For controls, we generated B6 WT sham (W-W) and PDL1−/− sham mice (P-P), which underwent through the same procedure of irradiation and BM cells infusion as the chimeric mice described above but received BM cells from the same strain (Figure 1). In aggregate, W-P mice had a predominant expression of PDL1 on APCs while P-W on the endothelium of the hearts.
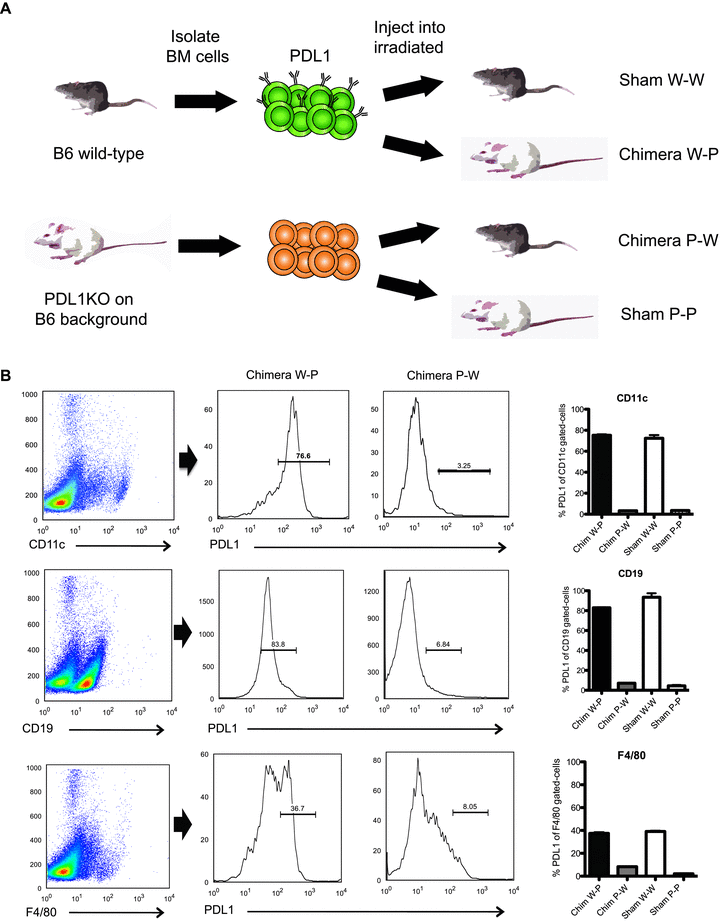
Generation of chimeric mice and expression of PDL1 on hematopoietic cells. (A) Lethally irradiated PDL1KO or WT mice were reconstituted with PDL1KO BM (P-P or P-W) or WT BM (W-P or W-W). (B) Six weeks after creation, chimerism was confirmed by fluorescent-activated cell sorter analysis for the percentage of PDL1-positive cells in blood and peripheral lymphoid organs. CD11c+, CD19+ and F4/80+ cells were gated (left column) and subsequently PDL1 expression was measured. The histograms shown are representative of at least three separate experiments per time point. On the far right column, the percentage of PDL1 expression on different APC populations are quantified and compared between chimeric animals and controls sham (W-W or P-P). Note W-W and W-P APCs show high levels of PDL1 expression in contrast to low expression in chimera P-W and sham P-P.
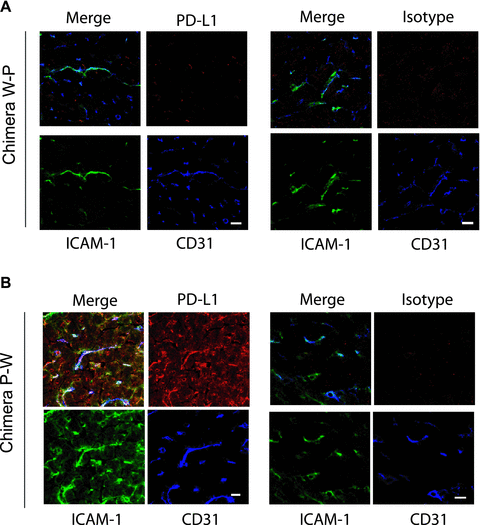
Analysis of PDL1 expression in cardiac allografts of chimeric mice. Immunohistochemistry demonstrated PDL1 staining expression on Chimera P-W hearts colocalized with CD31 and ICAM-1, consistent with predominant endothelial expression of PDL1 in the myocardium. While no PDL1 expression was seen on Chimera W-P. Isotype controls are shown on the right upper panels. Bars, 10 μm. Data are shown for one of three independent experiments.
PDL1 expression is upregulated on endothelium upon transplantation
In order to understand the expression pattern of PDL1 on the endothelium upon transplantation, we isolated endothelial cells from naïve chimera and transplanted animals 7 days after surgery. As expected, PDL1 expression was absent on W-P and present on P-W CD31+CD105+ endothelial cells (Figure 3A, B), confirming the immunohistochemistry findings. Upon transplantation, PDL1 was significantly upregulated on P-W endothelium (Figure 3C), while other markers of endothelium activation like ICAM-1 and B7-1 were similarly expressed on both groups (data not shown). This suggests that PDL1 upregulation on the endothelium of the allograft could play an important role in protecting the allograft against the alloimmune response.
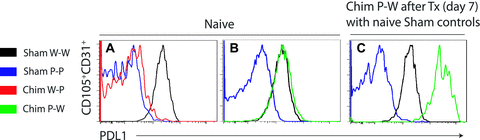
Endothelium PDL1 is upregulated upon transplantation. Flow cytometric analysis of endothelial cells (CD105+CD31+) from donor grafts before transplantation revealed that PDL1 is absent on Chimera W-P (A) and present on naïve Chimera P-W endothelial cells (B). Controls of sham naïve B6 WT (W-W) and PDL1KO (P-P) are shown in black and blue lines, respectively. Seven days after transplantation, endothelium PDL1 expression is significantly upregulated in Chimera P-W (C) as demonstrated by the right panel histogram. In the same histogram, naïve sham controls are shown. Histograms are from one experiment and are representative of three independent experiments.
PDL1 deficiency on donor nonhematopoietic cells prevents the induction of tolerance
We have previously shown the important role of intact recipient PDL1 in acquired transplantation tolerance (6). Since PDL1 is also expressed on both BM-derived and parenchymal cells in the donor allograft, the induction of tolerance could be dependent on PDL1 expression on either passenger leukocytes, nonlymphoid heart tissue or both. When B6 wild-type sham hearts (W-W) were transplanted into BALB/c recipients treated with CTLA4-Ig, allografts survived long term (MST > 100 days, n = 10) (Figure 4). However, when PDL1−/− sham hearts (P-P) were used as donors, all allografts were rejected despite CTLA4-Ig administration, reinforcing the key role of donor PDL1 for tolerance induction (MST = 9, n = 8; p < 0.0001). Subsequently, we transplanted hearts from chimeric mice into BALB/c recipients and treated them with CTLA4-Ig. Interestingly, the absence of PDL1 on nonhematopoietic cells in heart tissue (W-P) led to an early and accelerated rejection similar to the PDL1−/− sham hearts (MST = 7, n = 8; p = NS), while the presence of PDL1 solely on nonhematopoietic cells in heart tissue (P-W) delayed rejection; however it did not completely restore tolerance in all mice as seen in Figure 4 (n = 10, p < 0.0001 vs. sham P-P donor group).
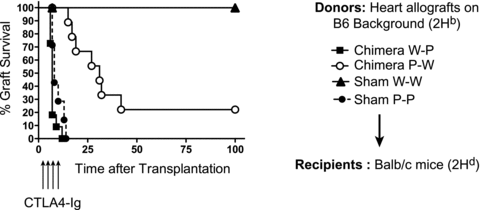
Absence of PDL1 expression on endothelial cells (W-P) significantly affected allograft survival in this model of acquired transplantation tolerance. While recipients of sham W-W hearts survived greater than 100 days, P-P sham hearts were all rejected by 10 days despite CTLA4-Ig. The lack of PDL1 on hematopoietic cells in donor hearts (P-W) also prevented complete tolerance with only a few grafts surviving >100 days (n = 8–10 per group).
Heart allografts lacking PDL1 on nonhematopoietic cells had significantly earlier and more severe rejection
The histology of the chimeric and sham allografts recovered at 1 week after transplantation was compared, at which time clinical rejection started to occur in some groups. CTLA4-Ig treatment was able to fully protect B6 WT sham allografts from rejection, while PDL1−/− sham hearts had significant cellular rejection and myocyte loss (Figure 5A, B). Chimera W-P also had similar rejection severity and myocyte loss when compared to PDL1−/− sham grafts. However, Chimera P-W had a significant milder alloimmune response at similar time point. Nonetheless, Chimera P-W grafts did develop worsening rejection overtime with significant difference when compared by histology to B6 sham controls at 100 days (Figure 5C). These findings suggest that donor nonhematopoietic PDL1 expression, particularly endothelium, plays a crucial role in early tolerance acquirement. Nonetheless, PDL1 on hematopoietic donor cells also contribute to acquired tolerance but at a later tempo.
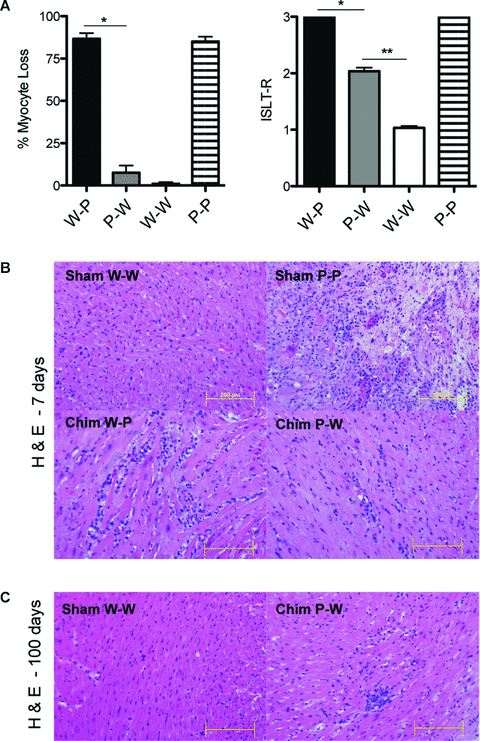
Histopathology analysis of cardiac allografts. (A) W-P and P-P hearts demonstrated significant myocyte loss and higher rejection scores when compared to P-W and W-W allografts (*p = 0.02, **p = 0.01). (B) Representative photomicrographs of hematoxylin-and-eosin staining at 7 days after transplantation showing no evidence of cellular infiltration in W-W sham heart in contrast to severe infiltration and necrosis on P-P sham graft. W-P graft also demonstrates significant cellular infiltration while P-W has a less aggressive picture at same time point. (C) Pathology (H & E staining) at 100 days after transplantation showing normal myocardium on W-W sham heart reflecting the tolerogenic state and mild cellular infiltration on surviving P-W cardiac allograft. Histology results presented are from one experiment and are representative of three independent experiments. Bars, 200 μm.
Absence of PDL1 on nonhematopoieitic cells of the allograft enhanced recipients’ alloreactivity
To further understand the mechanism by which PDL1 chimeric donors led to a difference in allograft outcome, we measured the frequency of alloreactive cytokine-producing cells 7 days after transplantation. Recipients of W-P grafts had significantly higher frequency of alloreactive T cells producing IFN-γ when compared to P-W donors (Figure 6A). In addition to that, IL-6 and Granzyme B production was also significantly upregulated, while no significant difference was present for IL-4 (Figure 6A). Taken together, these data show that the absence of PDL1 on nonhematopoietic cells of the allograft leads to increased alloreactivity in the recipient with significant polarization toward Th1 effector cells, which are known to be major mediators of allograft rejection.
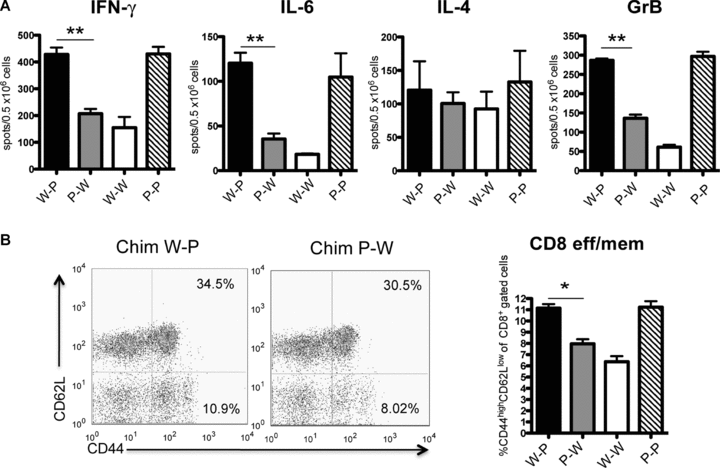
(A) ELISPOT analysis of IFN-γ, IL-4, IL-6 and Granzyme B cytokine profile of splenocytes derived from transplant recipients treated with CTLA4-Ig (1 week after transplantation). Recipients of W-P grafts showed a significant increase in the frequency of alloreactive IFN-γ-, GrB- and IL-6-producing splenocytes as compared with P-W donors (**p < 0.01). The frequency of IL-4-producing T cells was no different between groups. (B) Frequency of alloreactive CD8+ effector T cells in splenocytes derived from transplant recipients of chimeric donor hearts one week after transplantation. There was a higher frequency of CD8+CD44high CD62Llow in PDL1-deficient environment (n = 6 each group, *p = 0.03). Flow cytometry panels are representative examples of dot plots demonstrating the percentage of CD8+ effector memory cells in Chimera W-P and Chimera P-W donor groups. Data are representative of at least two independent experiments using a minimum of n = 3 mice per group. All measurements were done in triplicates.
Increased generation of effector T cells in hearts lacking endothelium PDL1 expression
Next, we assessed the effect of PDL1 expression in the allograft on the generation of alloreactive effector CD4+ and CD8+ T cells. Recipients of W-P donors had significantly higher CD8+ effector/memory cells (11.15%± 0.35%, p = 0.03) when compared to P-W donors (7.9%± 0.4%) (Figure 6B). However, no difference was observed in the frequency of CD4+ effector/memory cells (11.42 ± 2 vs. 10.18 ± 0.4, p = NS) or Tregs (8.35 ± 0.80 vs. 9.08 ± 0.20, p = NS). In the allografts of chimeric animals, a similar increase in CD8+ infiltrating T cells was observed in W-P when compared to P-W hearts (data not shown). These data suggest that PDL1 absence in the donor endothelium enhanced generation of effector T cells even in the presence of CTLA4-Ig.
Discussion
Our group and others have demonstrated the importance of the negative costimulatory pathway PD1:PDL1 in heart and skin transplantation with the use of an anti-PDL1 monoclonal antibody (MIH-6) (6,9–11). Transient blockade of PDL1 accelerated BALB/c cardiac allograft loss when recipients were deficient in either CD28 or B7 receptors (9). This antibody approach has multiple possible targets, including recipient and donor PDL1 receptors in hematopoietic and nonhematopoietic cells. Using PDL1-deficient mice, Tanaka et al. showed that PDL1−/− recipients had a significantly shortened graft survival after CTLA4-Ig administration when compared to WT recipients (6). Similarly, Wang et al. found that PDL1-deficient recipients were unable to be tolerized after combined anti-CD154 mAb and donor splenocyte transfusion (12). In the present study, we demonstrate for the first time a critical role of PDL1 expression in the donor cardiac allograft in acquired transplantation tolerance achieved through CTLA4-Ig administration in a fully MHC-mismatched model.
Through the generation of chimeric mice that expressed PDL1 either solely on hematopoietic cells or on nonhematopoietic cells of the heart (Figures 1 and 2), we were able to dissect the importance of PDL1 expression on different cell types in the donor allograft. Similarly, Yang et al. had shown that donor PDL1 deficiency was of great importance in protecting bm12 allografts from vasculopathy in B6 recipients (11). In this single MHC class II-mismatched model, allografts survive long term without immunosuppression but develop significant vasculopathy. However, PDL1-deficient recipients did not affect allograft survival in this single mismatched model (11), contrasting with the above outcomes in fully allogeneic model and questioning the validity of these findings in other models.
Donor PDL1 is a key mediator of T-cell tolerance within the heart, protecting the allograft against pathogenic alloreactive T cells in this fully allogeneic transplant model. The absence of PDL1 mainly on endothelium of the allograft led to early and accelerated rejection, abrogating the tolerogenic effects of CD28:B7 blockade and leading to similar survival as PDL1−/− sham hearts. Nonhematopoietic PDL1 expression has been reported to be of key importance in murine autoimmune diabetes, in which pancreatic islet cells lacking PDL1 led to earlier onset diabetes associated with significant CD4+ T-cell infiltration and activation (4) and endothelial PDL1 expression was shown to protect the myocardium against T-lymphocyte-induced myocarditis (13). Furthermore, PDL1 expression on sinusoidal endothelial cells and Kupffer cells are known to be essential for liver transplant spontaneous tolerance (14). Our studies show for the first time that PDL1 expression by the endothelium is required to achieve cardiac allograft tolerance in a fully allogeneic mismatched model. Moreover, donor APCs lacking PDL1 also affected the outcome of CTLA4-Ig treated animals preventing complete tolerance in most but not all recipients, suggesting a contributory role. This latter finding expands a prior observation that donor dendritic cells are important for tolerance development (15) and suggests that PDL1 on donor APCs might be involved in this process.
In the heart, PDL1 is constitutively expressed on resting cardiac endothelium and significantly upregulated in the setting of inflammation, especially when IFN-γ is present (2, 13, 16). Here we demonstrated by flow cytometry and immunohistochemical staining of hearts taken 7 days after transplantation that PDL1 staining was predominantly detected on endothelial cells of heart tissue and its expression was significantly upregulated in alloimmunity (Figure 3). The PDL1 upregulation is a mechanism in which the heart could protect itself from excessive inflammation and/or alloimmune response (2,13). It is worth noticing that the upregulation of PDL1 was especially significant in the chimeric P-W (Figure 3B), in which a stronger alloimmune response with higher IFN-γ was observed.
Our mechanistic data showed that chimeric donors that lacked PDL1 on the endothelium demonstrated increased alloreactivity with significant higher frequency of IFN-γ secreting cells and expansion of CD8+ effector memory cells (Figure 5). The ability of endothelial cells to enhance T-cell activation is well established (17,18), and has been attributed to the presence of MHC and costimulatory molecules on endothelial cells. Mouse endothelium has antigen-presenting capability and upregulates MHC class I and II in the setting of IFN-γ and can activate CD8+ T cells through direct allorecognition (19). Moreover, costimulation in trans could also play a role between endothelial cells of the donor heart and T cells from the recipient even in the absence of direct antigen presentation, since this type of interaction has been demonstrated to be important in the alloimmune response in vivo (20). Nevertheless, we acknowledge that our experimental design does not permit us to dissect how important transcostimulation versus ciscostimulation is in our in vivo model.
The role of costimulatory signals by the endothelium is consistent with previous reports showing that endothelial cells are capable of activating CD8+ T-cell response in vitro (16,19,21). Moreover, endothelial PDL1 has also been shown to downregulate T-cell activation in vitro (16) and the absence of negative PDL1 signaling can lead to unopposed positive costimulation, enhancing T-cell activation. The significant CD8+ T-cell expansion and alloreactivity in PDL1-deficient endothelium in our model suggest that this could be a potential mechanism important in tolerance development, especially since donor endothelial cells remain on the allograft indefinitely, resulting in a lasting stimulus for allorecognition in direct contact with circulating leukocytes. In addition, higher Granzyme B production in our study translates into enhance cytolytic activity of CD8+ T cells, in agreement with prior findings of PDL1 blockade of the endothelium in vitro (16). Another possible mechanism has been proposed by Kreisel et al. in which PDL1 expression on vascular endothelium could induce allogeneic regulatory T cells (22). However, we were unable to demonstrate any difference in Treg generation between chimeric donors in our model, making unlikely to be responsible for such a difference in allograft survival. Finally, in addition to its role in primary T-cell activation in the graft, nonhematopoietic donor cells expressing PDL1 could be regulating the function or further expansion of effector T cells that had been already activated in the peripheral lymphoid system and migrated to the graft, functioning as regulators of the alloimmune response intragraft.
Taken together, the present study demonstrates a key role of donor PDL1 expression in controlling the T-cell-mediated alloimmune response and in the induction of tolerance mediated by CD28:B7 blockade. These findings have important clinical implications for the understanding of physiologic mechanisms that promote transplantation tolerance and further reveal the complex interplay of positive and negative costimulatory pathways involving both endothelium and hematopoietic cells. Moreover, since CTLA4-Ig is currently approved for use in kidney transplantation, it is important to understand the signals that may abrogate its beneficial therapeutic effects and avoid inhibiting the expression or blockade of these signals in humans. Finally, targeting PDL1 expression on donor tissue via an antibody approach or gene therapy might permit the development of newer therapeutic strategies to achieve the ultimate goal of tolerance.
Acknowledgments
This work was supported by the following grants: research grant from the American Society of Transplantation to L.V.R., National Kidney Foundation grant to M.Y.; National Institute of Health (NIH) grant RO1 AI51559 to M.H.S.; and PO1AI56299 to A.H.S. and M.H.S.; T32 AI070085 to P.T.S.; N.N. is a recipient of the American Heart Association Scientist Development grant and NIH 1KO8 AI064335-01A2.
2We thank Olaf Boenisch for invaluable technical assistance.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




